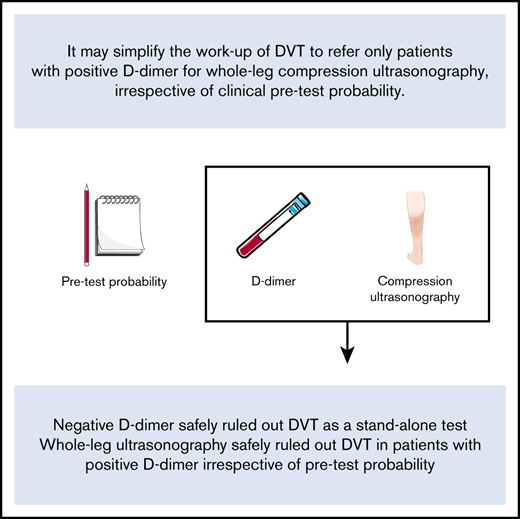
Key Points
Negative D-dimer safely ruled out DVT as a stand-alone test.
A single negative whole-leg ultrasonography safely ruled out DVT in patients with positive D-dimer irrespective of pretest probability.
Abstract
Guidelines for the diagnostic workup of deep vein thrombosis (DVT) recommend assessing the clinical pretest probability before proceeding to D-dimer testing and/or compression ultrasonography (CUS) if the patient has high pretest probability or positive D-dimer. Referring only patients with positive D-dimer for whole-leg CUS irrespective of pretest probability may simplify the workup of DVT. In this prospective management outcome study, we assessed the safety of such a strategy. We included consecutive outpatients referred to the Emergency Department at Østfold Hospital, Norway, with suspected DVT between February 2015 and November 2018. STA-Liatest D-Di Plus D-dimer was analyzed for all patients, and only patients with levels ≥0.5 µg/mL were referred for CUS. All patients with negative D-dimer or negative CUS were followed for 3 months to assess the venous thromboembolic rate. One thousand three hundred ninety-seven patients were included. Median age was 64 years (interquartile range, 52-73 years), and 770 patients (55%) were female. D-dimer was negative in 415 patients (29.7%) and positive in 982 patients (70.3%). DVT was diagnosed in 277 patients (19.8%). Six patients in whom DVT was ruled out at baseline were diagnosed with DVT within 3 months of follow-up for a thromboembolic rate of 0.5% (95% confidence interval, 0.2-1.2). A simple diagnostic approach with initial stand-alone D-dimer followed by a single whole-leg CUS in patients with positive D-dimer safely ruled out DVT. We consider this strategy to be a valuable alternative to the conventional workup of DVT in outpatients. This trial was registered at www.clinicaltrials.gov as #NCT02486445.
Introduction
Current guidelines for the diagnostic workup of deep vein thrombosis (DVT) recommend incorporating clinical pretest probability (C-PTP) assessment, D-dimer results, and compression ultrasonography (CUS).1,2 Each of the components has been widely assessed,3-5 although no 1 diagnostic strategy has been deemed superior to others. Guidelines recommend first assessing C-PTP using a validated prediction rule, followed by a high-sensitivity D-dimer assay for patients with non-high C-PTP, and either single whole-leg or proximal CUS for patients with high C-PTP or positive D-dimer. For proximal CUS, a repeat examination or negative D-dimer is required to rule out DVT in patients with moderate or high C-PTP, whereas a single negative proximal CUS suffices in patients with low C-PTP.
In a recent study, we found that negative stand-alone D-dimer safely ruled out DVT irrespective of C-PTP while necessitating fewer CUS examinations than if C-PTP had also been considered.6
We believe a diagnostic strategy in which only patients with positive D-dimer are referred for a single whole-leg CUS regardless of C-PTP may simplify the workup of DVT without compromising safety. As such, this prospective management trial aimed to determine the safety and feasibility of such a strategy.
