Key Points
In cancer-associated venous thromboembolism (VTE), the risk of recurrent VTE and major bleeding is similar with rivaroxaban and LMWH.
Rivaroxaban may be a reasonable alternative to LMWH in select patients with cancer-associated VTE.
Abstract
Guidelines provide differing recommendations regarding direct-acting oral anticoagulants vs low-molecular-weight heparin (LMWH) for treatment of cancer-associated thrombosis (CAT). This study was undertaken to evaluate the effectiveness and safety of rivaroxaban vs LMWH for treatment of CAT. Using US Surveillance, Epidemiology and End Results-Medicare–linked data from 2013 through 2016, we evaluated adults with active breast, lung, ovarian, or pancreatic cancer, who were admitted to the hospital or treated in the emergency department for CAT and were prescribed rivaroxaban or LMWH for outpatient anticoagulation. Patients with luminal gastrointestinal or genitourinary cancers were excluded. Rivaroxaban and LMWH users were 1:1 propensity score matched. Outcomes included the composite of recurrent thrombosis or major bleeding, each outcome separately, and mortality at 6 months, using an intent-to-treat approach. On-treatment analysis after 12 months was also performed. Proportional hazards models for the subdistribution of competing risk were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs). We included 529 rivaroxaban- and 529 LMWH-treated patients with CAT. Rivaroxaban was not associated with differences in risk of the composite outcome (HR, 0.71; 95% CI, 0.41-1.22), major bleeding (HR, 1.01; 95% CI, 0.50-2.01), or mortality (HR, 0.87; 95% CI, 0.70-1.07) vs LMWH, but it reduced recurrent thrombosis (HR, 0.37; 95% CI, 0.15-0.95). On-treatment analysis at 12 months showed similar results. Rivaroxaban may be a reasonable alternative to LMWH for patients with CAT without gastrointestinal or genitourinary cancer.
Introduction
Patients with active cancer are ∼5 times more likely to develop a venous thromboembolism (VTE) than those without.1 When a VTE occurs, patients with cancer carry up to a threefold higher rate of thrombosis recurrence and have approximately twice the risk of bleeding during anticoagulation.2,3 Therefore, when treating cancer-associated thrombosis (CAT), it is critical to use anticoagulants that optimize efficacy while minimizing bleeding risk.
Although guidelines4,5 include direct-acting oral anticoagulants (DOACs) as an alternative to low-molecular-weight heparin (LMWH) for treatment of CAT, specific recommendations for selection of candidates for DOAC treatment vary. The strength of the recommendation of DOACs to treat CAT is based on data from randomized controlled trials (RCTs) that compared them to LMWHs6,7 , with results suggesting that DOACs may reduce thrombosis risk but with potentially more frequent bleeding (particularly in those with gastrointestinal and genitourinary cancers).
Observational studies evaluating DOACs for CAT treatment have been published,8-11 but these studies were single arm,8,9 evaluated cancer subtypes not recommended for DOAC treatment (eg, gastrointestinal and genitourinary),9,10 were of limited sample size,8,9,11 and/or used heterogeneous definitions of active cancer.8,10 We sought to evaluate the effectiveness and safety of rivaroxaban vs LMWH for CAT treatment in select patients with active cancers that did not include luminal gastrointestinal or genitourinary malignancies.
