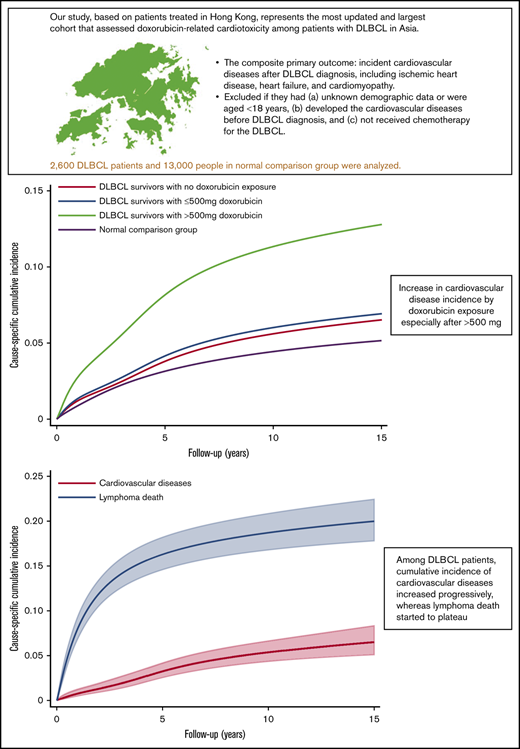
Key Points
Our study had the most updated and largest cohort to assess doxorubicin-related cardiotoxicity among patients with DLBCL in Asia.
A >500 mg absolute dose of doxorubicin, together with hypertension and history of aspirin use confer a particularly high risk of CVD incidence.
Abstract
Evidence regarding the dose-related impact of doxorubicin on subsequent cardiovascular diseases (CVDs) in Asian patients with diffuse large B-cell lymphoma (DLBCL) without preexisting CVDs is lacking. From a territory-wide electronic database in Hong Kong, we identified adults who were diagnosed with DLBCL and treated with chemotherapy between 2000 and 2018. We evaluated the patients for incident CVDs (including ischemic heart disease, heart failure, and cardiomyopathy). We evaluated the cause-specific cumulative incidence (csCI) of CVD with levels of doxorubicin exposure by using flexible parametric competing risk analysis and adjusting for demographics, comorbidities, therapeutic exposure, cardiovascular risk factors, and lifestyle factors. Controls were age- and sex-matched to DLBCL patients. We analyzed 2600 patients and 13 000 controls. The adjusted cause-specific hazard ratio (HR) for CVD in patients treated with >500 mg doxorubicin compared with non-doxorubicin regimens was 2.65 (95% confidence interval [CI], 1.23-5.74; P = .013). The 5-, 10-, and 15-year csCIs were 8.2%, 11.3%, and 12.8% in patients vs 3.1%, 4.4%, and 5.2% in controls, respectively. Hypertension (HR, 6.20; 95% CI, 0.79-48.44; P = .082) and use of aspirin/angiotensin-converting enzyme inhibitor/beta-blocker at baseline (HR, 2.13-4.63; P < .001 to .002) might confer a higher risk of subsequent CVDs. In this Hong Kong population-based study, doxorubicin exposure (absolute dose >500 mg), together with hypertension or baseline use of medication for cardiovascular risk factors, was found to be associated with an increase in csCIs of CVDs. Tailoring therapeutic strategies to underlying CVD risk factors and risk-adapted monitoring and follow-up of susceptible DLBCL patients are advisable.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma globally, constituting 25% to 40% of all cases in different geographic regions.1-4 The median age at diagnosis is ∼70 years.2 Effective modern therapeutic strategies have resulted in a 5-year survival exceeding 60% according to US population-based data.1 However, the therapeutic exposures responsible for long-term survival are also implicated in long-term sequelae among survivors. The mainstay of therapeutic regimens for the treatment of patients with DLBCL includes rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy (RT).5 This anthracycline-based chemotherapy regimen can increase the risk of cardiovascular sequelae; exposure to chest RT and preexisting cardiovascular risk factors may also further enhance the risk.6-19
However, to our knowledge, anthracycline-related cardiotoxicity has not been studied in an Asian population diagnosed with DLBCL. It has been well documented that the prevalence and management of major cardiovascular risk factors can vary markedly worldwide.20 There are also significant differences in health habits and environmental exposures among Asian populations compared with those in the Western world, which may influence the susceptibility of Western populations to treatment-related cardiotoxicity.21-23 An improved understanding of the interacting effects of preexisting comorbidities and lifestyle factors on the relationship between DLBCL therapy and cardiovascular diseases (CVDs) can guide upfront treatment decisions as well as long-term cardiac risk–based survivorship care tailored for this understudied population.
We aimed to determine the association between therapeutic exposure (doxorubicin and RT) and new-onset CVD among DLBCL survivors by analyzing population-based data, including information on major cardiovascular risk factors, comorbidities, and lifestyle factors in a population of DLBCL survivors and controls without cancer in Hong Kong.
Materials and methods
Data sources
We retrieved data from the Clinical Data Analysis and Reporting System (CDARS), an electronic medical database operated by the Hong Kong Hospital Authority. The CDARS was established in 1995 for audit and research purposes. The Hospital Authority is the only public health care provider, and it covers ∼90% of all secondary and tertiary care in Hong Kong, which has a population of nearly 7.5 million.24,25 Data on patient demographics, diagnoses, hospitalizations, treatments, laboratory results, and causes, times, and dates of death, are recorded in CDARS. In routine practice in the Hong Kong Hospital Authority, clinicians in clinical and hospital settings provide International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for each episode of attendance.26,27 In a previous study, these codes revealed a high coding accuracy in diagnosing myocardial infarction and stroke with positive predictive values of 85.4% (95% confidence interval [CI] 78.8%-90.6%) and 91.1% (95% CI, 83.2%-96.1%), respectively.28 Another study also demonstrated the reliability of the administrative database of the Hong Kong Hospital Authority to capture demographics and use of antidiabetic drugs with an almost perfect level of data completeness regarding demographics (100%) and drug prescription (99.98%).27 For other diagnoses, previous studies have also demonstrated high coding accuracy, with positive and negative predictive values exceeding 90%.29,30 Patient confidentiality is protected by unique anonymous identifiers that are linked to all data contained in the CDARS to facilitate data retrieval. Various high-quality population-based studies on CVDs, cancers, and medications have been published based on data retrieved from the CDARS.28,31,32 The study protocol was approved by the Research Ethics Committee of the New Territories West Cluster, Hong Kong Hospital Authority (reference no: NTWC/REC/19085).
