Key Points
A severely immunocompromised patient with MM and COVID19 who received a convalescent plasma product showed SARS-CoV-2 clearance.
The convalescent plasma showed humoral immunity against all structural SARS-CoV-2 proteins, which was successfully transferred to the patient.
Introduction
Coronavirus disease 2019 (COVID-19) has become the greatest global health challenge.1 Importantly, patients at an advanced age and with preexisting medical conditions,2-5 in particular cancer patients on immunosuppressive treatment, show a more severe disease and increased mortality.6-8 COVID-19 infection is caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).9 The virus consists of various nonstructural proteins and 4 major structural proteins: the surface-exposed spike (S), membrane (M), and envelope (E) proteins and the internal nucleocapsid (N) protein.9,10 The SARS-CoV-2 virus enters cells, such as pneumocytes in the lung,11 via binding of the receptor-binding domain (RBD) within its S1 protein12 to the angiotensin-converting enzyme-2 receptor.10,13
Recent findings indicate that infected patients develop spontaneous antibody-mediated immune responses against viral particles,10,14,15 and an apparent improvement in the clinical status was observed upon treatment with convalescent plasma containing anti–SARS-CoV-2 antibodies.16 Indeed, it was recently shown that patient-derived antibodies directed against the viral S protein are able to neutralize the SARS-CoV-2 virus.12 However, the exact targets of the clinically relevant antibodies within the polyclonal plasma remain unclear, the potential immunogenicity and clinical relevance of other viral proteins has not been investigated, and individual epitopes of the antibodies present in convalescent plasma and in the blood of COVID-19 patients have not been identified. In addition, it has never been demonstrated whether the complete antiviral immunity can be transferred and detected in the recipient after transfusing a convalescent plasma product.
Case description
Our patient ID359 is a 72-year-old female who was diagnosed with immunoglobulin G (IgG) κ multiple myeloma (MM) 10 years prior to her admission for COVID-19. She had received 4 prior lines of treatment, including 3 autologous stem cell transplants, steroids, cytotoxic chemotherapy, proteasome inhibitors, immunomodulatory drugs, and the anti-CD38 monoclonal antibody daratumumab. She was in partial remission after 8 cycles of carfilzomib/pomalidomide/dexamethasone and had received her most recent dose of carfilzomib ∼3 weeks prior to this admission; she still had active disease with a serum IgG κ monoclonal protein measuring 0.36 g/dL (Figure 1A), serum free κ light chains of 30.6 mg/L, and an elevated κ/λ ratio.
Compromised antiviral immunity in an MM patient with hypogammaglobulinemia. (A) Total IgG and IgM levels of patient ID359 on the day of COVID-19 diagnosis, as determined by immunoelectrophoresis. M indicates the level of total monoclonal protein in the γ region, and dotted lines indicate the reference range. (B) Reciprocal IgG titers against tetanus toxoid (TT; Millipore-Sigma, cat. no. 582231) and influenza H1N1 nucleoprotein (FLU; Sino Biological, cat. no. 11675-V08B) in patient ID359 plasma at COVID-19 diagnosis, as well as in 4 healthy donors (HD), as determined by ELISA. (C) IgG responses, expressed as optical density (OD) readings, of 6 COVID-19 patients (PAT) and 5 healthy donors (HD) against SARS-CoV-2 proteins S1 (ACROBiosystems; cat. no. S1N-C52H3), S2 (expressed in Expi293 cells), and N (BioVision; cat. no. P1523-50), the S1 RBD expressed in mammalian cells (mRBD; RayBiotech; cat. no. 230-30162) or produced synthetically (sRBD; LifeTein), positive-control proteins TT and FLU, and negative-control glutathione-S-transferase (GST; expressed in Expi293 cells), as determined by ELISA. Data represent the mean, and circles indicate technical replicates.