Methods
We used US Surveillance, Epidemiology, and End Results (SEER)-Medicare–linked data12 from 2013 through 2016, to identify adults diagnosed with active (primary or metastatic) breast, lung, ovarian, or pancreatic cancer, who were admitted to the hospital or attended the emergency department who had a condition that was assigned a primary diagnosis code for VTE (positive predictive value of 95% [95% CI, 93-97]),13 who had ≥12 months of continuous medical and prescription benefits before the CAT, and who received rivaroxaban or an LMWH as their first outpatient anticoagulant for the index event. The SEER-Medicare–linked database combines these 2 large sources of information into 1 data set that reports patients’ demographic, clinical, and mortality data. SEER contains information about an incident cancer diagnosis, cancer-directed surgery, and radiation therapy. Medicare is the federally funded program that provides health insurance for the elderly, persons with end-stage renal disease, and some disabled patients in the United States. Almost all Medicare beneficiaries have Part A coverage, which includes hospital, skilled-nursing facility, and hospice admissions and some home health care, and also elect to pay a monthly premium to enroll in Part B of Medicare, which covers physician and outpatient services. Approximately 70% of patients with Medicare also have Part D coverage, which covers many prescription medications.12 Because of limitations in SEER access, only data on the 5 cancers studied most frequently in RCTs of anticoagulation for CAT (excluding colorectal cancer, because of the high risk of bleeding) were available for inclusion in our analysis. Active cancer in our study was defined as cancer in current treatment, diagnosed within 6 months of the index CAT, or associated with metastatic disease (regardless of time from initial cancer diagnosis).5 Patients with primary or metastatic luminal gastrointestinal cancer (oral, esophageal, stomach, or colorectal) or genitourinary cancer (adrenal, bladder, kidney, prostate, or testicular) were excluded from this analysis, as these cancers carry an increased risk of bleeding per the guidance of the International Society of Thrombosis and Haemostasis (ISTH).5 Of the available DOACs, ISTH recommends either edoxaban or rivaroxaban for the treatment of CAT.5 At the time of our SEER-Medicare data request, the number of patients with CAT treated with edoxaban was small (fewer than the 11 required for reporting per the Cell Size Suppression Policy of the Centers for Medicare and Medicaid Services (https://www.resdac.org/articles/cms-cell-size-suppression-policy). Therefore, the analysis evaluated only rivaroxaban and LMWH.
Patients receiving rivaroxaban were matched 1:1 with patients receiving LMWH, based on propensity scores calculated via multivariable logistic regression.14 The propensity scores were estimated based on accepted risk factors for differential anticoagulation exposure including age (binary, <75, ≥75), sex (binary), white race (binary), pulmonary embolism (PE) as the index event (binary), cancer type (categorical), time from first cancer diagnosis to index VTE (categorical: <1 year, 1 to <2 years, 2 to <3 years, ≥3 years); comorbidities (binary); and comedications (binary), as listed in Table 1. The presence of residual differences in measured covariates after cohort matching (caliper = 0.2 standard deviation of the logit of the propensity score) were assessed by calculating standardized differences (<0.1 was considered well balanced for each covariate).13 Propensity score matching was performed with MatchIT in R v3.4.3 (The R-Project for Statistical Computing).