Methods
Study population and design
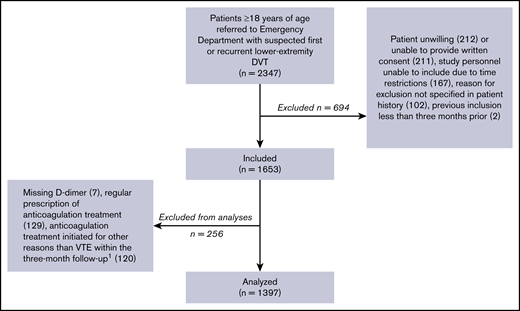
The Ri-Schedule study (clinicaltrials.gov NCT02486445) was a prospective outcome trial including consecutive outpatients referred from primary care to the Emergency Department at Østfold Hospital, Norway, between February 2015 and November 2018. Inclusion criteria were ≥18 years of age and referral for suspected first or recurrent DVT. Exclusion criteria were failure to consent, and previous inclusion in the study within the past 3 months. Furthermore, the following patients were excluded from analyses: patients with missing D-dimer results at baseline, patients who were prescribed anticoagulants for other indications than empiric anticoagulation for suspected DVT at the time of inclusion, and patients who were prescribed anticoagulants for indications other than venous thromboembolism (VTE) in the interval from inclusion until the end of the 3-month follow-up.
Interventions
Designated study personnel screened patients for inclusion. Included patients underwent clinical examination and blood admission tests including D-dimer. C-PTP was assessed according to the 3-tier Wells score for later analyses and before D-dimer was obtained,7 but was not used to guide further management. D-dimer was analyzed by the immunoturbidometric method of STA-Liatest D-Di Plus (Stago Diagnostics, Asnières, France). Positive D-dimer was defined as levels ≥0.5 µg/mL fibrinogen-equivalent units. Patients with D-dimer <0.5 µg/mL were considered not to have DVT, did not undergo CUS, and remained untreated at baseline.
Patients with positive D-dimer were referred for whole-leg CUS. The deep and saphenous veins were scanned with a linear probe (5-10 MHz). For first DVT, recurrent contralateral DVT, recurrent ipsilateral DVT with documented resorption of thrombus, or recurrent DVT without available images for comparison, the diagnostic criterion was incompressibility of the vein or a grayscale visualization of the thrombus.8 Recurrent ipsilateral DVT was defined as noncompressibility of, or visualization of, the thrombus in a venous segment not involved from reference CUS,9 as magnetic resonance direct thrombus imaging to distinguish between acute and chronic DVT was not established as an alternative at the time the study was designed.10
Patients diagnosed with DVT at baseline started anticoagulation treatment. Patients with suspected concurrent pulmonary embolism at baseline were managed according to hospital guidelines instead of according to the trial protocol.
Patients in whom we considered DVT to be ruled out either due to negative D-dimer or CUS were discharged to be followed up for 3 months. Patients were advised to seek medical attention if symptoms progressed, or if other symptoms of DVT or pulmonary embolism developed, in which case they would undergo diagnostic imaging. At the end of the follow-up period, patients were contacted by phone to determine whether they had been diagnosed with VTE since inclusion, and/or had been treated with anticoagulation during this time.
Objectives and end points
The study aimed to assess the safety and feasibility of a diagnostic strategy ruling out DVT in patients with negative D-dimer, and ruling out DVT with normal findings on a single whole-leg CUS for patients with positive D-dimer.
The primary end point was the failure rate of the strategy, defined as the proportion of patients in whom DVT had been ruled out (ie, patients with either negative D-dimer or normal CUS at baseline) who developed VTE or died of unknown cause possibly attributable to VTE within 3 months of follow-up out of all patients in whom DVT had been ruled out.
The secondary safety objectives were to assess the safety of stand-alone D-dimer and single whole-leg CUS, respectively. The secondary safety end points were determining
the failure rate of stand-alone D-dimer, defined as the proportion of patients with negative D-dimer at baseline who developed VTE or died of unknown cause possibly attributable to VTE within 3 months of follow-up out of all patients with negative D-dimer, and
the failure rate of whole-leg CUS, defined as the proportion of patients with normal CUS at baseline who developed VTE or died of unknown cause possibly attributable to VTE within 3 months of follow-up out of all patients with normal CUS.
All potential outcome events were adjudicated by an independent committee.
We have previously reported on the safety of stand-alone D-dimer for the exclusion of DVT in 913 of the patients included in this study, and compared its safety and feasibility to standard diagnostic workup, as well as age-adjusted D-dimer.6 Our findings were recently validated in a retrospective study.11
Statistical analyses
We would accept a failure rate of the strategy of 2% with an upper limit of the 95% confidence interval (CI) of 4%. This was based on the rate of symptomatic VTE within 3 months of a negative venography (1.3%; 95% CI, 0.2% to 4.4%), which is the reference standard against which diagnostic management studies of DVT are typically evaluated.2,12 For a power of 80% at a 5% significance level, we estimated a sample size of at least 500 patients in whom DVT was ruled out at baseline according to the strategy. As the study was part of the larger Ri-Schedule study addressing several objectives with different sample size calculations, we allowed for >500 patients to be included in the study.
