Key Points
Myeloablative haploBMT with PTCy has low rates of nonrelapse mortality and GVHD for pediatric and adult patients with hematologic malignancies.
Abstract
Promising results have been reported for patients with high-risk hematologic malignancies undergoing HLA-haploidentical bone marrow transplantation (haploBMT) with posttransplantation cyclophosphamide (PTCy), but there are few data on outcomes with myeloablative conditioning in this context. We report the results of a single-institution, prospective phase 2 trial of myeloablative haploBMT using busulfan-based or total body irradiation–based conditioning in 96 children or adults (median age, 42 years; range, 1-65 years) with high-risk hematologic malignancies. Recovery of neutrophils and platelets occurred at a median of 24 and 29 days. Engraftment of donor cells with chimerism >95% was achieved in 91%. The cumulative incidence of acute graft-versus-host disease (GVHD) grades II to IV and grades III to IV at day 100 was 11% and 4%, and of chronic GVHD at 6 and 12 months was 4% and 15%, with 6% moderate to severe. The cumulative incidence of nonrelapse mortality was 6% at 100 days and 11% at 1 year (19% in those aged >55 years). The cumulative incidence of relapse at 1 year was 35%; at 3 years, it was 43%. In multivariable analysis, relapse was associated with increased age (P = .02 for age 20-55 years and P = .02 for age >55 years) and with minimal residual disease before transplantation (P = .05). The overall survival at 1 and 3 years is 73% and 54%, and event-free survival at 1 and 3 years is 57% and 49%. We show that haploBMT with PTCy after myeloablative conditioning is safe and efficacious for adult and pediatric patients with hematologic malignancies. Careful consideration must be given to using myeloablative conditioning in patients age >55 years. This trial was registered at www.clinicaltrials.gov as #NCT00796562.
Introduction
HLA-haploidentical bone marrow transplantation (haploBMT) has increased significantly in the past decade with the development of novel methods to control the powerful allogeneic reactions generated in the HLA-mismatched setting. Posttransplantation cyclophosphamide (PTCy) has gained national and international acceptance as an effective, safe, and affordable method to perform haploBMT, with outcomes similar to those from HLA-matched donors in comparative studies that are largely retrospective or focused on the adult population.
HaploBMT with PTCy successfully addresses several limitations of allogeneic BMT. It provides near-universal donor availability (especially donor access for ethnic minorities), decreases toxicity while maintaining efficacy for patients with high-risk hematologic malignancies, and offers a platform that is transportable and affordable to centers around the globe, particularly in the setting of reduced-intensity conditioning (RIC).
After obtaining promising results with nonmyeloablative (NMA) haploBMT, several groups have explored more intense conditioning regimens. These regimens include fludarabine with busulfan (Bu) and thiotepa,1 fludarabine with melphalan and thiotepa or total body irradiation (TBI),2,3 fludarabine with TBI,4,5 and Bu and cyclophosphamide (Cy) with peripheral blood stem cells (PBSCs) for patients with hematologic malignancies.6 We report here results of a prospective, phase 2 clinical trial of haploBMT and PTCy after myeloablative conditioning (MAC) with Cy and Bu or Cy and TBI in 96 children and adults with high-risk hematologic malignancies.
Methods
Study design
The primary objective of this single-institution phase 2 study (#NCT00796562) was to characterize donor engraftment at day 60 after MAC haploBMT with PTCy in patients ages 6 months to 65 years with high-risk hematologic malignancies conditioned with Bu/Cy or Cy/TBI, with a hypothesis that graft failure would not exceed 10%. The trial was initially designed for patients in remission and with active relapse (n = 30). Analysis after 30 patients revealed only 1 graft failure. The decision was made to expand the trial to include only those patients who met standard BMT eligibility criteria (n = 44 additional patients in remission), with the exclusion of active relapse patients. Continued analysis of our primary objective always showed engraftment in at least 90% of the patients who had undergone transplant. We are reporting the patients undergoing transplant while in remission.
Prespecified secondary end points included nonrelapse mortality (NRM), acute graft-versus-host disease (aGVHD), chronic graft-versus-host disease (cGVHD), event-free survival (EFS), and overall survival (OS). All 96 consecutive haploBMT patients with high-risk hematologic malignancies treated with this approach while the trial was open are included. The Institutional Review Board (IRB) of the Johns Hopkins Hospital approved the study. Patients were either treated on the prospective IRB-approved institutional clinical trial (n = 74 [77%]), or following the current open study (n = 22 [23%]) if they were unable to be enrolled secondary to insurance coverage limitations. All patients signed consent. Separate IRB approval to include all patients in this report undergoing transplant on or following the current study was obtained.
Eligible patients had high-risk leukemia or lymphoma, as previously published.7,8 Criteria for BMT were based on institutional standards and included age 0.5 to 65 years, Eastern Cooperative Oncology Group performance status score ≤2, Karnofsky/Lansky score >70, left ventricular ejection fraction >45%, forced expiratory volume in 1 second and forced vital capacity ≥50% of predicted, total bilirubin level <2, and creatinine clearance >40 mL/min. Patients received a Bu/Cy conditioning regimen except those with acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma who received a Cy/TBI preparative regimen. Donors were ineligible if the recipient had anti-HLA donor-specific antibodies.
Transplantation platform
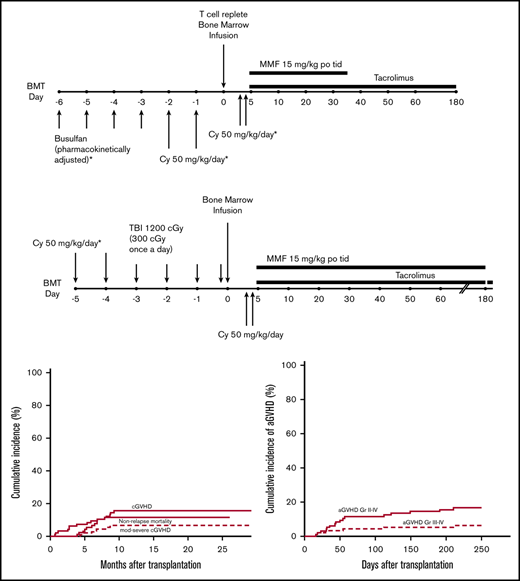
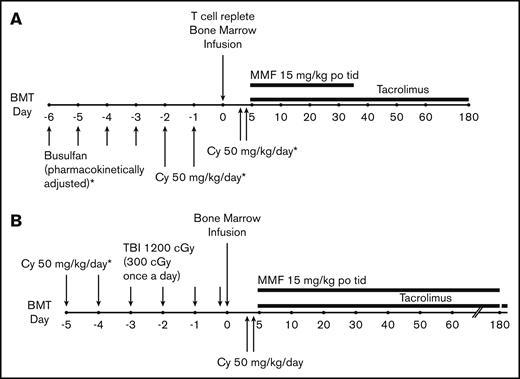
For the patients receiving Bu/Cy (Figure 1A), Bu was given every 6 hours for 4 consecutive days followed by Cy 50 mg/kg per day for 2 consecutive days. Bu dosing was started at either 0.8 mg/kg per dose, 32 mg/m2 per dose, or 1.1 mg/kg per dose IV per age and weight guidelines. Dosing was adjusted for the fifth and subsequent doses based on measured pharmacokinetic variables to achieve a targeted area under the concentration curve of 800 to 1400 mmol ⋅ min/L. Seizure prophylaxis with levetiracetam or fosphenytoin was administered for all patients aged >10 years receiving Bu. For the patients receiving Cy/TBI (Figure 1B), Cy 50 mg/kg per day was given for 2 consecutive days followed by 300 cGy of radiation once a day for 4 days (total of 1200 cGy). Allografts comprised unmanipulated bone marrow from an HLA-haploidentical (matched for at least one allele each of HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1) first-degree relative (full or half sibling, parent, or child) with a targeted dose of 4 × 108 marrow nucleated cells per kilogram of recipient ideal or actual body weight, whichever was lower. All patients received Cy 50 mg/kg per day on posttransplantation days 3 and 4. Mesna was administered on days of PTCy treatment in 4 divided doses at a total daily dose of 80% of the Cy dose. Additional GVHD prophylaxis consisted of mycophenolate mofetil 15 mg/kg orally 3 times per day (maximum total daily dose, 3 g) from days 5 through 35 and tacrolimus 0.015 mg/kg per dose 2 times daily, adjusted to maintain a serum trough level of 5 to 15 ng/mL, from days 5 to 180. Tacrolimus was given IV until the patient tolerated oral medications. Filgrastim was given only for delayed engraftment or life-threatening infection before engraftment. Supportive care was provided per institutional standard practice as previously described.8,9 Central nervous system (CNS) prophylaxis was left to the discretion of the treating physician for patients aged >25 years. Patients through age 25 years with acute myeloid leukemia (AML) and a history of CNS disease, and those with ALL regardless of history, received posttransplant CNS prophylaxis. No testicular prophylaxis was given during or after transplantation.