Compromised antiviral immunity in an MM patient with hypogammaglobulinemia. (A) Total IgG and IgM levels of patient ID359 on the day of COVID-19 diagnosis, as determined by immunoelectrophoresis. M indicates the level of total monoclonal protein in the γ region, and dotted lines indicate the reference range. (B) Reciprocal IgG titers against tetanus toxoid (TT; Millipore-Sigma, cat. no. 582231) and influenza H1N1 nucleoprotein (FLU; Sino Biological, cat. no. 11675-V08B) in patient ID359 plasma at COVID-19 diagnosis, as well as in 4 healthy donors (HD), as determined by ELISA. (C) IgG responses, expressed as optical density (OD) readings, of 6 COVID-19 patients (PAT) and 5 healthy donors (HD) against SARS-CoV-2 proteins S1 (ACROBiosystems; cat. no. S1N-C52H3), S2 (expressed in Expi293 cells), and N (BioVision; cat. no. P1523-50), the S1 RBD expressed in mammalian cells (mRBD; RayBiotech; cat. no. 230-30162) or produced synthetically (sRBD; LifeTein), positive-control proteins TT and FLU, and negative-control glutathione-S-transferase (GST; expressed in Expi293 cells), as determined by ELISA. Data represent the mean, and circles indicate technical replicates.
The patient underwent testing of a nasopharyngeal sample by SARS-CoV-2 polymerase chain reaction (PCR), following continuous exposure to an individual with known COVID-19 for ∼1 week, and was diagnosed with the infection. At the time of testing (day −3), she was asymptomatic; however, on the following day (day −2), she developed diarrhea and nausea. One day later (day −1), she developed substantial dyspnea, cough, wheezing, and, from home, she reported hypoxia with an oxygen saturation of 75% to 85%. The patient was admitted to the emergency room at the University of Utah. On admission, she was found to be in respiratory distress and was hypoxic and disoriented. Venous blood gas analysis showed hypoxia and hypercapnia. She was placed on oxygen via nasal cannula, and a chest radiograph showed streaky left basilar opacities pointing to viral pneumonia. Other abnormal laboratory results included leukopenia and lymphopenia and worsening of her chronic renal disease. The patient was diagnosed with acute hypoxic respiratory failure due to COVID-19 infection, without any clinical signs of cytokine storm, and viral pneumonia. She was admitted to the medical COVID unit where she received 2 to 3 L of oxygen via nasal cannula for the next 24 hours.
Evaluating her humoral immune system, the patient was found to have severe hypogammaglobulinemia with very low absolute levels of normal IgG and IgM (Figure 1A), consistent with her long-standing MM and several lines of immunosuppressive treatments, including a monoclonal antibody targeting CD38-expressing plasma cells. Remarkably, the low amount of total IgG that she did have consisted largely of myeloma-related monoclonal M protein (Figure 1A). Accordingly, she showed nonexistent antibody titers against influenza A virus and tetanus toxoid (Figure 1B). A routine ELISA screening for anti–COVID-19 antibodies demonstrated a complete absence of antiviral serological reactivity.
On day 0, based on the lack of clinical improvement, the US Food and Drug Administration clinical criteria for convalescent plasma administration under the expanded access program, and her severe immunosuppression, we transfused 1 200-mL unit of convalescent plasma from a donor who had recovered from COVID-19. Importantly, the patient did not receive any other treatment potentially having an effect on the course of COVID-19, such as steroids or antivirals. After transfusion of the convalescent plasma, the patient showed a dramatic clinical improvement, became asymptomatic, and was discharged home only 2 days later (day +3). The patient continued to be asymptomatic; a SARS-CoV-2 PCR performed on a nasopharyngeal sample on day +19 was negative, indicating complete viral clearance.
Methods
Serum samples
Patient and healthy donor samples were collected under protocol #45880, which was approved by the Institutional Review Board at the University of Utah. Written informed consent was obtained from participants prior to inclusion in the study.