The study outcomes are expressed as proportions with percentages and corresponding 95% CIs, calculated by the Clopper-Pearson exact method. Baseline characteristics are expressed as median with interquartile range (IQR) for continuous variables, and numbers and percentages for categorical variables. IBM SPSS Statistics software (version 25) was used.
Ethics
The study was approved by the Regional Committee for Medical and Health Research Ethics (REK; reference number 2014/377). The researchers adhered to the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, and the International Council for Harmonisation–Good Clinical Practice.
Results
Baseline results
In the 46 months of inclusion, 2347 patients aged ≥18 years, referred with first or recurrent lower-extremity DVT, were screened for participation (Figure 1).
Inclusion of patients.1Ninety-three due to superficial thrombophlebitis.
Of these, 1397 patients were included in the analyses. Their baseline characteristics are outlined in Table 1. DVT was diagnosed in 277 patients (19.8%): 187 of these DVT cases (67.5%) were proximal and 90 (32.5%) were isolated distal DVT. Thirty-three patients were included twice, and 1 patient was included 3 times. The diagnostic properties of the implemented strategy vs strategies including the Wells score in the diagnostic algorithm are shown in Table 2.
Demographic and clinical characteristics of patients
| . | All, N = 1397 . | DVT,* n = 277 . | No DVT,* n = 1120 . |
|---|---|---|---|
| Age, median (IQR), y | 64 (52-73) | 65 (53-73) | 64 (51-73) |
| Symptom duration, median (IQR), d | 7 (3-14) | 5 (3-7) | 7 (3-14) |
| Female sex, n (%) | 770 (55) | 114 (41) | 656 (59) |
| Positive D-dimer, n (%) | 982 (70) | 275 (99) | 707 (63) |
| Low probability for DVT,† n (%) | 383 (27) | 23 (8) | 360 (32) |
| Moderate probability for DVT,† n (%) | 670 (48) | 121 (44) | 549 (49) |
| High probability for DVT,† n (%) | 344 (25) | 133 (48) | 211 (19) |
| Positive D-dimer and/or high probability for DVT† | 1024 (73) | 276 (99) | 748 (67) |
| DVT likely,‡ n (%) | 698 (50) | 216 (78) | 482 (43) |
| DVT unlikely,‡ n (%) | 699 (50) | 61 (22) | 638 (57) |
| Positive D-dimer and/or DVT likely,‡ n (%) | 1105 (79) | 276 (99) | 829 (74) |
| Previous VTE, n (%) | 203 (15) | 73 (26) | 130 (12) |
| VTE in first-degree relatives, n (%) | 266 (19) | 57 (21) | 209 (19) |
| Active cancer within 6 mo, n (%) | 62 (4) | 21 (8) | 41 (4) |
| Travel >4 h, n (%) | 368 (26) | 76 (27) | 292 (26) |
| Immobilization due to trauma, n (%) | 64 (5) | 17 (6) | 47 (4) |
| Hormonal contraceptives, n (%) | 37 (3) | 8 (3) | 29 (3) |
| Hormone-replacement therapy, n (%) | 121 (9) | 15 (5) | 106 (10) |
| Known thrombophilia, n (%) | 45 (3) | 15 (5) | 30 (3) |
| Pregnancy or puerperium, n (%) | 19 (1) | 1 (0.4) | 18 (2) |
| Recent surgery, n (%) | 109 (8) | 32 (12) | 77 (7) |
| . | All, N = 1397 . | DVT,* n = 277 . | No DVT,* n = 1120 . |
|---|---|---|---|
| Age, median (IQR), y | 64 (52-73) | 65 (53-73) | 64 (51-73) |
| Symptom duration, median (IQR), d | 7 (3-14) | 5 (3-7) | 7 (3-14) |
| Female sex, n (%) | 770 (55) | 114 (41) | 656 (59) |
| Positive D-dimer, n (%) | 982 (70) | 275 (99) | 707 (63) |
| Low probability for DVT,† n (%) | 383 (27) | 23 (8) | 360 (32) |