Baseline characteristics of the 1:1 propensity score–matched study cohorts
| . | Rivaroxaban, % . | LMWH, % . | Standardized difference . |
|---|---|---|---|
| Demographics | |||
| Age, median (25%, 75%), y* | 73 (68, 78) | 72 (68, 78) | — |
| Age ≥75 y | 42.2 | 40.6 | 0.0306 |
| Female sex | 74.7 | 71.3 | 0.0780 |
| White race | 80.2 | 80.7 | −0.0142 |
| Index event PE (with or without DVT) | 43.5 | 45.4 | −0.0381 |
| Index DVT only | 56.5 | 54.6 | 0.0381 |
| Primary cancer site | |||
| Breast | 36.9 | 35.0 | 0.0392 |
| Lung | 43.5 | 46.3 | −0.0572 |
| Ovary | 6.2 | 6.8 | −0.0235 |
| Pancreas | 10.2 | 8.7 | 0.0501 |
| Other | 3.2 | 3.2 | 0 |
| Cancer stage at diagnosis† | |||
| Stage, median (25%, 75%)* | 2 (2, 3) | 2 (2, 3) | — |
| Stage II or III | 98.9 | 0.0569 | |
| Stage I or IV or unknown | — | — | ‡ |
| Time from first cancer diagnosis to index VTE, y§ | |||
| Median (25%, 75%)* | 0.64 (0.13, 2.31) | 0.59 (0.13, 2.28) | — |
| <1 | 57.8 | 56.7 | 0.0229 |
| 1 to <2 | 13.0 | 14.6 | −0.045 |
| 2 to <3 | 9.5 | 7.8 | 0.0578 |
| ≥3 | 19.7 | 21.0 | −0.0331 |
| Comorbidities and medications | |||
| Active cancer therapy | 73.0 | 72.4 | 0.0128 |
| Antiplatelet use | 7.9 | 7.4 | 0.0208 |
| CKD stage 3 or worse | 32.5 | 32.9 | −0.0080 |
| Coagulopathy | 9.3 | 8.1 | 0.0392 |
| Major bleed | ‡ | ‡ | 0 |
| Prior VTE | 24.2 | 24.2 | 0 |
| Surgery within 2 wk prior to index event | 2.5 | 3.0 | −0.0367 |
| Thrombocytopenia | 6.8 | 5.7 | 0.0446 |
| . | Rivaroxaban, % . | LMWH, % . | Standardized difference . |
|---|---|---|---|
| Demographics | |||
| Age, median (25%, 75%), y* | 73 (68, 78) | 72 (68, 78) | — |
| Age ≥75 y | 42.2 | 40.6 | 0.0306 |
| Female sex | 74.7 | 71.3 | 0.0780 |
| White race | 80.2 | 80.7 | −0.0142 |
| Index event PE (with or without DVT) | 43.5 | 45.4 | −0.0381 |
| Index DVT only | 56.5 | 54.6 | 0.0381 |
| Primary cancer site | |||
| Breast | 36.9 | 35.0 | 0.0392 |
| Lung | 43.5 | 46.3 | −0.0572 |
| Ovary | 6.2 | 6.8 | −0.0235 |
| Pancreas | 10.2 | 8.7 | 0.0501 |
| Other | 3.2 | 3.2 | 0 |
| Cancer stage at diagnosis† | |||
| Stage, median (25%, 75%)* | 2 (2, 3) | 2 (2, 3) | — |
| Stage II or III | 98.9 | 0.0569 | |
| Stage I or IV or unknown | — | — | ‡ |
| Time from first cancer diagnosis to index VTE, y§ | |||
| Median (25%, 75%)* | 0.64 (0.13, 2.31) | 0.59 (0.13, 2.28) | — |
| <1 | 57.8 | 56.7 | 0.0229 |
| 1 to <2 | 13.0 | 14.6 | −0.045 |
| 2 to <3 | 9.5 | 7.8 | 0.0578 |
| ≥3 | 19.7 | 21.0 | −0.0331 |
| Comorbidities and medications | |||
| Active cancer therapy | 73.0 | 72.4 | 0.0128 |
| Antiplatelet use | 7.9 | 7.4 | 0.0208 |
| CKD stage 3 or worse | 32.5 | 32.9 | −0.0080 |
| Coagulopathy | 9.3 | 8.1 | 0.0392 |
| Major bleed | ‡ | ‡ | 0 |
| Prior VTE | 24.2 | 24.2 | 0 |
| Surgery within 2 wk prior to index event | 2.5 | 3.0 | −0.0367 |
| Thrombocytopenia | 6.8 | 5.7 | 0.0446 |
N = 529 in both groups.
CKD, chronic kidney disease; DVT, deep vein thrombosis.
Continuous variable not included in the propensity score model in lieu of the categorized version.
Stages I, II, III, and IV and unknown were separately included in the propensity score model.
Data not reported or reported for the entire study cohort, to comply with the Cell Size Suppression Policy of the Centers for Medicare and Medicaid Services (available at: https://www.resdac.org/articles/cms-cell-size-suppression-policy). Variables were included in the propensity score–matching algorithm, as depicted in the table.
Patients may have had subsequent relapse or new metastatic disease.