Serum ELISA and titration
Ninety-six–well plates were coated with 5 μg/mL recombinant protein or peptides. ELISAs were performed as described previously.17
Pseudotyped virus-neutralization assay
Assays were performed as described, using plasmid HIV-1-δ-env-luc with pSARS-Cov-2-S for patient samples and MLV-Ψ(−)Env(-), l-luc-SN and pSARS-Cov-2-S for donor plasma.18
Results and discussion
To perform the first detailed analysis of convalescent plasma product and its effect on the anti–SARS-CoV-2 immunity in the recipient, we established an ELISA measuring antibody titers against the SARS-CoV-2 structural proteins. We obtained full-length recombinant proteins for the S1 protein, the RBD within the S1 protein, and the S2 and nucleocapsid (N) proteins. S1, S2, and RBD used in this study were expressed in human 293 cells to preserve structure and glycosylation patterns. Peptide libraries consisting of 20mers overlapping by 10 aa were synthesized for these SARS-CoV-2 proteins and for the M and E proteins. In addition, we obtained a 69-aa synthetic peptide comprising a highly conserved cryptic epitope in the RBDs of SARS-CoV-2 and severe acute respiratory syndrome coronavirus.19
We performed assay validation assessing sera from 6 patients with PCR-confirmed and symptomatic COVID-19 and from 5 healthy donors for antibodies against the different SARS-CoV-2 proteins and peptides, positive-control antigens influenza H1N1 nucleoprotein (FLU) and tetanus toxoid (TT), and the negative-control protein glutathione-S-transferase. We found that all patients showed strong antibody responses against S1 and the RBD (Figure 1C). In addition, all 5 patients showed antibody responses against the S2 and N proteins. Interestingly, the RBD produced synthetically was not recognized by any of the patient-derived antibodies, pointing to glycosylation-dependent and/or conformational epitopes as targets for the antiviral immune responses. Accordingly, we did not observe antibody responses against pooled M and E peptides (data not shown). With the exception of the mammalian cell–expressed RBD, which was recognized by serum from 1 healthy donor, none of the other SARS-CoV-2 proteins were recognized by sera from non–COVID-19 individuals (Figure 1C).
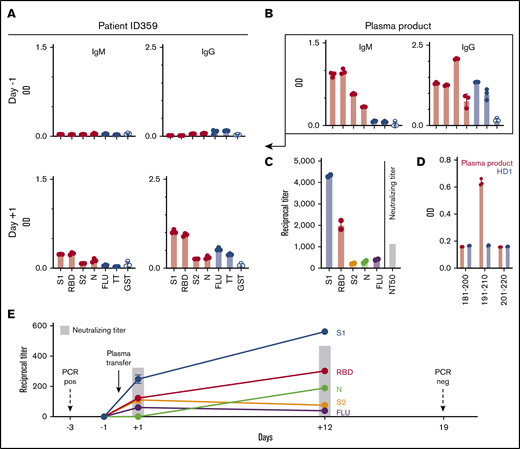
As expected, when screening the patient’s blood on the day of admission, we did not detect antibodies against any of the viral proteins (Figure 2A) or any neutralizing antibody activity (Figure 2E). It has previously been shown that, at least in the case of severe acute respiratory syndrome coronavirus, lung injury is associated with neutralizing antibody development20 and, it may be noteworthy that, in our patient, respiratory distress occurred despite a severely suppressed humoral immune system and the absence of anti–SARS-CoV-2 antibodies. In contrast, a <1-mL leftover from the frozen and thawed convalescent plasma product administered to the patient contained IgM and IgG antibodies (Figure 2B) and a low level of IgA antibodies (data not shown) against S1, RBD, S2, and N proteins, as well as IgG antibodies against positive-control proteins FLU and TT (Figure 2B-C). Importantly, the donor plasma also contained significant neutralizing activity (reciprocal 50% neutralizing titer [NT50]; Figure 2C). Analyzing the convalescent plasma product using overlapping synthetic peptide libraries we were able to identify 1 linear epitope within the S1 protein, N-terminal from the RBD, at aa position 191 to 210 (Figure 2D).
Transfer of SARS-CoV-2–specific immunity to MM patient by convalescent plasma infusion and subsequent viral clearance. (A) IgM and IgG antibody responses in patient ID359 plasma against SARS-CoV-2 proteins and control proteins at 1:20 serum dilution before and after convalescent plasma infusion, as determined by ELISA. (B) IgM and IgG responses at 1:20 plasma dilution against SARS-CoV-2 and control proteins in convalescent plasma product, as determined by ELISA. (C) Reciprocal IgG antibody titers against SARS-CoV-2 and control proteins and neutralizing antibody titers in the convalescent plasma product. (D) Epitope mapping of convalescent plasma product and plasma from a healthy donor (HD1) using synthetic S1 peptides, as determined by ELISA. 20mer peptides overlapping by 10 aa for SARS-CoV-2 S1, S2, M, and E sequences were synthetized (peptides & elephants, Hennigsdorf, Germany). Data represent the mean, and circles indicate technical replicates. (E) Reciprocal IgG titers against SARS-CoV-2 proteins and FLU, as well as neutralizing antibody activity in patient ID359 plasma, before and after convalescent plasma infusion. Antibody titers represent mean ± standard deviation from 2 technical replicates. GST, glutathione-S-transferase; neg, negative.