Case definitions and outcome ascertainment
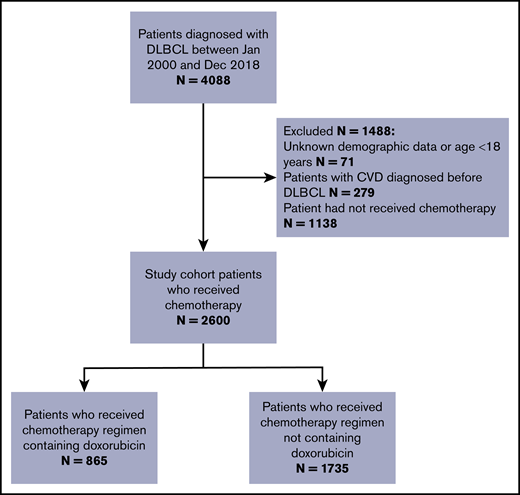
We used data from the CDARS to identify individuals histologically diagnosed with DLBCL between 31 December 2018 and 1 January 2000. Figure 1 shows the study criteria and the final number of patients who constituted the study cohort.33 The composite primary outcome was incident CVD after DLBCL diagnosis, including ischemic heart disease, heart failure, and cardiomyopathy clinically diagnosed during inpatient hospital visits or as the cause of death after lymphoma diagnosis (ICD-9 codes are provided in supplemental Table 1). Patients were excluded if they had unknown demographic data or were younger than age 18 years, if they developed CVD before DLBCL diagnosis, and if they had not received chemotherapy for the DLBCL (supplemental Table 2). The follow-up times for CVD continued until the first diagnosis of a CVD event, non-cardiac death, or censor date, whichever was earlier. We censored patients who remained alive and had not developed CVD by the end of follow-up on 30 September 2019. Follow-up started after the date of histologic lymphoma diagnosis and ended 15 years after diagnosis. We counted the events only if the diagnosis of CVD occurred beyond a landmark period of 9 months after lymphoma diagnosis because most patients have completed the first-line treatment by that time. Because of the heavily subsidized health care system in Hong Kong, patients with chronic diseases and serious complications (eg, myocardial infarction) are treated mostly in the Hong Kong Hospital Authority public health care system.34 Therefore, the Hong Kong Hospital Authority data should have captured nearly all hospital-managed CVD outcomes with dates available.
Flowchart outlining the inclusion and exclusion criteria (N = 2600), Hong Kong, 2000-2018.
Flowchart outlining the inclusion and exclusion criteria (N = 2600), Hong Kong, 2000-2018.
Preexisting cardiovascular risk factors, comorbidities, and other variables
The cardiovascular risk factors included hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease (COPD), alcohol-related diseases, atrial fibrillation, and history of depression (supplemental Table 3).35,36 Diabetes, hypertension, and dyslipidemia were determined by using a combination of ICD-9 codes and the prescriptions for medications for these conditions (supplemental Table 3). We used approaches similar to those adopted by Poulsen et al37 to determine COPD, smoking status, and alcohol-related diseases because these data were not directly available in the CDARS. COPD and smoking status were captured by ICD-9 codes 491, 492, 496, and V15.82.37 We identified alcohol-related diseases, which include hepatic and gastrointestinal diseases and neurologic and psychiatric diseases (ICD-9 codes 291, 303, 305.0, 571, 980).37
We included sex and age at diagnosis. Comorbid conditions before the lymphoma diagnosis were measured using the Royal College of Surgeons (RCS) adaptation of the Charlson Comorbidity Index (CCI).38 Overlapping cardiovascular risk factors were removed from the overall RCS score. The remaining comorbidities in the CCI (peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, liver disease, hemiplegia/paraplegia, renal disease, and AIDS/HIV infection) were combined into the score and examined as such. We extracted information regarding the need for medical fee waiver as a surrogate for lower socioeconomic status.
Normal comparison group
The normal comparison group of people selected from a non-cancer random sample without replacement from primary care clinics were sex- and age-matched (within 3 years) to DLBCL patients in a 5:1 ratio. The normal comparison group had no previous diagnosis of cancer or CVD. Both the DLBCL and normal comparison cohort have been retrieved from all 18 districts of Hong Kong.
Treatment information
The treatment data included chemotherapy regimens (doxorubicin- vs non-doxorubicin–based), rituximab, and the use of RT. The absolute prescribed doses of doxorubicin were determined from pharmacy records. The selected patients did not receive anthracyclines other than doxorubicin. Patients exposed to doxorubicin were grouped according to the absolute cumulative doses (≤500 or >500 mg, ∼300 mg/m2, or 6 cycles of doxorubicin-based treatment, assuming an average body surface area of 1.67 m2, which is a reasonable number based on local data).39
Statistical analysis
Descriptive statistics for demographics, follow-up duration, and prevalence of characteristics were generated for the DLBCL survivors and normal comparison group. Continuous variables were presented as medians with interquartile ranges and were compared using rank-sum tests, whereas categorical variables were presented as percentages and compared using χ2 tests. To investigate potential confounding by indication, we cross-tabulated preexisting cardiovascular risk factors with receipt of doxorubicin and tested for associations using χ2 tests.