Our primary analysis evaluated the composite incidence of recurrent VTE13 or major bleeding15 and all-cause mortality at 6 months, according to the intent-to-treat approach. Secondary outcomes included recurrent VTE and major bleeding, individually. Recurrent VTE was identified by using a previously validated set of International Classification of Diseases (ICD, 9th Revision) codes or cross-walked (ICD, 10th Revision) codes for hospital discharge diagnosis for DVT and/or PE in the primary coding position.13 Major bleeding was identified by using the validated Cunningham algorithm, which identifies bleeding-related hospitalizations in intracranial, gastrointestinal, genitourinary, and other locations with positive predictive values between 89% and 99%.15
Various sensitivity analyses were performed. The first was an on-treatment approach whereby patients were observed for up to 12 months or until the end point was reached, the index anticoagulant was switched or discontinued (30-day permissible gap), or the end of the SEER-Medicare–linked available data. Second, given that we included multiple cancers at high risk for VTE,16,17 we performed a sensitivity analysis to determine whether a patient’s history of previous VTE influenced outcome. Third, we performed stabilized inverse probability-of-treatment weighting (IPTW) to adjust for differences in baseline characteristics according to propensity scores. Stabilized IPTW was performed in generalized boosted models on the basis of 10 000 regression trees within the TWANG package in R statistical software v3.4.3. The variables entered in this model were the same as those used in the initial propensity score–matched analysis.
Baseline characteristics were analyzed by using descriptive statistics. Proportional hazards models for the subdistribution of competing risk were fit to compare incidences over time for the cohorts, by using cmprsk and riskRegression in R. Results of regression were reported as hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
We identified 2667 patients with cancer who had a CAT treated with rivaroxaban or LMWH. Of those, 108 patients were excluded for luminal gastrointestinal cancer, 534 for genitourinary cancer, and 269 for absence of active cancer, leaving 533 patients receiving rivaroxaban and 1223 receiving LMWH who met the inclusion criteria. Propensity score matching (Table 1) yielded 2 groups, each containing 529 patients who received rivaroxaban or LMWH (most receiving enoxaparin). The median age of rivaroxaban-treated patients was 73 years (25th, 75th percentiles; 68, 78) and of LMWH-treated patients was 72 years (68, 78). For the 12-month on-treatment population, there was no significant difference in treatment duration between the 2 cohorts (P = .43): patients receiving rivaroxaban were treated a median of 330 days (113, 365) and those treated with LMWH, a median of 286 days (115, 365). Nearly half (44.5%) of all the patients had PE, with or without DVT, as the index thrombosis, with the remainder having DVT only. Lung cancer (44.9%) was the most common primary cancer type, followed by breast (36.0%), pancreatic (9.5%), ovarian (6.5%), and other (3.2%). More than 98% of patients were diagnosed with stage II or III disease.
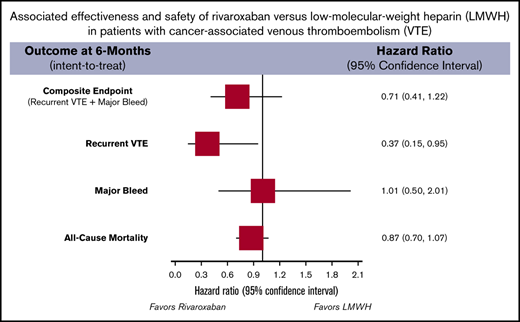
Rivaroxaban was not associated with a reduction in the composite end point or in all-cause mortality (Figure 1) vs LMWH. It was associated with a reduction in recurrent VTE risk (HR, 0.37; 95% CI, 0.15-0.95), but no significant difference in major bleeding (HR, 1.01; 95% CI, 0.50-2.01). Twelve-month on-treatment analysis results were similar for all outcomes.
Incidence and subdistribution HRs for the composite outcome of recurrent VTE or major bleeding and all-cause mortality of the 1:1 propensity score–matched analysis.