Treatment schemas. (A) Chemotherapy conditioning regimen. (B) TBI conditioning regimen. MMF, mycophenolate mofetil; po, by mouth; tid, thrice daily.
Treatment schemas. (A) Chemotherapy conditioning regimen. (B) TBI conditioning regimen. MMF, mycophenolate mofetil; po, by mouth; tid, thrice daily.
Definitions and end points
Neutrophil recovery time was defined as the number of days from BMT to the first of 3 consecutive days with an absolute neutrophil count >0.5 × 109/L. Platelet recovery time was defined as the number of days from BMT until the platelet count was >20 × 109/L without platelet transfusion in the preceding 7 days. Primary graft failure was defined as <5% donor chimerism measured on day 60. Secondary graft failure was defined as initial achievement of at least 5% donor chimerism, followed by its sustained loss with <5% donor chimerism. For study purposes, the designation of graft failure was based on analysis of sorted CD3+ T cells. GVHD was diagnosed by using standard criteria.10,11 aGVHD was treated per our institutional standard practice, which included corticosteroids as first-line therapy. Pretransplantation disease status was evaluated before the start of conditioning and included, at a minimum, bone marrow aspiration and biopsy with marrow flow cytometry and cytogenetics for patients with leukemia, or computed tomography (CT) and/or a positron emission tomography (PET) scan for patients with lymphoma. Morphologic complete remission (CR) of leukemia was determined by using standard criteria and was defined as <5% blasts on bone marrow biopsy specimens and absence of active extramedullary disease or evidence of disease on CT and/or PET imaging. Patients not in morphologic CR were considered to have active disease. Untreated myelodysplastic syndrome (MDS) patients or MDS patients without response to pretransplantation therapy were considered to have active disease regardless of blast count. Minimal residual disease (MRD) in patients in morphologic CR was defined as any disease detectable by flow cytometry, cytogenetics, fluorescence in situ hybridization, and/or polymerase chain reaction (PCR), or by CT and/or PET imaging.
Investigators blinded to patient outcomes defined pretransplantation remission status. Posttransplantation disease status was first assessed by bone marrow biopsy at ∼day 60. However, patients with circulating blasts, delayed engraftment, or other clinical concerns for relapse had their disease status evaluated before day 60. Relapse was defined as any detectable disease posttransplantation, including at flow cytometric, cytogenetic, and/or the molecular level. Therapies to prevent relapse such as tyrosine kinase inhibitors for Philadelphia chromosome positive ALL or chronic myeloid leukemia, or sorafenib for FLT3–internal tandem duplication disease, were permitted. Patients were followed up for infectious complications for 6 months post-BMT. Cytomegalovirus (CMV) reactivation was defined as a PCR >150 copies/mL based on the level of detection of the institutional assay. Antiviral therapy for CMV reactivation was initiated when the PCR was >500 to 1000 copies/mL on 2 occasions ∼1 week apart.
Statistical analysis
Proportions are reported with exact 95% binomial confidence intervals (CIs). EFS and OS were estimated with the method of Kaplan and Meier and compared by using the log-rank statistic or the proportional hazards regression model. Outcomes with competing risk were assessed with the proportional subdistribution hazard regression model for competing risks.12 Statistical analyses were performed by using SAS version 9.2 (SAS Institute Inc., Cary, NC) and R version 3.0 (R Foundation for Statistical Computing, Vienna, Austria). For EFS, an event was defined as relapse or death from any cause. OS was defined from transplantation date to the date of event occurrence or censored at last follow-up for patients without an observed event. NRM was a competing risk for relapse and vice versa. Competing risks for GVHD were defined as graft failure, relapse, donor lymphocyte infusion, or death from any cause. Univariate analysis included MRD status, CMV at risk (patient and/or donor CMV immunoglobulin G–positive), nucleated cell/kg (<4, 4-5, >5 × 108), age (<20 years [n = 16], 20-55 years [n = 54], >55 years [n = 26]), ABO incompatibility (none, minor, major), sex mismatch (none, female to male, male to female), diagnosis (AML, ALL, other), and disease risk index (DRI) (low, intermediate, high). Multivariate analyses were conducted for all patients by including variables derived from stepwise procedures based on P < .1. Significance in multivariate analyses or in direct comparisons between groups was based on P < .05.
Results
Patient, donor, and allograft characteristics
Patient, donor, and allograft characteristics are summarized in Table 1. The median patient age was 42 years (range, 1-65 years), and the median donor age was 37 years (range, 11-77 years). The median number of HLA allele mismatches was 4/10 (range, 2-5). Twenty-three patients (24%) received a TBI-based preparative regimen. The DRI13 assignments included 6 patients (6%) in the low-risk group, 61 patients (64%) in the intermediate-risk group, and 29 patients (30%) in the high-risk group. Twelve of the patients with AML (29%) and 7 of the patients with ALL (33%) had evidence of MRD before haploBMT. Eight (67%) of the patients with MDS were untreated, and 5 (44%) of the patients with lymphoma had evidence of disease pre-BMT. Median follow-up of surviving patients is 39 months (range, 12-79 months).