With the hypothesis that doxorubicin exposure drives the differences in CVD outcomes between groups, we used a cause-specific hazard framework to deal with the competing risks of non-cardiac mortality and to derive cause-specific hazard ratios (HRs).40,41 We evaluated the cause-specific cumulative incidence (csCI) of CVD with levels of doxorubicin exposure using flexible parametric competing risk analysis.42-45 We considered non-cardiac mortality as a competing risk when comparing csCIs between lymphoma survivors and the normal comparison group.42,43,46,47 The csCI of CVD was derived after adjusting for age, sex, race, year of diagnosis, need for medical fee waiver, cumulative dose of doxorubicin, receipt of RT, rituximab administration, preexisting cardiovascular risk factors (COPD or smoking, alcohol-related diseases, atrial fibrillation, hypertension, hyperlipidemia, dyslipidemia, diabetes mellitus, and depression), use of aspirin, beta blockers, and angiotensin-converting enzyme (ACE) inhibitors (supplemental Table 3), and RCS comorbidity score. The csCIs of CVD for DLBCL patients and the normal comparison group are illustrated by using cumulative incidence plots. In secondary analyses, we included the csCI plots of each CVD end point (ie, heart failure, cardiomyopathy, and ischemic heart disease) for DLBCL patients and the normal comparison group. We computed the ratios of predicted cumulative incidence of lymphoma death and CVD among DLBCL patients at 5, 10, and 15 years and computed their respective 95% confidence intervals (CIs) by bootstrapping. We performed a sensitivity analysis to test whether the results were robust with respect to the absence of landmark period and doxorubicin dose cutoff. We used Stata v.16.1 (StataCorp, College Station, TX), including the command stpm2 (version 1.7.4) to perform the statistical analyses and fit the flexible parametric survival models.48,49
Results
The characteristics of the DLBCL cohort (N = 2600) and the normal comparison group (N = 13 000) are detailed in Table 1. The median age at diagnosis for the DLBCL cohort was 63 years (interquartile range [IQR], 53-73 years) and 56.0% were male. As of September 30, 2019, the median follow-up time from the index date for the entire lymphoma survivor cohort was 6.8 years (IQR, 3.6-10.5 years), providing 13 352 person-years of follow-up. Beyond 9 months from diagnosis, 118 (4.5%) of the DLBCL survivors developed CVDs, with a median interval to CVD of 3.9 years (IQR, 1.8-6.0 years). Among DLBCL survivors, most deaths were a result of lymphoma (53.8%), followed by pulmonary disorders (17.0%), and infections (3.8%). Prescription of doxorubicin was associated with age, cardiovascular risk factors, and social factors such that patients who were older (odds ratio [OR] per 1-year increase in age, 0.96; 95% CI, 0.96-0.97; P < .001), had diabetes (OR, 0.70; 95% CI, 0.57-0.86; P < .001), hypertension (OR, 0.73; 95% CI, 0.61-0.88; P < .001), dyslipidemia or hyperlipidemia (OR, 0.69; 95% CI, 0.56-0.85; P < .001), ACE inhibitor use (OR, 0.74; 95% CI, 0.61-0.92; P = .005), or received medical fee waiver (OR, 0.61; 95% CI, 0.44-0.86; P = .004) were less commonly administered doxorubicin. The median age of patients who received doxorubicin vs those who did not was 57 vs 66 years, respectively (rank-sum test P < .001). Supplemental Table 4 provides the characteristics of patients who received different regimens.
Characteristics of DLBCL survivors (N = 2600) and matched general population comparison group (N = 13 000) in Hong Kong, 2000-2018
| Characteristic . | All lymphoma patients (N = 2600) . | Normal comparison group (n = 13 000) . | P . | Lymphoma patients categorized by CVD status (N = 2600) . | P . | |
|---|---|---|---|---|---|---|
| CVD (n = 175) . | No CVD (n = 2425) . | |||||
| Patient factors | ||||||
| Median age at lymphoma diagnosis (IQR), y | 63 (53-73) | 63 (54-72) | —* | 70 (62-79) | 62 (52-72) | <.001 |
| Sex | —* | .875 | ||||
| Male | 1456 (56.0) | 7 280 (56.0) | 99 (56.6) | 1357 (56.0) | ||
| Female | 1144 (44.0) | 5 720 (44.0) | 76 (43.4) | 1068 (44.0) | ||
| Race/ethnicity | .001 | .068 | ||||
| Chinese | 2484 (95.5) | 12 586 (96.8) | 172 (98.3) | 2312 (95.3) | ||
| Non-Chinese | 116 (4.5) | 414 (3.2) | 3 (1.7) | 113 (4.7) | ||
| RCS comorbidity scores | <.001 | <.001 | ||||
| 0 | 1710 (65.8) | 8 871 (68.2) | 69 (39.4) | 1641 (67.7) | ||
| 1 | 664 (25.5) | 2 631 (20.2) | 61 (34.9) | 603 (24.9) | ||
| ≥2 | 226 (8.7) | 1 498 (11.5) | 45 (25.7) | 181 (7.5) | ||
| Median follow-up time for alive patients (IQR), y | 6.8 (3.6-10.5) | 7.8 (4.4-10.9) | <.001 | 5.3 (1.9-7.5) | 6.9 (3.6-10.6) | <.001 |