Incidence and subdistribution HRs for the composite outcome of recurrent VTE or major bleeding and all-cause mortality of the 1:1 propensity score–matched analysis.
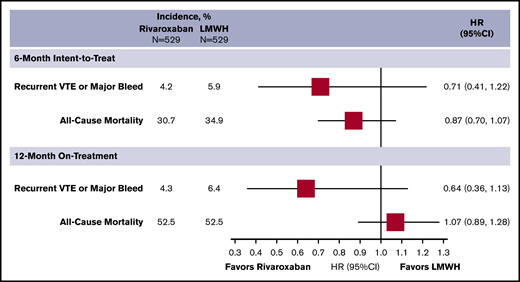
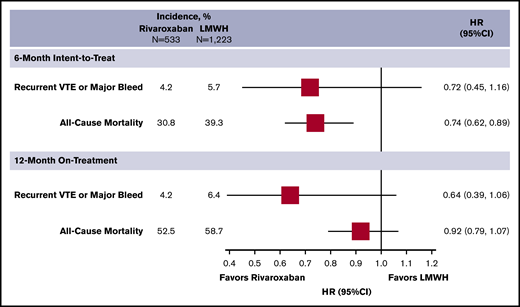
Sensitivity analysis using stabilized IPTW showed that baseline characteristics (Table 2) and outcomes/HRs (Figure 2) remained generally consistent, compared with the matched analysis. Although there was no difference in the composite end point or major bleeding (HR, 0.63; 95% CI, 0.39-1.06, and HR, 1.06; 95% CI, 0.58-1.91, respectively), rivaroxaban was associated with a reduction in recurrent VTE (HR, 0.38; 95% CI, 0.16-0.90). The 12-month on-treatment analysis provided similar results.
Baseline characteristics of the stabilized IPTW analysis study cohorts (sensitivity analysis)
| . | Rivaroxaban, % (N = 533) . | LMWH, % (N = 1223) . | Standardized difference . |
|---|---|---|---|
| Demographics | |||
| Age ≥75 y | 40.3 | 39.5 | 0.016 |
| Female sex | 72.1 | 72.0 | 0.001 |
| White race | 78.5 | 78.3 | 0.005 |
| Index event PE (with or without DVT) | 49.4 | 49.8 | −0.009 |
| Index DVT only | 50.6 | 50.2 | 0.009 |
| Primary cancer site | |||
| Breast | 29.9 | 29.0 | 0.018 |
| Lung | 44.1 | 43.8 | 0.006 |
| Ovary | 7.0 | 7.1 | −0.004 |
| Pancreas | 15.2 | 15.7 | −0.013 |
| Other | 3.9 | 4.5 | −0.027 |
| Cancer stage at diagnosis* | |||
| Stage II or III | 98.5 | 0.006 | |
| Stage I or IV or unknown | † | ||
| Time from first cancer diagnosis to index VTE, y‡ | |||
| <1 | 62.6 | 62.6 | 0.001 |
| 1 to <2 | 12.5 | 12.6 | −0.002 |
| 2 to <3 | 7.5 | 7.5 | 0 |
| ≥3 | 17.4 | 17.4 | 0 |
| Comorbidities and medications | |||
| Active cancer therapy | 69.9 | 69.9 | 0 |
| Antiplatelet use | 6.4 | 6.3 | 0.004 |
| CKD stage 3 or worse | 31.5 | 32.4 | −0.019 |
| Coagulopathy | 9.7 | 10.0 | −0.01 |
| Major bleed | † | † | −0.044 |
| Prior VTE | 24.1 | 24.1 | 0.001 |
| Surgery within 2 wk prior to index event | 2.9 | 3.0 | −0.007 |
| Thrombocytopenia | 6.4 | 6.9 | −0.023 |
| . | Rivaroxaban, % (N = 533) . | LMWH, % (N = 1223) . | Standardized difference . |
|---|---|---|---|
| Demographics | |||
| Age ≥75 y | 40.3 | 39.5 | 0.016 |
| Female sex | 72.1 | 72.0 | 0.001 |
| White race | 78.5 | 78.3 | 0.005 |
| Index event PE (with or without DVT) | 49.4 | 49.8 | −0.009 |
| Index DVT only | 50.6 | 50.2 | 0.009 |