Patient, donor, and allograft characteristics (N = 96)
| Characteristic . | Value . |
|---|---|
| Age at BMT, median (range), y | 42 (1-65) |
| Patient sex, male, n (%) | 56 (58) |
| Disease, n (%) | |
| AML | 41 (43) |
| CR1 | |
| MRDneg | 19 |
| MRDpos | 9 |
| CR ≥2 | |
| MRDneg | 10 |
| MRDpos | 3 |
| ALL | 20 (21) |
| CR1 | |
| MRDneg | 9 |
| MRDpos | 2 |
| CR ≥2 | |
| MRDneg | 5 |
| MRDpos | 4 |
| Mixed lineage leukemia | 5 (5) |
| MDS | 11 (11.5) |
| Active | 8 |
| Lymphoma | 11 (11.5) |
| CR | 6 |
| Partial remission | 5 |
| Chronic myeloid leukemia | 4 (4) |
| Other | 4 (4) |
| DRI, n (%) | |
| Low | 6 (6) |
| Intermediate | 61 (64) |
| High | 29 (30) |
| Conditioning, n (%) | |
| Bu/Cy | 73 (76) |
| Cy/TBI | 23 (24) |
| Donor age, median (range), y | 37 (11-77) |
| Donor sex, male, n (%) | 44 (46) |
| Sex mismatch, n (%) | |
| Female donor to male recipient | 30 (31) |
| Male donor to female recipient | 18 (19) |
| None | 48 (50) |
| Graft cell counts | |
| Nucleated cells/kg infused × 108 | 4.8 (2.4-11.2) |
| CD34+/kg infused × 106 | 4.8 (0.3-35) |
| CD3+/kg infused × 107 | 4.6 (3.7-11.6) |
| CMV at risk, n (%) | 53 (55) |
| Median no. of HLA allele mismatches | 4/10 |
| ABO incompatibility, n (%) | |
| Major | 8 (8) |
| Minor | 15 (16) |
| None | 73 (76) |
| Characteristic . | Value . |
|---|---|
| Age at BMT, median (range), y | 42 (1-65) |
| Patient sex, male, n (%) | 56 (58) |
| Disease, n (%) | |
| AML | 41 (43) |
| CR1 | |
| MRDneg | 19 |
| MRDpos | 9 |
| CR ≥2 | |
| MRDneg | 10 |
| MRDpos | 3 |
| ALL | 20 (21) |
| CR1 | |
| MRDneg | 9 |
| MRDpos | 2 |
| CR ≥2 | |
| MRDneg | 5 |
| MRDpos | 4 |
| Mixed lineage leukemia | 5 (5) |
| MDS | 11 (11.5) |
| Active | 8 |
| Lymphoma | 11 (11.5) |
| CR | 6 |
| Partial remission | 5 |
| Chronic myeloid leukemia | 4 (4) |
| Other | 4 (4) |
| DRI, n (%) | |
| Low | 6 (6) |
| Intermediate | 61 (64) |
| High | 29 (30) |
| Conditioning, n (%) | |
| Bu/Cy | 73 (76) |
| Cy/TBI | 23 (24) |
| Donor age, median (range), y | 37 (11-77) |
| Donor sex, male, n (%) | 44 (46) |
| Sex mismatch, n (%) | |
| Female donor to male recipient | 30 (31) |
| Male donor to female recipient | 18 (19) |
| None | 48 (50) |
| Graft cell counts | |
| Nucleated cells/kg infused × 108 | 4.8 (2.4-11.2) |
| CD34+/kg infused × 106 | 4.8 (0.3-35) |
| CD3+/kg infused × 107 | 4.6 (3.7-11.6) |
| CMV at risk, n (%) | 53 (55) |
| Median no. of HLA allele mismatches | 4/10 |
| ABO incompatibility, n (%) | |
| Major | 8 (8) |
| Minor | 15 (16) |
| None | 73 (76) |
Engraftment
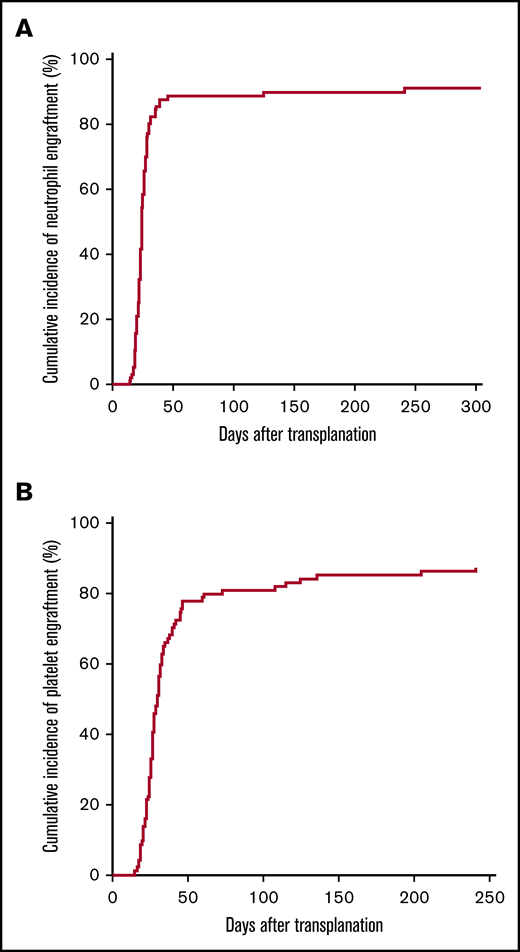
Neutrophils and platelets recovered at a median of 24 and 29 days, respectively. Cumulative incidence of neutrophil and platelet recovery at day 28 was 76% (95% CI, 0.7, 0.9) and 46% (95% CI, 0.4, 0.6), respectively, and overall were 91% (95% CI, 0.9, 1) and 87% (95% CI, 0.8, 1) (Figure 2). The cumulative incidence of neutrophil and platelet recovery at day 28 according to preparative regimen (Bu/Cy and Cy/TBI) is given in supplemental Figures 1A and 2A. Engraftment of donor cells with donor chimerism >95% at day 60 was achieved in 80 (91%) of 88 evaluable patients (95% CI, 83, 96). Patients who did not achieve >95% donor chimerism included 4 patients with AML and 1 patient with MDS; all 5 had received a Bu/Cy preparative regimen. All 5 patients were >55 years old; their donors ranged in age from 22 to 51 years, and grafts all contained at least 4 × 108 nucleated cells/kg.
Cumulative incidence of neutrophil and platelet engraftment. (A) Neutrophil engraftment. (B) Platelet engraftment.
Cumulative incidence of neutrophil and platelet engraftment. (A) Neutrophil engraftment. (B) Platelet engraftment.
Graft-versus-host disease
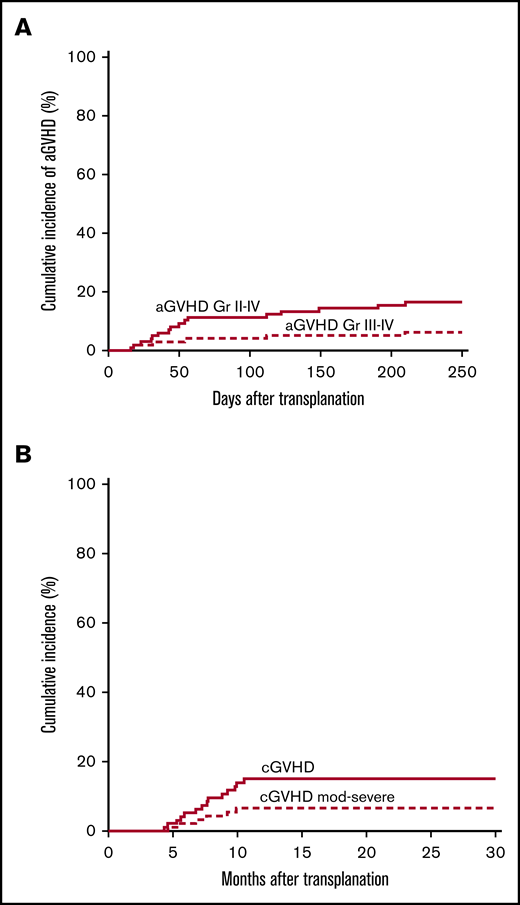
The cumulative incidence of aGVHD grades II to IV and grades III to IV at day 100 was 11% (95% CI, 0.05, 0.18) and 4% (95% CI, 0, 0.08), respectively (Figure 3A). The cumulative incidence of cGVHD at 6 and 12 months was 4% (95% CI, 0, 0.08) and 15% (95% CI, 0.08, 0.22), with 6% (95% CI, 0.01, 0.12) moderate to severe according to National Institutes of Health consensus criteria (Figure 3B).10 The cumulative incidence of aGVHD and cGVHD according to preparative regimen (Bu/Cy and Cy/TBI) is given in supplemental Figures 1B and 2B.