| Year of diagnosis | — | <.001 | ||||
| 2000-2004 | 398 (15.3) | — | 37 (21.1) | 361 (14.9) | ||
| 2005-2009 | 697 (26.8) | — | 68 (38.9) | 629 (25.9) | ||
| 2010-2014 | 870 (33.5) | — | 51 (29.1) | 819 (33.8) | ||
| 2015-2018 | 635 (24.4) | — | 19 (10.9) | 616 (25.4) | ||
| Fee waiver recipients (surrogate for lower socioeconomic status) | 200 (7.7) | 3 186 (24.5) | <.001 | 22 (12.6) | 178 (7.3) | .012 |
| COPD or smoker | 645 (24.8) | 5 662 (43.6) | <.001 | 72 (41.1) | 573 (23.6) | <.001 |
| Alcohol-related diseases | 18 (0.7) | 98 (0.8) | .739 | 0 (0.0) | 18 (0.7) | .253 |
| Diabetes mellitus | 598 (23.0) | 4 708 (36.2) | <.001 | 71 (40.6) | 527 (21.7) | <.001 |
| Hypertension | 1797 (69.1) | 10 393 (80.0) | <.001 | 173 (98.9) | 1624 (67.0) | <.001 |
| Dyslipidemia/hyperlipidemia | 586 (22.5) | 6 349 (48.8) | <.001 | 84 (48.0) | 502 (20.7) | <.001 |
| Anxiety or depressive disorders | 436 (16.8) | 3 435 (26.4) | <.001 | 37 (21.1) | 399 (16.4) | .109 |
| Atrial fibrillation | 31 (1.2) | 514 (4.0) | <.001 | 7 (4.0) | 24 (1.0) | <.001 |
| ACE inhibitor use | 574 (22.1) | 5 269 (40.5) | <.001 | 102 (58.3) | 472 (19.5) | <.001 |
| Beta blocker use | 792 (30.5) | 5 436 (41.8) | <.001 | 119 (68.0) | 673 (27.8) | <.001 |
| Aspirin use | 602 (23.2) | 5 266 (40.5) | <.001 | 131 (74.9) | 471 (19.4) | <.001 |
| Treatment factors | ||||||
| Chemotherapy | — | — | .761 | |||
| Regimens containing doxorubicin | ||||||
| >500 mg | 166 (6.4) | — | 13 (7.4) | 153 (6.3) | ||
| ≤500 mg | 699 (26.9) | — | 44 (25.2) | 655 (27.0) | ||
| Non-doxorubicin regimens | 1735 (66.7) | — | 118 (67.4) | 1617 (66.7) | ||
| RT | 308 (11.9) | — | — | 25 (14.3) | 283 (11.7) | .301 |
| Rituximab | 1980 (76.2) | — | — | 126 (72.0) | 49 (28.0) | .182 |
| Characteristic . | All lymphoma patients (N = 2600) . | Normal comparison group (n = 13 000) . | P . | Lymphoma patients categorized by CVD status (N = 2600) . | P . | |
|---|---|---|---|---|---|---|
| CVD (n = 175) . | No CVD (n = 2425) . | |||||
| Patient factors | ||||||
| Median age at lymphoma diagnosis (IQR), y | 63 (53-73) | 63 (54-72) | —* | 70 (62-79) | 62 (52-72) | <.001 |
| Sex | —* | .875 | ||||
| Male | 1456 (56.0) | 7 280 (56.0) | 99 (56.6) | 1357 (56.0) | ||
| Female | 1144 (44.0) | 5 720 (44.0) | 76 (43.4) | 1068 (44.0) | ||
| Race/ethnicity | .001 | .068 | ||||
| Chinese | 2484 (95.5) | 12 586 (96.8) | 172 (98.3) | 2312 (95.3) | ||
| Non-Chinese | 116 (4.5) | 414 (3.2) | 3 (1.7) | 113 (4.7) | ||
| RCS comorbidity scores | <.001 | <.001 | ||||
| 0 | 1710 (65.8) | 8 871 (68.2) | 69 (39.4) | 1641 (67.7) | ||
| 1 | 664 (25.5) | 2 631 (20.2) | 61 (34.9) | 603 (24.9) | ||
| ≥2 | 226 (8.7) | 1 498 (11.5) | 45 (25.7) | 181 (7.5) | ||
| Median follow-up time for alive patients (IQR), y | 6.8 (3.6-10.5) | 7.8 (4.4-10.9) | <.001 | 5.3 (1.9-7.5) | 6.9 (3.6-10.6) | <.001 |
| Year of diagnosis | — | <.001 | ||||
| 2000-2004 | 398 (15.3) | — | 37 (21.1) | 361 (14.9) | ||
| 2005-2009 | 697 (26.8) | — | 68 (38.9) | 629 (25.9) | ||
| 2010-2014 | 870 (33.5) | — | 51 (29.1) | 819 (33.8) | ||
| 2015-2018 | 635 (24.4) | — | 19 (10.9) | 616 (25.4) | ||
| Fee waiver recipients (surrogate for lower socioeconomic status) | 200 (7.7) | 3 186 (24.5) | <.001 | 22 (12.6) | 178 (7.3) | .012 |
| COPD or smoker | 645 (24.8) | 5 662 (43.6) | <.001 | 72 (41.1) | 573 (23.6) | <.001 |
| Alcohol-related diseases | 18 (0.7) | 98 (0.8) | .739 | 0 (0.0) | 18 (0.7) | .253 |
| Diabetes mellitus | 598 (23.0) | 4 708 (36.2) | <.001 | 71 (40.6) | 527 (21.7) | <.001 |
| Hypertension | 1797 (69.1) | 10 393 (80.0) | <.001 | 173 (98.9) | 1624 (67.0) | <.001 |
| Dyslipidemia/hyperlipidemia | 586 (22.5) | 6 349 (48.8) | <.001 | 84 (48.0) | 502 (20.7) | <.001 |
| Anxiety or depressive disorders | 436 (16.8) | 3 435 (26.4) | <.001 | 37 (21.1) | 399 (16.4) | .109 |
| Atrial fibrillation | 31 (1.2) | 514 (4.0) | <.001 | 7 (4.0) | 24 (1.0) | <.001 |
| ACE inhibitor use | 574 (22.1) | 5 269 (40.5) | <.001 | 102 (58.3) | 472 (19.5) | <.001 |
| Beta blocker use | 792 (30.5) | 5 436 (41.8) | <.001 | 119 (68.0) | 673 (27.8) | <.001 |
| Aspirin use | 602 (23.2) | 5 266 (40.5) | <.001 | 131 (74.9) | 471 (19.4) | <.001 |
| Treatment factors | ||||||
| Chemotherapy | — | — | .761 | |||
| Regimens containing doxorubicin | ||||||
| >500 mg | 166 (6.4) | — | 13 (7.4) | 153 (6.3) | ||
| ≤500 mg | 699 (26.9) | — | 44 (25.2) | 655 (27.0) | ||
| Non-doxorubicin regimens | 1735 (66.7) | — | 118 (67.4) | 1617 (66.7) | ||
| RT | 308 (11.9) | — | — | 25 (14.3) | 283 (11.7) | .301 |
| Rituximab | 1980 (76.2) | — | — | 126 (72.0) | 49 (28.0) | .182 |
All data are no. (%) unless otherwise indicated.