| Moderate probability for DVT,† n (%) | 670 (48) | 121 (44) | 549 (49) |
| High probability for DVT,† n (%) | 344 (25) | 133 (48) | 211 (19) |
| Positive D-dimer and/or high probability for DVT† | 1024 (73) | 276 (99) | 748 (67) |
| DVT likely,‡ n (%) | 698 (50) | 216 (78) | 482 (43) |
| DVT unlikely,‡ n (%) | 699 (50) | 61 (22) | 638 (57) |
| Positive D-dimer and/or DVT likely,‡ n (%) | 1105 (79) | 276 (99) | 829 (74) |
| Previous VTE, n (%) | 203 (15) | 73 (26) | 130 (12) |
| VTE in first-degree relatives, n (%) | 266 (19) | 57 (21) | 209 (19) |
| Active cancer within 6 mo, n (%) | 62 (4) | 21 (8) | 41 (4) |
| Travel >4 h, n (%) | 368 (26) | 76 (27) | 292 (26) |
| Immobilization due to trauma, n (%) | 64 (5) | 17 (6) | 47 (4) |
| Hormonal contraceptives, n (%) | 37 (3) | 8 (3) | 29 (3) |
| Hormone-replacement therapy, n (%) | 121 (9) | 15 (5) | 106 (10) |
| Known thrombophilia, n (%) | 45 (3) | 15 (5) | 30 (3) |
| Pregnancy or puerperium, n (%) | 19 (1) | 1 (0.4) | 18 (2) |
| Recent surgery, n (%) | 109 (8) | 32 (12) | 77 (7) |
At baseline visit.
According to the original, 3-level Wells score.
According to the modified, 2-level Wells score.
Diagnostic performance of stand-alone D-dimer vs other strategies (n = 1397)
| . | D-dimer . | Age-adjusted DD+ . | C-PTP adjusted DD+ . | ||
|---|---|---|---|---|---|
| ≥0.5 mg/L . | or Wells score ≥3 . | or Wells score ≥2 . | |||
| Positive predictive value,*n | 278/982 | 279/1024 | 279/1105 | 273/928 | 271/917 |
| Estimate, % | 28.3 | 27.2 | 25.2 | 29.4 | 29.6 |
| 95% CI | 25.5-31.2 | 24.5-30.1 | 22.7-27.9 | 26.5-32.5 | 26.6-32.6 |
| VTE within 3 mo despite negative workup,†n | 3/415 | 2/371 | 2/290 | 8/469 | 10/480 |
| Estimate, % | 0.7 | 0.5 | 0.7 | 1.7 | 2.1 |
| 95% CI | 0.1-2.1 | 0.1-1.9 | 0.1-2.5 | 0.7-3.3 | 1.0-3.8 |
| Required D-dimer tests according to strategy,‡n | 1397/1397 | 1053/1397 | 699/1397 | 1053/1397 | 1053/1397 |
| Estimate, % | 100 | 75.3 | 50.0 | 75.4 | 75.4 |
| 95% CI | 99.7-100 | 73.0-77.6 | 47.4-52.7 | 73.0-77.6 | 73.0-77.6 |
| Required CUS examinations according to strategy,§n | 982/1397 | 1024/1397 | 1105/1397 | 928/1397 | 917/1397 |
| Estimate, % | 70.3 | 73.3 | 79.1 | 66.4 | 65.6 |
| 95% CI | 67.8-72.7 | 70.9-75.6 | 76.9-81.2 | 63.9-68.9 | 63.1-68.1 |
| . | D-dimer . | Age-adjusted DD+ . | C-PTP adjusted DD+ . | ||
|---|---|---|---|---|---|
| ≥0.5 mg/L . | or Wells score ≥3 . | or Wells score ≥2 . | |||
| Positive predictive value,*n | 278/982 | 279/1024 | 279/1105 | 273/928 | 271/917 |
| Estimate, % | 28.3 | 27.2 | 25.2 | 29.4 | 29.6 |
| 95% CI | 25.5-31.2 | 24.5-30.1 | 22.7-27.9 | 26.5-32.5 | 26.6-32.6 |
| VTE within 3 mo despite negative workup,†n | 3/415 | 2/371 | 2/290 | 8/469 | 10/480 |
| Estimate, % | 0.7 | 0.5 | 0.7 | 1.7 | 2.1 |
| 95% CI | 0.1-2.1 | 0.1-1.9 | 0.1-2.5 | 0.7-3.3 | 1.0-3.8 |
| Required D-dimer tests according to strategy,‡n | 1397/1397 | 1053/1397 | 699/1397 | 1053/1397 | 1053/1397 |
| Estimate, % | 100 | 75.3 | 50.0 | 75.4 | 75.4 |
| 95% CI | 99.7-100 | 73.0-77.6 | 47.4-52.7 | 73.0-77.6 | 73.0-77.6 |
| Required CUS examinations according to strategy,§n | 982/1397 | 1024/1397 | 1105/1397 | 928/1397 | 917/1397 |
| Estimate, % | 70.3 | 73.3 | 79.1 | 66.4 | 65.6 |
| 95% CI | 67.8-72.7 | 70.9-75.6 | 76.9-81.2 | 63.9-68.9 | 63.1-68.1 |
DD, D-dimer.