Acute and chronic GVHD. (A) Cumulative incidence of aGVHD grades II to IV and grades III to IV. (B) Overall and moderate to severe cGVHD.
Acute and chronic GVHD. (A) Cumulative incidence of aGVHD grades II to IV and grades III to IV. (B) Overall and moderate to severe cGVHD.
Nonrelapse mortality
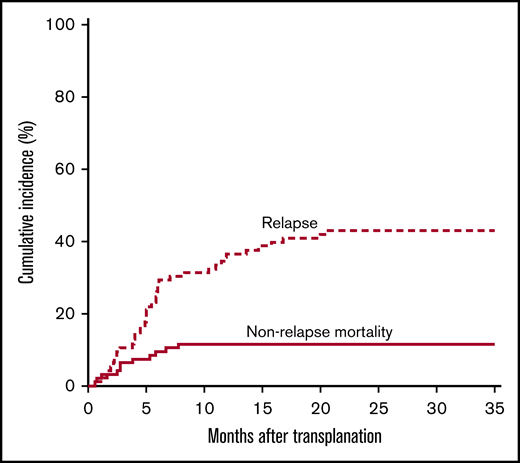
The cumulative incidence of NRM (Figure 4) was 6% (95% CI, 0.01, 0.11) at 100 days and 11% (95% CI, 0.05, 0.18) at 1 year. Causes of death in these 11 patients included GVHD (n = 2, 62 and 36 years old), cardiomyopathy (n = 1, doxorubicin associated, autopsy proven, 63 years old), sinusoidal obstruction syndrome (n = 2, 42 and 22 years old), drug-induced liver injury (n = 1, 29 years old), infection (gram-negative sepsis and disseminated adenovirus, n = 1, 22 months old; invasive rhinocerebral fungus, n = 1, 53 years old; multiple infections with multiorgan system failure, n = 1, 65 years old), and unknown (n = 2, 60 and 63 years old). The 1-year cumulative incidence of NRM was 19% (95% CI, 0.04, 0.35) for patients aged >55 years, 9% for patients aged 20 to 55 years (95% CI, 0.01, 0.17), and 6% for patients aged <20 years (95% CI, −0.06, 0.18). The cumulative incidence of NRM according to preparative regimen (Bu/Cy and Cy/TBI) is given in supplemental Figures 1C and 2C.
Relapse
The cumulative incidence of relapse at 1 year was 35% (95% CI, 0.26, 0.45), and at 3 years it was 43% (95% CI, 0.33, 0.53) (Figure 4). In a multivariate analysis of relapse, increasing age (hazard ratio [HR] of 5.4 [95% CI, 1.4, 21.4; P = .02] for age 20-55 years and HR of 5.5 [95% CI, 1.3, 23.3; P = .02] for age >55 years) was associated with increased relapse, as was having MRD pre-BMT (HR, 1.9; 95% CI, 1, 3.4; P = .05) (Table 2). The small number of patients and events in the low-risk DRI group limits our ability to estimate the effect of DRI across all levels. Comparing high-risk with other DRI, the association between DRI and relapse was significant (HR, 1.94; 95% CI, 1.05, 3.58; P = .03). The effect of DRI on relapse remained significant in multivariable analysis adjusted for age and MRD, or age alone (data not shown).
Multivariate regression models: relapse
| Variable . | SDHR . | LCL . | UCL . | P . |
|---|---|---|---|---|
| Age <20 | 1.0 | |||
| Age 20-55 y | 5.4 | 1.4 | 21.4 | .02 |
| Age >55 y | 5.5 | 1.3 | 23.3 | .02 |
| MRD– | 1.0 | |||
| MRD+ | 1.9 | 1 | 3.4 | .05 |
| Variable . | SDHR . | LCL . | UCL . | P . |
|---|---|---|---|---|
| Age <20 | 1.0 | |||
| Age 20-55 y | 5.4 | 1.4 | 21.4 | .02 |
| Age >55 y | 5.5 | 1.3 | 23.3 | .02 |
| MRD– | 1.0 | |||
| MRD+ | 1.9 | 1 | 3.4 | .05 |
LCL, lower confidence interval; UCL, upper confidence interval; SDHR, subdistribution HR.
Survival
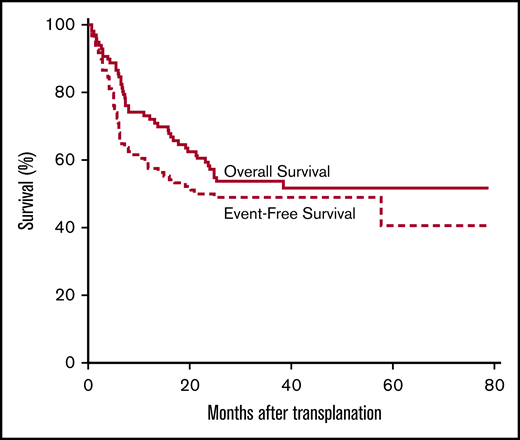
The overall survival at 1, 2, and 3 years is 73% (95% CI, 65, 82), 57% (95% CI, 48, 68), and 54% (95% CI, 44, 65), respectively. The EFS at 1, 2, and 3 years is 57% (95% CI, 48, 69), 50% (95% CI, 41, 61), and 49% (95% CI, 40, 60) (Figure 5). The OS differed according to refined DRI categorization, with an HR of 4.4 (95% CI, 0.59, 33.1) in the high-risk group and 2.9 (95% CI, 95% CI, 0.4, 21.6) in the intermediate-risk group compared with the low-risk group. The EFS differed according to refined DRI categorization, with an HR of 5 (95% CI, 0.66, 37.2) in the high-risk group and 3.4 (95% CI, 0.5, 24.6) in the intermediate-risk group compared with the low-risk group. For patients aged >55 years, the OS at 1, 2, and 3 years was 65% (95% CI, 49, 86), 46% (95% CI, 30, 70), and 41% (95% CI, 26, 66) and the EFS was 46% at 1 year (95% CI, 30, 70) and 38% (95% CI, 24, 63) at 2 and 3 years. In those aged >55 years (n = 26), 5 patients died of NRM and 10 from relapse; for those aged 20 to 55 years (n = 54), 5 patients died of NRM and 23 from relapse; and for those aged <20 years (n = 16), 1 died of NRM and 1 from relapse.