People in the normal comparison group were sex- and age-matched to lymphoma patients.
Those in the normal comparison group compared with DLBCL survivors were significantly more likely to have hypertension (80.0% vs 69.1%; P < .001), diabetes (36.2% vs 23.0%; P < .001), dyslipidemia or hyperlipidemia (48.8% vs 22.5%; P < .001), anxiety or depressive disorder (26.4% vs 16.8%; P < .001), be smokers (43.6% vs 24.8%; P < .001), or require a medical fee waiver (24.5% vs 7.7%; P < .001) (Table 1).
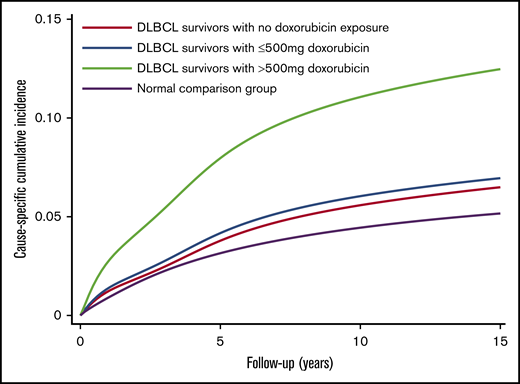
After multivariable adjustment (Table 2), patients treated with >500 mg absolute dose of doxorubicin had an approximately threefold increased risk of CVD compared with patients treated with regimens that did not contain doxorubicin (adjusted cause-specific HR, 2.55; 95% CI, 1.18-5.53; P = .017). The cumulative incidence curves reflected the results of the regression analysis. The patients who had received >500 mg absolute dose of doxorubicin had 5-, 10- and 15-year csCIs of CVD of 8.0%, 11.1%, and 12.5% respectively; the corresponding incidence rates for patients treated with ≤500 mg were 4.2%, 6.0%, and 7.0%, respectively; and those for patients treated with non-doxorubicin regimens, the corresponding incidence rates were 3.8%, 5.6%, and 6.5%, respectively. The incidence estimates for the normal comparison group were 3.1%, 4.4%, and 5.2%, respectively (Figure 2).
Cause-specific HRs for new-onset cardiovascular disease among patients with DLBCL from unadjusted and adjusted analyses including with and without 9-mo landmark period (N = 2600) in Hong Kong, 2000-2018
| Characteristic* . | No landmark period . | 9-mo landmark period . | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI)† . | P . | Adjusted HR (95% CI)† . | P . | Unadjusted HR (95% CI)† . | P . | Adjusted HR (95% CI)† . | P . | |
| Treatment factors | ||||||||
| Chemotherapy | ||||||||
| Doxorubicin ≤500 mg vs non-doxorubicin regimen | 0.86 (0.61-1.22) | .405 | 0.98 (0.66-1.44) | .902 | 0.89 (0.59-1.36) | .599 | 1.21 (0.75-1.94) | .437 |
| Doxorubicin >500 mg vs non-doxorubicin regimen | 1.03 (0.58-1.82) | .927 | 1.43 (0.76-2.71) | .270 | 1.16 (0.60-2.25) | .651 | 2.55 (1.18-5.53) | .017 |
| Receipt of RT | 1.24 (0.80-1.90) | .333 | 0.81 (0.47-1.41) | .464 | 1.07 (0.61-1.87) | .805 | 0.59 (0.30-1.15) | .120 |
| Rituximab | 0.82 (0.58-1.14) | .244 | 0.60 (0.39-0.93) | .023 | 0.81 (0.54-1.21) | .302 | 0.68 (0.41-1.15) | .154 |
| Patient factors | ||||||||
| Age at lymphoma diagnosis (per 1-y increase) | 1.05 (1.04-1.06) | <.001 | 1.01 (1.00-1.03) | .138 | 1.05 (1.04-1.06) | <.001 | 1.01 (0.99-1.03) | .409 |
| Sex (male vs female) | 1.15 (0.85-1.56) | .350 | 1.08 (076-1.54) | .645 | 1.12 (0.78-1.61) | .536 | 1.01 (0.66-1.55) | .966 |
| RCS comorbidity score‡ | ||||||||
| 1 vs 0 | 2.49 (1.76-3.52) | <.001 | 1.16 (0.77-1.76) | .473 | 2.41 (1.57-3.70) | <.001 | 1.11 (0.67-1.83) | .687 |
| 2 vs 0 | 5.24 (3.59-7.66) | <.001 | 1.01 (0.59-1.72) | .967 | 5.86 (3.75-9.17) | <.001 | 0.99 (0.53-1.94) | .974 |
| Year of diagnosis | ||||||||
| 2005-2009 vs 2000-2004 | 1.16 (0.77-1.75) | .471 | 1.16 (0.72-1.89) | .540 | 1.02 (0.64-1.62) | .921 | 1.03 (0.60-1.76) | .917 |
| 2010-2014 vs 2000-2004 | 0.83 (0.53-1.28) | .396 | 0.92 (0.52-1.64) | .776 | 0.76 (0.45-1.26) | .281 | 0.76 (0.40-1.44) | .400 |
| 2015-2018 vs 2000-2004 | 0.70 (0.39-1.24) | .221 | 1.08 (0.54-2.16) | .823 | 0.20 (0.06-0.67) | .009 | 0.28 (0.07-1.10) | .068 |
| Fee waiver recipients (surrogate for lower socioeconomic status) | 1.74 (1.11-2.72) | .015 | 1.12 (0.66-1.90) | .661 | 1.84 (1.09-3.13) | .023 | 1.14 (0.62-2.11) | .675 |
| COPD or smoker | 1.94 (1.43-2.63) | <.001 | 1.27 (0.89-1.81) | .192 | 2.12 (1.48-3.05) | <.001 | 1.32 (0.86-2.02) | .203 |