Number of DVT in all patients requiring workup according to each strategy; true positive/true positive + false positive.
According to the criteria ruling out DVT in each strategy; false negative/false negative + true negative.
Required in all patients or non–high-risk patients.
Required if positive D-dimer or high-risk patients.
Outcomes
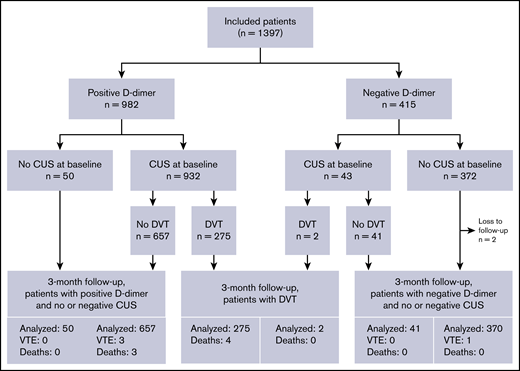
Outcomes are summarized in Figure 2. Six of the 1113 patients with negative D-dimer and/or normal CUS were diagnosed with DVT within 3 months for a failure rate of 0.5% (95% CI, 0.2-1.2).
Three of 415 patients with negative D-dimer were diagnosed with DVT: 2 proximal and 1 distal. These had Wells scores of −1, 1, and 3. As such, the failure rate for D-dimer as a stand-alone test was 0.7% (95% CI, 0.1-2.1).
Of the 698 patients with normal CUS at baseline, 3 patients were diagnosed with DVT within the 3-month follow-up, with Wells scores of 1, 3, and 4. As such, the failure rate for whole-leg CUS was 0.4% (95% CI, 0.1-1.3).
Two patients were lost to follow-up. They both had negative D-dimer and did not undergo CUS at baseline.
Additionally, 3 patients in whom DVT was ruled out by a normal CUS at baseline died within the 3-month follow-up: none underwent autopsy. VTE was adjudicated to not be the likely cause of death in any of the 3 patients, but cannot be definitely ruled out as autopsies were not performed. Considering these 5 cases as failures would have yielded 11 failures out of 1113 patients (1.0; 95% CI, 0.5-1.8) with negative workup: a figure with an upper bound of the 95% CI still well below the predefined acceptable safety margin.
As for the feasibility outcome of adherence, CUS was not performed in 50 of the 982 patients with positive D-dimer (5.1%) and was performed in 43 of the 415 patients with negative D-dimer (10.4%). As such, 1304 of 1397 patients (93.3%; 95% CI, 91.9-94.6) were managed according to protocol. In all cases in which CUS was not performed despite positive D-dimer, the suspicion of DVT was discarded after evaluation by the emergency department attending physician. Reasons given by physicians for requesting CUS despite negative D-dimer are listed in Table 3.