Characteristics associated in multivariable analysis with worse survival included age (HR of 5.7 [95% CI, 1.4, 23.4; P = .02] for age 20-55 years and HR of 6.9 [95% CI, 1.6, 30; P = .01] for age >55 years), and donor or recipient CMV seropositivity (HR, 2; 95% CI, 1, 3.7; P = .03) (Table 3). In addition, age was associated with an inferior EFS (HR, 4 [95% CI, 1.2, 13; P = .02] for age 20-55 years and HR of 4.3 [95% CI, 1.3, 14.8, P = .02] for age >55 years), as was patient CMV seropositivity (HR, 1.8; 95% CI, 1.1, 3.2; P = .03) (Table 4).
Multivariate regression models: OS
| Variable . | HR . | LCL . | UCL . | P . |
|---|---|---|---|---|
| Age <20 y | 1.0 | |||
| Age 20-55 y | 5.7 | 1.4 | 24 | .02 |
| Age >55 y | 6.9 | 1.6 | 30 | .01 |
| CMV– | 1.0 | |||
| CMV at risk | 2.0 | 1.1 | 3.7 | .03 |
| Variable . | HR . | LCL . | UCL . | P . |
|---|---|---|---|---|
| Age <20 y | 1.0 | |||
| Age 20-55 y | 5.7 | 1.4 | 24 | .02 |
| Age >55 y | 6.9 | 1.6 | 30 | .01 |
| CMV– | 1.0 | |||
| CMV at risk | 2.0 | 1.1 | 3.7 | .03 |
Multivariate regression models: EFS
| Variable . | HR . | LCL . | UCL . | P . |
|---|---|---|---|---|
| Age <20 | 1.0 | |||
| Age 20-55 y | 4.0 | 1.2 | 13 | .02 |
| Age >55 y | 4.3 | 1.3 | 14.8 | .02 |
| CMV– | 1.0 | |||
| Patient CMV+ | 1.8 | 1.1 | 3.2 | .03 |
| Variable . | HR . | LCL . | UCL . | P . |
|---|---|---|---|---|
| Age <20 | 1.0 | |||
| Age 20-55 y | 4.0 | 1.2 | 13 | .02 |
| Age >55 y | 4.3 | 1.3 | 14.8 | .02 |
| CMV– | 1.0 | |||
| Patient CMV+ | 1.8 | 1.1 | 3.2 | .03 |
In a univariate analysis, there were no differences in OS (HR, 1.3; 95% CI, 0.6, 2.8; P = .4) or EFS (HR, 1.4; 95% CI, 0.7, 2.7; P = .4) between subjects who received Bu/Cy vs those who received Cy/TBI (supplemental Figures 1D and 2D). However, our study was not powered to detect differences between Bu/Cy and Cy/TBI preparative regimens, the trial was nonrandomized (all myeloid patients received Bu/Cy, and patients with ALL/lymphoblastic lymphoma received Cy/TBI), and these post hoc estimates of treatment effects are exploratory, especially in light of the high correlation between the diagnosis-assigned treatments.
Infections
Tables 5-10 summarize the infections that occurred within 180 days after transplantation.
Summary of infections
| Variable . | No. of episodes . | No. of patients with ≥1 episode/no. at risk . | % . | Median days to infection (range) . |
|---|---|---|---|---|
| Any infection type* | 143 | 65/96 | 68 | 48 (1-180) |
| Viral* | 28 | 26/96 | 27 | 71.5 (1-172) |
| Fungal | 19 | 17/96 | 18 | 66 (3-180) |
| Bacterial | 96 | 57/96 | 59 | 44.5 (1-180) |
| CMV viremia† | 27 | 27/53‡ | 51 | 41 (9-131) |
| Hemorrhagic cystitis | 19 | 19/96 | 20 | 24 (4-141) |
| Variable . | No. of episodes . | No. of patients with ≥1 episode/no. at risk . | % . | Median days to infection (range) . |
|---|---|---|---|---|
| Any infection type* | 143 | 65/96 | 68 | 48 (1-180) |
| Viral* | 28 | 26/96 | 27 | 71.5 (1-172) |
| Fungal | 19 | 17/96 | 18 | 66 (3-180) |
| Bacterial | 96 | 57/96 | 59 | 44.5 (1-180) |
| CMV viremia† | 27 | 27/53‡ | 51 | 41 (9-131) |
| Hemorrhagic cystitis | 19 | 19/96 | 20 | 24 (4-141) |
Does not include CMV or urine BK/adenovirus.
CMV viremia defined as >150 copies/mL on PCR.
CMV at-risk defined as donor CMV-positive, recipient CMV-positive, or both donor and recipient positive during pre-BMT evaluation, and includes 1 patient who was neither donor nor recipient positive but had CMV viremia.
Viral infections
| Characteristic . | Value . |
|---|---|
| No. of episodes | 28 |
| No. of patients with ≥1 episode (%) | 25 (26) |
| Median days to infection (range) | 71.5 (1-172) |
| Upper respiratory tract infection, n | |
| Rhinovirus | 13 |
| Parainfluenza | 3 |
| Respiratory syncytial virus | 2 |
| Influenza A | 3 |
| Human metapneumovirus | 1 |
| Lower respiratory tract infection, n | |
| Parainfluenza | 1 |
| Encephalitis, n | |
| Varicella zoster | 1 |
| Human herpes virus 6 | 1 |
| Blood, n | |
| Adenovirus | 1 |
| Other, n | |
| Varicella zoster (vesicle fluid) | 1 |
| Donor hepatitis C transmission | 1 |
| Characteristic . | Value . |
|---|---|
| No. of episodes | 28 |
| No. of patients with ≥1 episode (%) | 25 (26) |
| Median days to infection (range) | 71.5 (1-172) |
| Upper respiratory tract infection, n | |
| Rhinovirus | 13 |
| Parainfluenza | 3 |
| Respiratory syncytial virus | 2 |
| Influenza A | 3 |
| Human metapneumovirus | 1 |
| Lower respiratory tract infection, n | |
| Parainfluenza | 1 |
| Encephalitis, n | |
| Varicella zoster | 1 |
| Human herpes virus 6 | 1 |
| Blood, n | |
| Adenovirus | 1 |
| Other, n | |
| Varicella zoster (vesicle fluid) | 1 |
| Donor hepatitis C transmission | 1 |
Table does not include CMV or urine BK virus and adenovirus.