| Primary cancer site | |||
| Breast | 29.9 | 29.0 | 0.018 |
| Lung | 44.1 | 43.8 | 0.006 |
| Ovary | 7.0 | 7.1 | −0.004 |
| Pancreas | 15.2 | 15.7 | −0.013 |
| Other | 3.9 | 4.5 | −0.027 |
| Cancer stage at diagnosis* | |||
| Stage II or III | 98.5 | 0.006 | |
| Stage I or IV or unknown | † | ||
| Time from first cancer diagnosis to index VTE, y‡ | |||
| <1 | 62.6 | 62.6 | 0.001 |
| 1 to <2 | 12.5 | 12.6 | −0.002 |
| 2 to <3 | 7.5 | 7.5 | 0 |
| ≥3 | 17.4 | 17.4 | 0 |
| Comorbidities and medications | |||
| Active cancer therapy | 69.9 | 69.9 | 0 |
| Antiplatelet use | 6.4 | 6.3 | 0.004 |
| CKD stage 3 or worse | 31.5 | 32.4 | −0.019 |
| Coagulopathy | 9.7 | 10.0 | −0.01 |
| Major bleed | † | † | −0.044 |
| Prior VTE | 24.1 | 24.1 | 0.001 |
| Surgery within 2 wk prior to index event | 2.9 | 3.0 | −0.007 |
| Thrombocytopenia | 6.4 | 6.9 | −0.023 |
In the match analysis, stages I, II, III, and IV and unknown were all matched separately.
Data not reported or reported for the entire study cohort, to comply with the Cell Size Suppression Policy of the Centers for Medicare and Medicaid Services (available at: https://www.resdac.org/articles/cms-cell-size-suppression-policy). Variables were included in the propensity score–matching algorithm, as depicted in the table.
Patients may have had subsequent relapse or new metastatic disease.
Incidence and subdistribution HRs for the composite outcome of recurrent VTE or major bleeding and all-cause mortality of the stabilized IPTW sensitivity analysis.
Incidence and subdistribution HRs for the composite outcome of recurrent VTE or major bleeding and all-cause mortality of the stabilized IPTW sensitivity analysis.
Data were available for 423 patients with a history of VTE (129 rivaroxaban, 294 LMWH). Results of this subanalysis were similar to those of the analysis of the overall study population in both the 6-month intent-to-treat and 12-month on-treatment analyses. There was no difference between rivaroxaban and LMWH at 6 or 12 months for composite end point (HR, 0.35; 95% CI, 0.08-1.54, and HR, 0.15; 95% CI, 0.02-1.14), all-cause mortality (HR, 0.74; 95% CI, 0.52-1.05, and HR, 1.08; 95% CI, 0.80-1.45), or major bleeding (HR, 0.66; 95% CI, 0.14-3.15, and HR, 0.29; 95% CI, 0.04-2.31), respectively. No patients in the rivaroxaban cohort experienced recurrence of VTE at 6 or 12 months.