Reasons given by physicians for requesting CUS despite negative D-dimer
| . | Total, N . | No DVT, n . | DVT, n . |
|---|---|---|---|
| No recorded reason in patient files | 14 | 14 | 0 |
| Strong suspicion of DVT due to specific symptoms or signs | 14 | 12 | 2 |
| Evaluate extent of suspected thrombophlebitis to determine treatment | 2 | 2 | 0 |
| Evaluate other suspected diagnosis than DVT | 7 | 7 | 0 |
| High C-PTP | 4 | 4 | 0 |
| Lack of alternative diagnosis to DVT | 2 | 2 | 0 |
| Total | 43 | 41 | 2 |
| . | Total, N . | No DVT, n . | DVT, n . |
|---|---|---|---|
| No recorded reason in patient files | 14 | 14 | 0 |
| Strong suspicion of DVT due to specific symptoms or signs | 14 | 12 | 2 |
| Evaluate extent of suspected thrombophlebitis to determine treatment | 2 | 2 | 0 |
| Evaluate other suspected diagnosis than DVT | 7 | 7 | 0 |
| High C-PTP | 4 | 4 | 0 |
| Lack of alternative diagnosis to DVT | 2 | 2 | 0 |
| Total | 43 | 41 | 2 |
Reasons as recorded in patient files.
Discussion
Principal findings
In this prospective management study, we found that our simple approach of performing a single whole-leg CUS only in patients with D-dimer ≥0.5 µg/mL and withholding CUS in patients with D-dimer <0.5 µg/mL was a safe strategy associated with a low failure rate. The upper limit of the 95% CI of 1.2% was well below the predefined accepted 3-month VTE rate of 4%. Moreover, both components of the strategy, stand-alone D-dimer and whole-leg CUS, had comparably low failure rates, similar to previous literature.6,13,14 To our knowledge, this is the first study to assess the clinical outcomes of withholding anticoagulation treatment in a diagnostic strategy combining stand-alone D-dimer and single whole-leg CUS.
Many management strategies incorporating C-PTP assessment, D-dimer results, and various CUS techniques have been studied.5,15-18 These lay the foundation for existing recommendations of the diagnostic workup of DVT.1,2 With some variation, the general recommendation is to conduct either proximal or whole-leg CUS in all patients with a high likelihood of DVT or positive D-dimer. Ruling out DVT on the basis of normal CUS or otherwise negative D-dimer when adhering to 1 of these strategies is associated with a VTE rate similar to that found in our study: between 0.4% and 2.0%,7,15,17,19-24 with an upper limit of the 95% CI of mostly ≤2.2%.
Guidelines currently recommend against using stand-alone D-dimer to rule out DVT. However,1,2 the studies upon which they are based were largely not prospective outcome studies using clinical follow-up as reference standard, and were instead based on D-dimer assessment against reference imaging at inclusion for the whole study population or for patients with high C-PTP.3,25-28 This approach does not necessarily reflect clinical outcomes after a follow-up period, and may result in detecting clinically insignificant DVT with subsequent risk of overdiagnosis. Moreover, generalizing failure rates of D-dimer yielded by universal imaging of high-risk populations to the general outpatient population may not be appropriate, and most prospective outcome studies of outpatients do not have a DVT prevalence nearing the ≥50% defined as a high-risk population.18 Rathbun et al conducted 2 studies withholding diagnostic workup in patients with negative D-dimer for suspected first and recurrent DVT, respectively.29,30 They found failure rates for stand-alone D-dimer of 0.0% (95% CI, 0-4.4) and 0.75% (95% CI, 0.02-4.1). However, the study populations were relatively small compared with ours with 81 and 134 patients with negative D-dimer. Moreover, in both studies, there were patients in whom VTE could not be definitely ruled out, yielding a worst-case upper limit of the 95% CI of 11.4%.
With the failure rate of our strategy being 0.5% (95% CI, 0.2-1.2) and well within our preaccepted safety margin, we would not suggest systematic follow-up of patients with negative D-dimer or negative CUS; the latter is in line with current guidelines.1,2,31 This does not preclude individual exceptions, and all patients with negative workup were encouraged to contact health care providers for renewed evaluation if they experienced recurring, persisting, or worsening symptoms, or symptoms of PE.
In addition to comparable safety, we believe our strategy has several advantages over current diagnostic algorithms and should therefore be seen as a valuable alternative. First, it may reduce the amount of CUS examinations, thereby decreasing cost,30 time, and resources required for the management of individual patients. According to current guidelines, all patients in the high-risk group should be referred for CUS, as well as patients with positive D-dimer in the low- or moderate-risk groups. Because our strategy entails referring only patients with positive D-dimer irrespective of pretest probability, fewer CUS examinations would be required in the group conventionally stratified as high risk. Notably, several recent guidelines have applied and/or stated their preference for the 2-tier Wells score in their recommendations.1,31,32 The modified score classifies a larger proportion of patients as high risk than the original 3-tier Wells score, resulting in more required CUS examinations. Although we did not conduct a formal comparison between the strategies, obtaining the Wells score at inclusion enabled us to retrospectively assess the diagnostic properties of strategies including the Wells score in the diagnostic algorithm (Table 2).