Fungal infections
| Characteristic . | Value . |
|---|---|
| No. of episodes | 19 |
| No. of patients with ≥1 episode (%) | 17 (18) |
| Median days to infection (range) | 66 (3-180) |
| Pneumonia, n | |
| Proven | 2 |
| Probable | 8 |
| Possible | 3 |
| Disseminated, n | |
| Candida species | 4 |
| Rhinocerebral, n | |
| Rhizopus | 1 |
| Sinusitis, n | |
| Candida krusei | 1 |
| Characteristic . | Value . |
|---|---|
| No. of episodes | 19 |
| No. of patients with ≥1 episode (%) | 17 (18) |
| Median days to infection (range) | 66 (3-180) |
| Pneumonia, n | |
| Proven | 2 |
| Probable | 8 |
| Possible | 3 |
| Disseminated, n | |
| Candida species | 4 |
| Rhinocerebral, n | |
| Rhizopus | 1 |
| Sinusitis, n | |
| Candida krusei | 1 |
Bacterial infections
| Characteristic . | Value . |
|---|---|
| No. of episodes | 96 |
| No. of patients with ≥1 episode (%) | 57 (59) |
| Median days to infection (range) | 44.5 (1-180) |
| Blood, n | 51 |
| Coagulase-negative staphylococci | 17 |
| Klebsiella pneumoniae | 11 |
| Vancomycin-resistant enterococci | 8 |
| Pseudomonas aeruginosa | 3 |
| Streptococcus viridans | 3 |
| Other | 9 |
| Stool, n | 29 |
| Clostridioides difficile | 29 |
| Urine, n | 13 |
| Enterococcus | 6 |
| K pneumoniae | 4 |
| Escherichia coli | 2 |
| P aeruginosa | 1 |
| Sputum, n | 3 |
| E coli | 1 |
| Methicillin-resistant Staphylococcus aureus | 1 |
| Stenotrophomonas maltophilia | 1 |
| Characteristic . | Value . |
|---|---|
| No. of episodes | 96 |
| No. of patients with ≥1 episode (%) | 57 (59) |
| Median days to infection (range) | 44.5 (1-180) |
| Blood, n | 51 |
| Coagulase-negative staphylococci | 17 |
| Klebsiella pneumoniae | 11 |
| Vancomycin-resistant enterococci | 8 |
| Pseudomonas aeruginosa | 3 |
| Streptococcus viridans | 3 |
| Other | 9 |
| Stool, n | 29 |
| Clostridioides difficile | 29 |
| Urine, n | 13 |
| Enterococcus | 6 |
| K pneumoniae | 4 |
| Escherichia coli | 2 |
| P aeruginosa | 1 |
| Sputum, n | 3 |
| E coli | 1 |
| Methicillin-resistant Staphylococcus aureus | 1 |
| Stenotrophomonas maltophilia | 1 |
Hemorrhagic cystitis
| Characteristic . | Value . |
|---|---|
| Total no. of BK- or adenovirus-positive patients (%) | 37 (39) |
| No. of patients with hemorrhagic cystitis (%) | 19 (20) |
| BK virus positive | 17 |
| Adenovirus positive | 1 |
| Both BK and adenovirus negative | 1 |
| No. of hemorrhagic cystitis cases by grade (%) | |
| Grade I | 4 (21) |
| Grade II | 6 (32) |
| Grade III | 5 (26) |
| Grade IV | 4 (21) |
| Median days to hemorrhagic cystitis (range) | 24 (4-141) |
| Characteristic . | Value . |
|---|---|
| Total no. of BK- or adenovirus-positive patients (%) | 37 (39) |
| No. of patients with hemorrhagic cystitis (%) | 19 (20) |
| BK virus positive | 17 |
| Adenovirus positive | 1 |
| Both BK and adenovirus negative | 1 |
| No. of hemorrhagic cystitis cases by grade (%) | |
| Grade I | 4 (21) |
| Grade II | 6 (32) |
| Grade III | 5 (26) |
| Grade IV | 4 (21) |
| Median days to hemorrhagic cystitis (range) | 24 (4-141) |
CMV reaction
| Characteristic . | Value . |
|---|---|
| No. of at-risk patients with CMV viremia*(%) | 27/53 (51) |
| No. of low-risk† patients with viremia (%) | 12/23 (52) |
| No. of high-risk‡ patients with viremia (%) | 15/30 (50) |
| Median days to viremia (range) | 41 (9-98) |
| Median weeks to clear viremia | 3 |
| No. of at-risk patients with suspected CMV disease (%) | 4/53 (7.5) |
| Proven colitis | 1 |
| Probable pneumonitis | 1 |
| Possible pneumonitis | 2 |
| Characteristic . | Value . |
|---|---|
| No. of at-risk patients with CMV viremia*(%) | 27/53 (51) |
| No. of low-risk† patients with viremia (%) | 12/23 (52) |
| No. of high-risk‡ patients with viremia (%) | 15/30 (50) |
| Median days to viremia (range) | 41 (9-98) |
| Median weeks to clear viremia | 3 |
| No. of at-risk patients with suspected CMV disease (%) | 4/53 (7.5) |
| Proven colitis | 1 |
| Probable pneumonitis | 1 |
| Possible pneumonitis | 2 |
CMV viremia defined as >150 copies/mL on PCR.
CMV low-risk defined as both donor and recipient CMV-positive during pre-BMT evaluation and includes 1 patient who was neither donor nor recipient positive but had CMV viremia.
CMV high-risk defined as either donor or recipient CMV-positive during pre-BMT evaluation.
Pediatric/adolescent and young adult patient, donor, and allograft characteristics (n = 29)
| Characteristic . | Value . |
|---|---|
| Age at BMT, median (range), y | 14 (0-24) |
| Patient sex, male, n (%) | 16 (55) |
| Disease, n (%) | |
| AML | 6 (21) |
| CR1 | |
| MRDneg | 2 |
| MRDpos | 1 |
| CR ≥2 | |
| MRDneg | 3 |
| MRDpos | 0 |
| ALL | 10 (35) |
| CR1 | |
| MRDneg | 3 |
| MRDpos | 1 |
| CR ≥2 | |
| MRDneg | 3 |
| MRDpos | 3 |
| Mixed lineage leukemia | 2 (7) |
| MDS | 3 (10) |
| Active | 3 |
| Lymphoma | 7 (24) |
| CR | 4 |
| Partial remission | 3 |
| Other | 1 (3) |
| DRI, n (%) | |
| Low | 1 (4) |
| Intermediate | 16 (55) |
| High | 12 (41) |
| Conditioning, n (%) | |
| Bu/Cy | 16 (55) |
| Cy/TBI | 13 (45) |
| Donor age, median (range), y | 33 (11-58) |
| Donor sex, male, n (%) | 9 (31) |
| Sex mismatch, n (%) | |
| Female donor to male recipient | 12 (41) |
| Male donor to female recipient | 5 (18) |
| None | 12 (41) |
| Graft cell counts | |
| Nucleated cells/kg infused × 108 | 5.5 (3-12) |
| CD34+/kg infused × 106 | 5.4 (2.5-12.4) |
| CD3+/kg infused × 107 | 5.4 (2.3-11.67) |
| CMV at risk, n (%) | 18 (62) |
| Median no. of HLA allele mismatches | 4/10 |
| ABO incompatibility, n (%) | |
| Major | 3 (10) |
| Minor | 1 (3) |
| None | 25 (86) |
| Characteristic . | Value . |
|---|---|
| Age at BMT, median (range), y | 14 (0-24) |
| Patient sex, male, n (%) | 16 (55) |
| Disease, n (%) | |
| AML | 6 (21) |
| CR1 | |
| MRDneg | 2 |
| MRDpos | 1 |
| CR ≥2 | |
| MRDneg | 3 |
| MRDpos | 0 |
| ALL | 10 (35) |
| CR1 | |
| MRDneg | 3 |
| MRDpos | 1 |
| CR ≥2 | |
| MRDneg | 3 |
| MRDpos | 3 |
| Mixed lineage leukemia | 2 (7) |
| MDS | 3 (10) |
| Active | 3 |
| Lymphoma | 7 (24) |
| CR | 4 |
| Partial remission | 3 |
| Other | 1 (3) |
| DRI, n (%) | |
| Low | 1 (4) |
| Intermediate | 16 (55) |
| High | 12 (41) |
| Conditioning, n (%) | |
| Bu/Cy | 16 (55) |
| Cy/TBI | 13 (45) |
| Donor age, median (range), y | 33 (11-58) |
| Donor sex, male, n (%) | 9 (31) |
| Sex mismatch, n (%) | |
| Female donor to male recipient | 12 (41) |
| Male donor to female recipient | 5 (18) |
| None | 12 (41) |
| Graft cell counts | |
| Nucleated cells/kg infused × 108 | 5.5 (3-12) |
| CD34+/kg infused × 106 | 5.4 (2.5-12.4) |
| CD3+/kg infused × 107 | 5.4 (2.3-11.67) |
| CMV at risk, n (%) | 18 (62) |
| Median no. of HLA allele mismatches | 4/10 |
| ABO incompatibility, n (%) | |
| Major | 3 (10) |
| Minor | 1 (3) |
| None | 25 (86) |
Hemorrhagic cystitis
Hemorrhagic cystitis was documented in 19 (20%) patients at a median time of 24 days posttransplantation, with 15 (79%) of 19 cases grade II or higher and 9 (48%) of 19 cases grade III or higher (Table 9). Eighty-nine percent of patients with hemorrhagic cystitis tested positive for BK virus.