Transfer of SARS-CoV-2–specific immunity to MM patient by convalescent plasma infusion and subsequent viral clearance. (A) IgM and IgG antibody responses in patient ID359 plasma against SARS-CoV-2 proteins and control proteins at 1:20 serum dilution before and after convalescent plasma infusion, as determined by ELISA. (B) IgM and IgG responses at 1:20 plasma dilution against SARS-CoV-2 and control proteins in convalescent plasma product, as determined by ELISA. (C) Reciprocal IgG antibody titers against SARS-CoV-2 and control proteins and neutralizing antibody titers in the convalescent plasma product. (D) Epitope mapping of convalescent plasma product and plasma from a healthy donor (HD1) using synthetic S1 peptides, as determined by ELISA. 20mer peptides overlapping by 10 aa for SARS-CoV-2 S1, S2, M, and E sequences were synthetized (peptides & elephants, Hennigsdorf, Germany). Data represent the mean, and circles indicate technical replicates. (E) Reciprocal IgG titers against SARS-CoV-2 proteins and FLU, as well as neutralizing antibody activity in patient ID359 plasma, before and after convalescent plasma infusion. Antibody titers represent mean ± standard deviation from 2 technical replicates. GST, glutathione-S-transferase; neg, negative.
On the morning after treatment with convalescent plasma (day +1), we were able to detect IgM and IgG antibody responses (Figure 2A), but no IgA antibodies (data not shown), against all tested viral proteins in the patient’s blood, albeit at a lower level compared with the plasma product. The plasma product evidenced the highest IgG antibody titers against SARS-CoV-2 proteins S1 and RBD and lower antibody titers against S2 and N (Figure 2D). Following plasma transfer, the patient showed a similar distribution pattern (Figure 2E), confirming that the humoral immunity had indeed been passively transferred to the recipient. Importantly, the transfer of the donor-derived antiviral immunity also resulted in neutralizing antibody activity in the patient’s blood (Figure 2E). The antibodies against the different viral proteins were still detectable in the patient 12 days after transfusion of the convalescent plasma, at titers even higher than on the day after treatment, with a higher neutralizing activity (Figure 2E) and new low-level antiviral IgA antibodies (data not shown), indicating the development of at least some autologous SARS-CoV-2–specific immunity.
In summary, our data indicate that transfer of convalescent plasma is safe, even in severely immunocompromised patients with hematologic malignancies who are under treatment for their malignancy. We also show here for the first time that the complete humoral immunity against the different viral target proteins can successfully be transferred and detected in the recipient and that antibody levels are maintained over time. Finally, our observations indicate that these patients may derive clinical benefit from such a treatment, although this will need to be confirmed by randomized clinical trials.
Data sharing requests should be sent to Djordje Atanackovic (e-mail: djordje.atanackovic@hci.utah.edu).
Acknowledgments
This work was supported by a grant from the CrowdCare Foundation (D.A.) and by a seed grant from the University of Utah Vice President for Research and the Immunology, Inflammation, and Infectious Disease Initiative (V.P.). This research was supported by a grant from the CrowdCare Foundation Inc.
Authorship
Contribution: T.L. designed the study, performed analyses, analyzed data, and wrote the manuscript; R.M. performed analyses, provided samples, and wrote the manuscript; V.P. performed analyses and wrote the manuscript; Y.Z., E.T.L., and S.V.A. performed analyses; E.S.S. provided samples and performed analyses; A.M.S., R.C.B., and H.D.M. wrote the manuscript; M. Steinbach obtained clinical data and wrote the manuscript; K.G.H. analyzed data and wrote the manuscript; T.B.M. and M. Sajadi provided samples and wrote the manuscript; P.R.S. provided samples and analyzed data; A.P.R. analyzed data and wrote the manuscript; and D.A. designed the study, provided samples, obtained clinical data, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Djordje Atanackovic, Department of Medicine/Bone Marrow Transplant, University of Maryland Greenebaum Comprehensive Cancer Center, Bressler Research Building, Room 9-011, 655 W Baltimore St, Baltimore, MD 21201; email: datanackovic@som.umaryland.edu.