Taking Wells score into consideration for our patients would have resulted in 3.0% and 8.8% more CUS according to the 3- and 2-tier scores, respectively, for a similar failure rate. In a recent retrospective study of 1765 patients, Rinde et al similarly found that stand-alone D-dimer would have required 9.6% less CUS than D-dimer incorporated with the 2-tier Wells score for a similar failure rate (1.8% [95% CI, 0.8% to 3.5%] vs 1.6% [95% CI, 0.5% to 3.6%], respectively).33
Recent attempts to increase specificity and reduce the number of unnecessary CUS examinations include increasing thresholds for positive D-dimer in older patients or in patients with low C-PTP.34 In our study, both strategies would have required CUS in 66% of patients because of positive D-dimer or high C-PTP, 4% less than our strategy albeit at the cost of a slightly higher failure rate (Table 2).
In addition to reducing CUS examinations by omitting clinical prediction rules, our strategy obviates the repeat examinations required in the case of a negative proximal CUS in moderate- or high-risk patients with positive D-dimer.1,2,32 As for choice of modality in CUS, both whole-leg and proximal CUS are acceptable options,19,21 and there is no favored consensus.1,2 Disadvantages of whole-leg CUS include being more skill- and resource-demanding, in addition to the potential disadvantage of treating distal DVT that would otherwise resolve without complications. However, we prefer whole-leg CUS to obviate repeat testing, and provide alternative explanations for the patient’s symptoms.
A second advantage of our strategy is avoiding the challenges with clinical prediction rules. These include the inherent weakness of subjectivity,35,36 not widely validated interrater reliability,2 and incorrect use. The latter may partly result from the fact that, in some emergency departments, standard blood samples including D-dimer are obtained before clinical evaluation to improve efficiency. Knowledge of D-dimer results prior to C-PTP assessment may influence scoring,37 contrary to the intended use. Lastly, real-life data show varying or limited adherence to prediction rules in clinical practice.16,38-41 The GARFIELD-VTE registry found that <5% of patients underwent C-PTP evaluation before imaging.42,43 We believe that simplifying the workup may increase clinical adherence and usefulness, supported by the 93% adherence to the strategy in our study. Additionally, the clinician’s familiarity with a score as well as clinical experience would be of less importance with our strategy.
Strengths, limitations, and clinical implications
Strengths of our study include its relatively large patient number, prospective design, standardized assessment and collection of data, and few losses to follow-up. The baseline prevalence of DVT of 19.8% was in the same range or higher than similarly designed studies.14,29,30,44 C-PTP was fairly evenly distributed between low, moderate, and high subgroups. All of these factors diminish the risk of an artificially high negative predictive value that a low prevalence could yield.
Our trial has several limitations, 1 being its monocentric design due to feasibility considerations. This may in turn adversely affect the generalizability of our findings, and external validity remains to be established. Nonetheless, consisting of consecutive outpatients with an overall intermediate DVT prevalence,1 a fairly even distribution of different C-PTP subgroups, as well as comparable failure rates to other studies examining stand-alone D-dimer, we believe our findings are likely to be generalizable to other emergency department populations with similar or lower prevalence.
Only 1 D-dimer assay was studied, which could limit extrapolation of our findings to other assays, such as point-of-care devices. As the negative predictive value of the STA-Liatest has been found to be comparable to that of other high-sensitivity assays,3 we expect these to be similarly safe granted internal quality control measures are in place.
Moreover, the study was not powered to include sample sizes for high-risk subgroups that would have benefited from clear management guidance, as this would have warranted a larger population and scope than feasible for the study. The strategy may be less specific for inpatients,45 or in other conditions or situations in which D-dimer could be expected to be increased, such as in pregnant patients and in patients with cancer46 who comprise 4% and 1% of the study population, respectively. False-negative D-dimer could occur in patients with DVT on anticoagulation treatment. Although its effects on D-dimer are still largely unknown,1 some studies have suggested decreasing D-dimer levels after initiation of anticoagulation therapy.47 For this reason, patients on a regular prescription of anticoagulants were excluded in this study.