CMV reactivation
Of the 53 at-risk patients, 27 (51%) developed CMV reactivation at a median of 41 days posttransplantation (Table 10). One CMV seronegative patient with a seronegative donor developed CMV viremia during conditioning, possibly from unscreened blood products received at an outside hospital.
Viral infections
Twenty-eight episodes of non-CMV, non-BK viral infection occurred in 25 patients (26%) (Table 6). The median time to viral infection was 72 days posttransplantation. The majority (79%) were upper respiratory tract infections.
Fungal infections
Bacterial infections
Bacterial infections were documented for 57 patients (59%) (Table 8). The leading causes of bacteremia included coagulase-negative Staphylococcus (n = 17) and Klebsiella pneumoniae (n = 11).
Results for children and adolescents and young adults
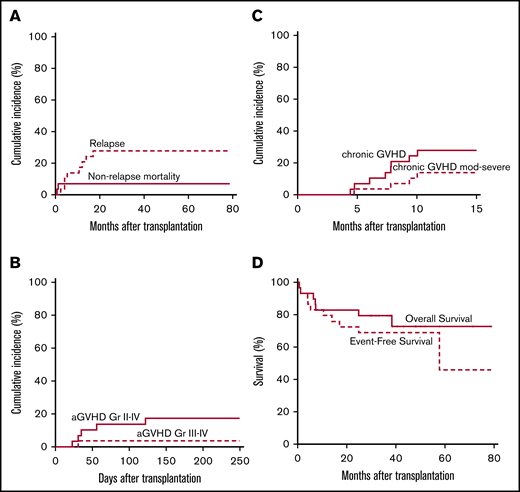
Patient, donor, and allograft characteristics are summarized in Table 11. Twenty-nine patients aged <25 years with a median age of 14 years (range, 1-24 years) are included. Neutrophil and platelet recovery occurred at a median of 24 and 29 days, respectively. Donor cell engraftment at day 60 with >95% donor chimerism occurred in 26 (96%) of 27 evaluable patients (95% CI, 81, 99.9). The cumulative incidence of NRM at 1 year was 7% (95% CI, not applicable, 0.2), and of aGVHD grades II to IV and grades III to IV at 8 months it was 17% (95% CI, 0.03, 0.3) and 4% (95% CI, not applicable, 0.1), respectively; the cumulative incidence of cGVHD at 1 year overall and moderate to severe was 28% (95% CI, 0.1, 0.4) and 14% (95% CI, 0.01, 0.3). The cumulative incidence of relapse at 1 year was 17% (95% CI, 0.03, 0.3), and at 3 years it was 28% (95% CI, 0.1, 0.4). OS and EFS at 3 years were 79% (95% CI, 66, 96) and 69% (95% CI, 54, 88) (Figure 6).
Pediatric/adolescent and young adult results. (A) Cumulative incidence of NRM and relapse. (B) Cumulative incidence of aGVHD grades II to IV and III to IV. (C) Cumulative incidence of overall and moderate to severe cGVHD. (D) OS and EFS.
Pediatric/adolescent and young adult results. (A) Cumulative incidence of NRM and relapse. (B) Cumulative incidence of aGVHD grades II to IV and III to IV. (C) Cumulative incidence of overall and moderate to severe cGVHD. (D) OS and EFS.
Discussion
We present results of a single-institution, prospective, phase 2 trial of MAC haploBMT with PTCy for hematologic malignancies that uniquely combines adult and pediatric patients, a Bu- or TBI-based preparative regimen, bone marrow grafts, and comprehensive data on post-BMT infections. Our high rates of donor engraftment and low rates of NRM and GVHD show the safety of this platform. Our results are comparable to other successful MAC haploBMT with PTCy regimens that have used a fludarabine/TBI preparative regimen with PBSCs, fludarabine/Bu/Cy or thiotepa with bone marrow or PBSCs, and Bu/fludarabine using bone marrow or PBSCs, all for adult acute leukemia and/or MDS patients in CR or with active disease.3-5,15 It is challenging to directly compare results because of differences in diagnoses, disease status, conditioning regimen, graft source, and patient age, but higher rates of engraftment, GVHD, and survival, and lower rates of relapse, in some of these studies can potentially be explained by the use of PBSCs, differences in the conditioning regimen, and/or a correlation between the development of GVHD and a graft vs leukemia effect.16 Further studies to prospectively test these possibilities are needed. There are now numerous publications on the use of haploBMT with PTCy after NMA and RIC, using both PBSCs and bone marrow, with safety and efficacy data comparable to HLA-matched donors.1,6,17-36 However, the absolute number of, and longitudinal experience with, patients who have undergone haploBMT after MAC still pales in comparison with those who have received MAC alloBMT from HLA-matched donors and cord blood. This underscores the importance of continuing to investigate outcomes of MAC haploBMT with PTCy and lays the groundwork for prospective randomized controlled BMT trials using MAC, different donor sources, and various haplo graft methods, especially for the pediatric and adolescent/young adult populations.
Several aspects of our data warrant further discussion. First, we continue to see low rates of aGVHD and cGVHD. This provides an opportunity to shorten the duration of posttransplant tacrolimus in future trials using MAC regimens, as has already been safely accomplished in the NMA setting.37 This is especially important given the fact that relapse remains a challenge after BMT regardless of donor type or conditioning regimen intensity. Incorporating novel agents early posttransplant to prevent and/or treat relapse is optimized when patients are off immunosuppression and without GVHD.