| Diabetes mellitus | 2.65 (1.96-3.59) | <.001 | 1.16 (0.79-1.72) | .442 | 2.36 (1.62-3.43) | <.001 | 0.85 (0.53-1.36) | .495 |
| Hypertension | 46.70 (11.58-188.25) | <.001 | 6.62 (1.45-30.10) | .014 | 68.14 (9.52-487.82) | <.001 | 6.21 (0.80-48.43) | .081 |
| Dyslipidemia/hyperlipidemia | 2.86 (2.12-3.86) | <.001 | 1.01 (0.71-1.44) | .953 | 3.28 (2.29-4.71) | <.001 | 1.18 (0.77-1.82) | .444 |
| Anxiety or depressive disorders | 1.38 (0.96-1.98) | .085 | 0.98 (0.66-1.47) | .941 | 1.36 (0.88-2.12) | .167 | 0.90 (0.56-1.43) | .650 |
| ACE inhibitor use | 5.24 (3.87-7.09) | <.001 | 1.95 (1.30-2.93) | .001 | 5.52 (3.82-7.97) | <.001 | 2.13 (1.32-3.42) | .002 |
| Beta blocker use | 5.14 (3.74-7.07) | <.001 | 1.66 (1.12-2.44) | .011 | 7.07 (4.69-10.65) | <.001 | 2.18 (1.32-3.60) | .002 |
| Aspirin use | 9.71 (6.90-13.67) | <.001 | 4.35 (2.80-6.76) | <.001 | 11.60 (7.51-17.93) | <.001 | 4.63 (2.54-8.43) | <.001 |
| Characteristic* . | No landmark period . | 9-mo landmark period . | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI)† . | P . | Adjusted HR (95% CI)† . | P . | Unadjusted HR (95% CI)† . | P . | Adjusted HR (95% CI)† . | P . | |
| Treatment factors | ||||||||
| Chemotherapy | ||||||||
| Doxorubicin ≤500 mg vs non-doxorubicin regimen | 0.86 (0.61-1.22) | .405 | 0.98 (0.66-1.44) | .902 | 0.89 (0.59-1.36) | .599 | 1.21 (0.75-1.94) | .437 |
| Doxorubicin >500 mg vs non-doxorubicin regimen | 1.03 (0.58-1.82) | .927 | 1.43 (0.76-2.71) | .270 | 1.16 (0.60-2.25) | .651 | 2.55 (1.18-5.53) | .017 |
| Receipt of RT | 1.24 (0.80-1.90) | .333 | 0.81 (0.47-1.41) | .464 | 1.07 (0.61-1.87) | .805 | 0.59 (0.30-1.15) | .120 |
| Rituximab | 0.82 (0.58-1.14) | .244 | 0.60 (0.39-0.93) | .023 | 0.81 (0.54-1.21) | .302 | 0.68 (0.41-1.15) | .154 |
| Patient factors | ||||||||
| Age at lymphoma diagnosis (per 1-y increase) | 1.05 (1.04-1.06) | <.001 | 1.01 (1.00-1.03) | .138 | 1.05 (1.04-1.06) | <.001 | 1.01 (0.99-1.03) | .409 |
| Sex (male vs female) | 1.15 (0.85-1.56) | .350 | 1.08 (076-1.54) | .645 | 1.12 (0.78-1.61) | .536 | 1.01 (0.66-1.55) | .966 |
| RCS comorbidity score‡ | ||||||||
| 1 vs 0 | 2.49 (1.76-3.52) | <.001 | 1.16 (0.77-1.76) | .473 | 2.41 (1.57-3.70) | <.001 | 1.11 (0.67-1.83) | .687 |
| 2 vs 0 | 5.24 (3.59-7.66) | <.001 | 1.01 (0.59-1.72) | .967 | 5.86 (3.75-9.17) | <.001 | 0.99 (0.53-1.94) | .974 |
| Year of diagnosis | ||||||||
| 2005-2009 vs 2000-2004 | 1.16 (0.77-1.75) | .471 | 1.16 (0.72-1.89) | .540 | 1.02 (0.64-1.62) | .921 | 1.03 (0.60-1.76) | .917 |
| 2010-2014 vs 2000-2004 | 0.83 (0.53-1.28) | .396 | 0.92 (0.52-1.64) | .776 | 0.76 (0.45-1.26) | .281 | 0.76 (0.40-1.44) | .400 |
| 2015-2018 vs 2000-2004 | 0.70 (0.39-1.24) | .221 | 1.08 (0.54-2.16) | .823 | 0.20 (0.06-0.67) | .009 | 0.28 (0.07-1.10) | .068 |
| Fee waiver recipients (surrogate for lower socioeconomic status) | 1.74 (1.11-2.72) | .015 | 1.12 (0.66-1.90) | .661 | 1.84 (1.09-3.13) | .023 | 1.14 (0.62-2.11) | .675 |
| COPD or smoker | 1.94 (1.43-2.63) | <.001 | 1.27 (0.89-1.81) | .192 | 2.12 (1.48-3.05) | <.001 | 1.32 (0.86-2.02) | .203 |
| Diabetes mellitus | 2.65 (1.96-3.59) | <.001 | 1.16 (0.79-1.72) | .442 | 2.36 (1.62-3.43) | <.001 | 0.85 (0.53-1.36) | .495 |
| Hypertension | 46.70 (11.58-188.25) | <.001 | 6.62 (1.45-30.10) | .014 | 68.14 (9.52-487.82) | <.001 | 6.21 (0.80-48.43) | .081 |
| Dyslipidemia/hyperlipidemia | 2.86 (2.12-3.86) | <.001 | 1.01 (0.71-1.44) | .953 | 3.28 (2.29-4.71) | <.001 | 1.18 (0.77-1.82) | .444 |
| Anxiety or depressive disorders | 1.38 (0.96-1.98) | .085 | 0.98 (0.66-1.47) | .941 | 1.36 (0.88-2.12) | .167 | 0.90 (0.56-1.43) | .650 |
| ACE inhibitor use | 5.24 (3.87-7.09) | <.001 | 1.95 (1.30-2.93) | .001 | 5.52 (3.82-7.97) | <.001 | 2.13 (1.32-3.42) | .002 |