Discussion
In our study of >1050 patients with active cancer who experienced VTE, we found a reduction in recurrent thrombosis with no significant impact on the composite outcome of recurrent VTE or major bleeding, major bleeding alone, or all-cause mortality with rivaroxaban vs LMWH. Our clinical findings are consistent with prior RCTs of anticoagulation for CAT treatment and meta-analyses.6,7,18,19 A network meta-analysis of RCTs of anticoagulation for CAT treatment18 suggested that DOACs could reduce recurrent thrombosis risk by 28% (95% CI, 4-44) without increasing bleeding vs LMWH (HR, 1.14; 95% CI, 0.64-2.03). Of the RCTs included in the network meta-analysis, 2 were head-to-head comparisons of a DOAC with LMWH.6,7 SELECT-D7 reported less recurrent VTE with rivaroxaban vs dalteparin (HR, 0.43; 95% CI, 0.19-0.99). Although not well powered for bleeding outcomes, SELECT-D did not show a significant difference in major bleeding (HR, 1.83; 95% CI, 0.68-4.96).7 Hokusai VTE-Cancer6 showed a trend toward a reduced risk of recurrent thrombosis with edoxaban vs dalteparin (HR, 0.71; 95% CI, 0.48-1.06), but with a significant increased risk of major bleeding (HR, 1.77; 95% CI, 1.03-3.04). Recently, the Caravaggio Investigators19 reported similar results, with a reduction in recurrent VTE in patients randomized to apixaban or dalteparin (HR, 0.63; 95% CI, 0.37-1.07) and no difference in major bleeding risk (HR, 0.82; 95% CI, 0.40-1.69). Notably, each of these 3 RCTs6,7,19 enrolled patients with gastrointestinal or genitourinary cancers, and subanalysis from Hokusai6 demonstrated that patients with those cancer types were more likely to bleed when treated with edoxaban than when receiving dalteparin (13.2% vs 2.4% for gastrointestinal and 13.2% vs 0% for urothelial cancers, respectively). Consequently, many CAT guidance documents list rivaroxaban and other factor Xa inhibitors as reasonable alternatives to LMWH in patients who are not at high risk of bleeding because of gastrointestinal and genitourinary cancer site involvement.4,5
Interestingly, before propensity score matching, our data set included 1 rivaroxaban-treated patient with CAT per ∼2.3 patients treated with an LMWH. This frequent use of rivaroxaban occurred despite the lack of guideline endorsement for CAT treatment during this study’s time frame. However, the use of rivaroxaban at these levels is not entirely surprising. A study by Khorana and colleagues20 analyzing US MarketScan claims data between 1 August 2013 and 31 July 2014 showed that 28.3% of patents with newly diagnosed cancer in whom anticoagulation treatment was initiated received warfarin, 19.8% DOACs (mainly rivaroxaban), and 48.5% LMWH. Comparison with data from earlier years suggested the increased use of DOACs came mostly at the expense of prescriptions for warfarin. As part of the prospective Xa inhibition with rivaroxaban for long-term and initial anticoagulation in a VTE (XALIA) registry study,21 a subanalysis of 587 patients with cancer found that 24.9% were initially prescribed rivaroxaban, 5.1% switched early (within 14 days) to rivaroxaban, and 62.0% received LMWH alone or were started on an LMWH and transitioned to a vitamin K antagonist (8% received an alternative anticoagulant). Taken together, the results of these studies suggest that DOACs were used somewhat frequently, despite not being recommended by the guidelines; however, both also concluded that DOAC use was more frequent in patients at lower risk of detrimental outcomes, whereas LMWHs were more often used in those at higher risk. These studies also suggest that the decision to use DOACs may also have been driven in part by demographics of the select patients (advanced age, living conditions) and their unwillingness to be adhere to an injectable agent. Prior real-world studies8-11 have evaluated DOACs (mostly rivaroxaban) or compared them to LMWHs in patients with CAT and found results generally consistent with the 2 RCTs. These studies, however, frequently included luminal gastrointestinal and/or genitourinary cancers (comprising up to one-third of a study population), had a small DOAC-treated sample size, and/or used heterogeneous (or unclear) definitions of active cancer8,10,11 that are inconsistent with current guidance.5