Second, the incidence of significant bacterial, fungal, and viral infections and reactivations (specifically CMV) in this study is similar to other published alloBMT studies in the HLA-matched and haplo with PTCy settings.38-44 Deaths as a result of infection continue to be low after haploBMT with PTCy. Hemorrhagic cystitis that is largely mediated by BK virus and occurs peri-engraftment has a reported incidence in haploBMT with PTCy from 20% to 70%.42-44 Importantly, our observed incidence of hemorrhagic cystitis was not different from what we find in our matched transplants not receiving PTCy.45 Albeit not usually life-threatening, BK-associated hemorrhagic cystitis significantly contributes to morbidity post-BMT, and strategies to decrease its incidence are warranted. We did not observe any cases of posttransplant Epstein-Barr virus lymphoproliferative disease.46
Third, our study includes a significant pediatric and adolescent/young adult population and an extensive report of infectious complications. Published pediatric prospective data using haploBMT with PTCy are limited. Our results in this age group compare favorably with BMT outcomes using HLA-matched donors and cord blood, as well as αβ T cell–depleted haploBMTs.47,48 The perceived increase in cGVHD rates in pediatric patients is most likely not a real phenomenon (particularly given our recent Pediatric Blood and Marrow Transplant Consortium data49 ) and rather due to the small numbers of patients included. One possible explanation is that pediatric patients tapered their tacrolimus from day 150 to 180 and adult patients stopped their tacrolimus at day 180. More recent trials have all patients stopping their tacrolimus at day 180, without any taper. This includes the completed Pediatric Blood and Marrow Transplant Consortium trial in which we saw 1 patient of 31 with extensive cGVHD.49
The interpretation of the results of this study is limited by patient heterogeneity. One important variable that requires further investigation includes the intensity of the preparative regimen that is required, as data suggest that MAC may not provide improved disease-free survival for certain disease types and status.21,50,51 Table 12 summarizes our institutional NMA and MAC results. Results are similar, but any direct comparisons are limited because MRD was not routinely tested in the early years of these studies, and there were no specific criteria to assign MAC vs NMA, lending to provider bias. Ongoing clinical trials will try to help prospectively identify outcomes that conditioning regimen intensity might affect. A recent Center for International Blood and Marrow Transplant Research publication of a large number of MAC vs RIC haploBMT with PTCy-treated patients revealed no differences in NRM, with lower disease-free survival and higher relapse after RIC for patients 18 to 54 years of age. In patients aged 55 to 70 years, there were no differences in disease-free survival, and relapse and NRM were lower using RIC. The authors concluded that MAC regimens are preferred for AML, ALL, and MDS; reduced-intensity regimens should be reserved for those unable to tolerate myeloablation.52 We also observed a higher NRM in patients aged >55 years compared with younger patients, and careful consideration must be given to using MAC in this age group. Better defining the age and additional factors associated with improved outcomes using MAC is important for future studies.
MAC and NMA HaploBMT with PT/Cy at Johns Hopkins
| Study . | Study period, N, age range (median, y) . | HSC source . | Cond. intensity . | Graft failure, % . | aGVHD grade II-IV, % . | aGVHD grade III-IV, % . | cGVHD, % . | NRM, % . | Relapse, % . | PFS/ EFS, % . | OS, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| McCurdy et al33 | 2002-2012, N = 372, 18-75 (55) | BM | NMA | 8 | 32 | 4 | 13 | 11 | 46 | 40 | 50 |
| Klein et al34 | 2003-2015, N = 40, 1-25 (20) | BM | NMA | 9 | 33 | 5 | 23 (7 moderate to severe) | 13 | 52 | 43 | 56 |
| Current study | 2008-2014, N = 96, 1-65 (42) | BM | MAC | 9 | 11 | 4 | 15 (6 moderate to severe) | 11 | 43 | 52 | 54 |
| Study . | Study period, N, age range (median, y) . | HSC source . | Cond. intensity . | Graft failure, % . | aGVHD grade II-IV, % . | aGVHD grade III-IV, % . | cGVHD, % . | NRM, % . | Relapse, % . | PFS/ EFS, % . | OS, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| McCurdy et al33 | 2002-2012, N = 372, 18-75 (55) | BM | NMA | 8 | 32 | 4 | 13 | 11 | 46 | 40 | 50 |
| Klein et al34 | 2003-2015, N = 40, 1-25 (20) | BM | NMA | 9 | 33 | 5 | 23 (7 moderate to severe) | 13 | 52 | 43 | 56 |
| Current study | 2008-2014, N = 96, 1-65 (42) | BM | MAC | 9 | 11 | 4 | 15 (6 moderate to severe) | 11 | 43 | 52 | 54 |
BM, bone marrow; Cond., conditioning; HSC, hematopoietic stem cell; PFS, progression-free survival.
As previously described, we observed a higher cumulative incidence of relapse in patients who were MRD positive and with a higher DRI, and we observed more NRM in patients aged >55 years.53-56 All of the graft failures occurred in patients conditioned with Bu/Cy. Thus age, conditioning regimen (chemotherapy-based vs TBI-based), graft source (ie, bone marrow vs peripheral blood), and posttransplant relapse prevention therapies require careful consideration. It will be important to include such powered analyses in future trials. In addition, in our trial, we used standard donor selection criteria. In future studies, it will be important to incorporate killer-immunoglobulin-like receptor alloreactivity, donor age and sex, and other donor characteristics in the analysis to optimize outcomes, especially because the number of HLA mismatches does not negatively affect haploBMT with PTCy outcomes.57-59
It is difficult to directly compare our results vs those of other haploBMT platforms such as megadose CD34+ selection60 and αβ T cell–depleted haploBMTs47,48 without randomized controlled trials. Results with PTCy are potentially associated with lower infectious complications and have reported success in both adult and pediatric patients. In contrast, successful megadose CD34+ and αβ T cell–depleted haploBMTs have been primarily successfully reported in pediatric patients. We look forward to the generation of additional data using these sophisticated cell-processing platforms and to further clarifying their advantages and limitations in both pediatric and adult populations, while acknowledging the unique transportability and accessibility afforded by PTCy.
In conclusion, our data indicate that haploBMT with PTCy after MAC offers a widely available platform that is safe and efficacious to transplant patients with high-risk hematologic malignancies.
Requests for data sharing may be submitted to the corresponding author (Heather J. Symons; e-mail: hsymons2@jhmi.edu).
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Cancer Institute (P01 CA015396 [R.J.J.] and P30 CA006973), the Giant Food Children’s Cancer Research Fund, and Otsuka Pharmaceuticals.
Authorship
Contribution: H.J.S. designed and conducted the trial, treated patients, performed the analysis, and wrote the paper; E.J.F. designed and conducted the trial and treated patients; M.Z. designed the trial, performed the analysis, and wrote the paper; Y.C performed the analysis and wrote the paper; K.C. and R.J.J. conducted the trial, treated patients, and wrote the paper; and A.C., C.G., O.K., N.L., E.T.Z., R.A., J.B.-M., I.B., R.B., A.D., I.G., M.S., L.S., B.D.S., and L.L. treated patients.
Conflict-of-interest-disclosure: None of the authors received any financial support for this trial. Currently, H.J.S. is on the speaker bureau for Jazz Pharmaceuticals. K.C. receives research support, is a consultant for, and is on the speaker bureau and advisory board for Jazz Pharmaceuticals. J.B.-M. is on a Drug Safety Monitoring Board for Incyte. I.G. receives research support from Merck Inc., Amgen, Amphivena, Celgene, and Genentech. L.L. receives research support from Genentech and Merck, serves on the speaker bureau for Merck, serves as a consultant and is on the advisory board for AbbVie, and is a patent holder for WindMIL Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Heather J. Symons, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans St, CRB I 2M-52, Baltimore, MD 21287; e-mail: hsymons2@jhmi.edu.
References
Author notes
The full-text version of this article contains a data supplement.