We cannot eliminate the possibility that removing C-PTP assessment led to more DVT being diagnosed and treated. In the event of low C-PTP, physicians might be more inclined not to refer the patient for CUS despite positive D-dimer, or to dismiss an uncertain radiologic finding. However, when performed correctly, falsely interpreted CUS examinations for first DVT are rare. For suspected recurrent ipsilateral DVT, magnetic resonance direct thrombus imaging is an alternative to distinguish between acute and chronic DVT, which would reduce the risk of falsely interpreted CUS in these patients.10
Regardless, our strategy should only be used when DVT is suspected and where D-dimer is useful. Conversely, false-positive results could similarly occur with current recommendations referring all perceived high-risk patients for CUS regardless of D-dimer as this is based on subjective evaluation.
To our knowledge, comparisons between alternative D-dimer thresholds, such as stand-alone, age-adjusted, and C-PTP–adjusted D-dimer, have been retrospective.6,11,34 For future research, a prospective multicenter study with a head-to-head comparison of the various strategies would be useful in determining the optimal strategy. Future research efforts aimed at obviating unnecessary diagnostic workup altogether would further advance the management of patients with suspected DVT, for instance, by identifying new biomarkers and/or developing machine-learning strategies. Increased knowledge of which DVT should be managed conservatively or pharmacologically would aid these efforts.
In conclusion, a single negative whole-leg CUS safely ruled out DVT in patients with positive D-dimer, and negative D-dimer safely ruled out DVT, both irrespective of C-PTP. We consider this strategy to be a valuable alternative to the conventional diagnostic workup of DVT in outpatients.
Deidentified individual participant data that underlie the reported results may be requested after publication to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose, to achieve aims in the approved proposal. Information regarding accessing data and obtaining study protocol can be directed to corresponding author, Synne G. Fronas, at s.g.fronas@gmail.com.
Acknowledgments
The authors thank Brynhild Jørgensen, Jens Stene-Johansen, Mohamed Qarbosh, Håkon Rørberg, Mathias Perminow, Adeline Svendsen, and Bitte Therese Broch for enrollment and follow-up of patients; Åse-Berit Mathisen for laboratory assistance; and Heidi Hassel Pettersen and Christina Roaldsnes for study coordination.
This work was supported in part by research funding from Bayer, the South-Eastern Norway Regional Health Authority, and the Østfold Hospital Trust.
Authorship
Contribution: S.G.F. participated in study design, data acquisition, and management of the trial, analyzed and interpreted the data, and drafted and revised the manuscript; C.T.J. participated in data acquisition, daily management of the study, data analyses, and revision of the manuscript; A.E.A.D. participated in protocol drafting, study management, interpretation and revision of the manuscript; H.S.W. participated in protocol drafting, data interpretation, and revision of the manuscript; J.G. participated in study management and drafting and revising of the manuscript; N.R. participated in data acquisition and study management, and revision of the manuscript; R.H. participated in study design, input regarding end-point selection and statistical analyses, and revision of the manuscript; F.A K. participated in data analyses and interpretation and revision of the manuscript; and W.G. was trial manager, was responsible for the design, planning, initiation, and conduction of the study, and participated in data acquisition and interpretation and revision of the manuscript.
Conflict-of-interest disclosure: S.G.F. and C.T.J. report grants from Bayer, the South-Eastern Norway Regional Health Authority, and the Østfold Hospital Trust to the conduct of the study (no personal fees). A.E.A.D. reports grants and personal fees from Pfizer AS and personal fees from Bristol-Myers Squibb and Novartis Norway AS, outside of the submitted work. F.A.K. reports research grants from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Actelion, the Dutch Heart Foundation, and the Dutch Thrombosis Association, outside of the submitted work. W.G. reports grants from Bayer, the South-Eastern Norway Regional Health Authority and Østfold Hospital Trust for the conduct of this study; grants from Bayer, Bristol-Myers Squibb, and Novartis, outside of the submitted work; and participation on an advisory board for Amgen and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Synne G. Fronas, Clinic of Internal Medicine, Department of Emergency Medicine, Østfold Hospital Trust, Kalnesveien 300, 1714 Grålum, Norway; e-mail: s.g.fronas@gmail.com.