| Beta blocker use | 5.14 (3.74-7.07) | <.001 | 1.66 (1.12-2.44) | .011 | 7.07 (4.69-10.65) | <.001 | 2.18 (1.32-3.60) | .002 |
| Aspirin use | 9.71 (6.90-13.67) | <.001 | 4.35 (2.80-6.76) | <.001 | 11.60 (7.51-17.93) | <.001 | 4.63 (2.54-8.43) | <.001 |
Race, alcohol-related diseases, and atrial fibrillation were not adjusted in the model because most of the patients were Hong Kong Chinese and too few patients had alcohol-related diseases and atrial fibrillation.
Cause-specific HRs by competing risk analyses.
Included peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, liver disease, hemiplegia/paraplegia, renal disease, AIDS/HIV infection.
csCIs of cardiovascular diseases for DLBCL survivors, Hong Kong, 2000-2018. csCIs of cardiovascular diseases were estimated by levels of exposure to doxorubicin (N = 2600) and in the normal comparison group (N = 13 000) with non-cardiac death as a competing risk.
csCIs of cardiovascular diseases for DLBCL survivors, Hong Kong, 2000-2018. csCIs of cardiovascular diseases were estimated by levels of exposure to doxorubicin (N = 2600) and in the normal comparison group (N = 13 000) with non-cardiac death as a competing risk.
Aspirin use (HR, 4.63; 95% CI, 2.54-8.43; P < .001), ACE inhibitor use (HR, 2.13; 95% CI, 1.32-3.42; P = .002), beta blocker use (HR, 2.18; 95% CI, 2.54-8.43; P < .001), and hypertension (HR, 6.21; 95% CI, 0.80-48.43; P = .081) at baseline were also associated with subsequent CVDs. Patients with preexisting hypertension who used aspirin and who received >500 mg of doxorubicin had 5-, 10-, and 15-year csCIs of CVD of 12.1%, 18.4%, and 21.1%, respectively (supplemental Figure 1).
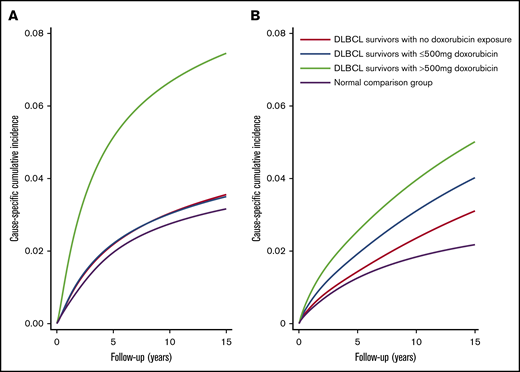
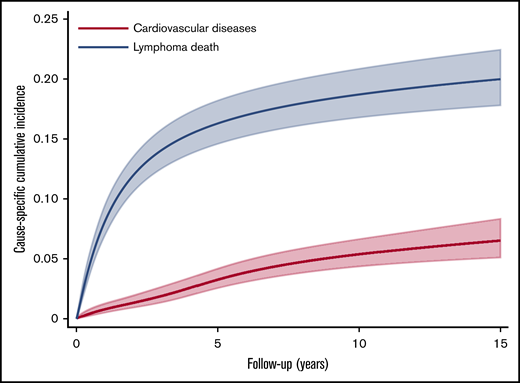
The csCI plots of CVD end points (ie, heart failure/cardiomyopathy and ischemic heart disease) for DLBCL patients and the normal comparison group showed association between doxorubicin and outcomes similar to those in the main analysis (Figure 3A-B). The ratios of predicted cumulative incidence of lymphoma death and CVD among DLBCL patients at 5, 10, and 15 years were 4.99 (95% CI, 4.95-5.03; P < .001), 3.48 (95% CI, 3.47-3.48; P < .001), and 3.08 (95% CI, 3.07-3.08; P < .001), respectively (Figure 4). Repeated analyses with and without landmark periods produced largely consistent results (Table 2; supplemental Figure 2). Analysis of different cutoff doses of doxorubicin did not change our main findings. We found that 42 lymphoma patients and 149 people from the normal comparison group had one of the major autoimmune diseases (supplemental Table 3). A χ2 test between aspirin and major autoimmune diseases showed weak evidence of association (P = .635).
csCIs of ischemic heart disease, heart failure, and cardiomyopathy for DLBCL survivors, Hong Kong, 2000-2018. csCIs of ischemic heart disease (A) and heart failure and cardiomyopathy (B) were estimated by levels of exposure to doxorubicin (N = 2600) and in normal comparison group (N = 13 000) with non-cardiac death as competing risk.
csCIs of ischemic heart disease, heart failure, and cardiomyopathy for DLBCL survivors, Hong Kong, 2000-2018. csCIs of ischemic heart disease (A) and heart failure and cardiomyopathy (B) were estimated by levels of exposure to doxorubicin (N = 2600) and in normal comparison group (N = 13 000) with non-cardiac death as competing risk.
csCIs of cardiovascular diseases in comparison with lymphoma death among DLBCL survivors, Hong Kong, 2000-2018. csCIs of cardiovascular diseases were estimated (N = 2600) with death as a result of lymphoma as a competing risk.
csCIs of cardiovascular diseases in comparison with lymphoma death among DLBCL survivors, Hong Kong, 2000-2018. csCIs of cardiovascular diseases were estimated (N = 2600) with death as a result of lymphoma as a competing risk.