By using this SEER-Medicare–linked data set, we were able to leverage data from both cancer registries and longitudinal claims information for patients receiving Medicare.22 Still, our study has limitations worth discussing. Because of the retrospective cohort design and use of claims data, various biases may have affected our results.23 Sampling bias may have occurred as a result of our exclusion of some cancers that are found more frequently in younger populations.22,23 Moreover, because of limitations in SEER data access, only the 5 most frequently occurring cancers studied in RCTs of anticoagulation for CAT (excluding colorectal, because of high bleeding risk) could be included in our analysis. This limitation most likely resulted in an oversampling of some cancers (ie, breast and lung). Although misclassification bias is also a concern in retrospective claims analyses, we attempted to attenuate its presence by using validated coding schema (whenever possible) to identify covariates and outcomes.13,15,24,25 Specifically, to identify recurrent VTE, we limited identification of thrombotic events to the presence of at least 1 of a validated set of VTE-associated billing codes restricted to the primary coding position during a hospital encounter. In prior studies, this coding schema showed a positive predictive value of 95% for identifying VTE.13 Numerous other real-world studies evaluating the comparative effectiveness of anticoagulation to prevent CAT have used this same or a similar coding schema.10,26 A validated coding algorithm was also used in the identification of major bleeding.15 Confounding bias may also be a concern in any nonrandomized study. To reduce the risk of confounding bias, our base case analysis used 1:1 propensity score matching to balance key baseline covariates between the rivaroxaban and LMWH cohorts (though residual confounding is still possible). Our use of propensity score matching would naturally result in the inclusion of only those LMWH users who resembled rivaroxaban users in key baseline characteristics (ie, less severe disease and perceived lower risk of recurrent thrombosis). To address this possible limitation, we also performed stabilized IPTW to adjust for confounding, while maintaining the full LMWH population. Results were similar between the 2 approaches. For both these propensity score–based approaches, all covariates entered into the propensity score model were binary or categorical. In many cases, this selection of covariates was required because of our reliance on billing codes instead of laboratory data (eg, glomerular filtration rate data were not available, but chronic kidney disease staging could be determined by billing code). The use of categorized instead of continuous covariates inevitably leads to data loss and can potentially result in less precise matching (although this did not appear to occur in our study for age, cancer stage, or time since initial cancer diagnosis). Last, because of our use of SEER-Medicare–linked data and our inclusion of only 1 DOAC and rivaroxaban and the infrequent use of other LMWHs besides enoxaparin, our study’s findings are most generalizable to an elderly US population with cancers treated with rivaroxaban or enoxaparin.12
Our study of select patients with CAT, without luminal gastrointestinal or genitourinary malignancies because of the higher risk of bleeding, found rivaroxaban to be associated with a reduced risk of recurrent VTE vs LMWH without a significant impact on the composite outcome, major bleeding, or all-cause mortality. These data support the recommendation that DOACs, including rivaroxaban, are reasonable alternatives to LMWHs for treatment of CAT when used in accordance with guidelines.
Data used in this study were obtained from SEER under an agreement with C.I.C. and C.G.K. and are therefore not publicly available.
Authorship
Contribution: O.S.C., C.I.C., and C.G.K. conceptualized and designed the study; O.S.C., C.I.C., C.G.K., and T.J.B. analyzed the data; O.S.C., C.G.K., and C.I.C. wrote the manuscript; O.S.C., C.G.K., N.M.K., G.H.L., T.J.B., and C.I.C. contributed to data analysis; and all authors substantially contributed to the project, read and approved the manuscript, and assumed responsibility for the contents of the manuscript.
Conflict-of-interest disclosure: C.I.C. has received research funding and consulting fees from Bayer AG and Janssen Pharmaceuticals. N.M.K. has received consultancy fees and travel support from Janssen Pharmaceuticals, Coherus Biosciences, Halozyme, Myriad Genetics, Mylan, Celldex, and Pfizer. G.H.L. has received research funding from Amgen. The remaining authors declare no competing financial interests.
Correspondence: Craig I. Coleman, University of Connecticut, School of Pharmacy, 69 North Eagleville Rd, Unit 3092, Storrs, CT 06269; e-mail: craig.coleman@hhchealth.org.