Discussion
Our analysis of a contemporary cohort of 2600 patients diagnosed with DLBCL without previous heart disease and 13 000 age- and sex-matched normal comparison group (all derived from a Hong Kong population electronic medical records database) revealed an increased risk of cancer survivors developing CVDs, despite their lower prevalence of underlying CVD risk factors compared with the normal comparison group. Among the patients with DLBCL, the receipt of a cumulative absolute doxorubicin dose of 500 mg (∼300 mg/m2) was associated with a threefold increased risk of CVD compared with that for treatment with non-doxorubicin regimens. We found that preexisting hypertension and aspirin use were associated with higher subsequent risk of CVD. We hypothesize that history of aspirin on the medication list is likely a surrogate for high cardiovascular risk. This is a secondary finding supplementary to the main results largely because of data sparsity and multiple comparisons in end points. Aspirin could be prescribed for other medical conditions such as autoimmune diseases. However, our sensitivity analysis has revealed weak association between aspirin use and autoimmune diseases. The ratios of lymphoma death and CVD among DLBCL patients decreased with time, implying that CVD might be a larger health burden than lymphoma itself as follow-up time increases.
The association between doxorubicin and cardiac risks is well-established in the Western world. In patients with aggressive non-Hodgkin lymphoma, a Surveillance, Epidemiology, and End Results (SEER)-Medicare study that included 9438 patients with DLBCL showed that doxorubicin, older age, presence of comorbidity, hypertension, diabetes, preexisting atherosclerotic disease, and preexisting heart disease were significantly associated with subsequent risks of congestive heart failure (CHF).18 A Danish registry study of 2508 non-Hodgkin lymphoma survivors found that cumulative doxorubicin dose (per 100 mg/m2), older age, male sex, prediagnosis CVD risk factors, and prediagnosis of intrinsic heart disease were significantly associated with the risk of CHF.10 The authors also reported the 5-year risks of CHF by age, sex, preexisting heart disease, and CVD risk factors after a median follow-up time of 2.5 years.10 Unlike in previous studies, our study excluded patients who did not receive chemotherapy and those with preexisting cardiovascular events, so that the very frail were excluded from our analysis. Despite our more stringent inclusion criteria, we still detected significantly increased CVD risks among DLBCL survivors; at a median follow-up of 6.8 years, the 10-year estimated csCI of CVD was 18.4% in patients with cardiac risk factors who had received >500 mg absolute dose of doxorubicin.
In our selected study cohort, hypertension was the most prevalent preexisting cardiac risk factor, and its presence was associated with an estimated sixfold increased risk of subsequent CVD, although the wide confidence interval reflects the limited power, and the association could have been weakened because we included in the model ACE inhibitors and beta blockers, which are commonly used for hypertension. Anthracyclines can induce abnormal cell signaling and cytotoxic molecules that are in common with those produced by hypertension.50,51 The resultant oxidative stress and inflammatory processes induce damage to the cardiovascular system.51 Our finding is consistent with the pathophysiology that hypertension can exacerbate the cardiotoxic effects of doxorubicin.50 The most prevalent CVD risk factor in Asia is hypertension.52 Therefore, Asian countries are faced with the challenge of large numbers of hypertensive patients who also have very low awareness of this silent risk factor and a history of low rates of blood pressure control.52-54 Our findings underscore the importance of tailoring DLBCL treatment decisions on the basis of population-specific data and the prevalence of preexisting cardiac risk factors, including a history of hypertension and/or other cardiovascular risk factors. This suggests that aggressive control of blood pressure and close follow-up of DLBCL survivors after exposure to doxorubicin maybe more clinically important.
For posttreatment cardiac surveillance, the American Society of Clinical Oncology Clinical Practice Guidelines recommend that an echocardiogram be performed between 6 and 12 months after completion of cancer-directed therapy in asymptomatic patients at increased risk of cardiac dysfunction.55 The European Society of Medical Oncology recommends consideration of left ventricular ejection fraction assessment at 6 to 12 months and possibly 2 years posttreatment, as well as periodic reassessment thereafter in asymptomatic patients with normal baseline ejection fraction after anthracycline-based chemotherapy.56 Cardiology authorities have also published guidelines on monitoring after anthracyclines. They recommended a more frequent assessment of cardiac function57,58 : document baseline left ventricular ejection fraction, repeat measurement after doxorubicin dose reaches 240 mg/m2, and repeat measurement for each additional 50 mg/m2, then reassess 6 months after completing therapy. Our finding that most CVDs developed within the first few years after treatment (median, ∼4 years) supports these recommendations of cardiac evaluation early posttreatment. Implementation of such stringent risk stratifications and early frequent cardiac assessment could have potentially allowed earlier detection of some of the cardiotoxicities and potentially the ability to prevent CVD events in the future. We propose that the follow-up of patients in this period by oncologists and primary care physicians should focus not only on disease relapse but also on the evaluation for and prompt treatment of cardiac risk factors, with cardiac screening of asymptomatic patients.
Our study has several limitations. The data were subject to confounding by indication in that participants with subclinical medical conditions not measurable by our methodology may have been less likely to receive doxorubicin-containing regimens. In our determination of outcome events, we restricted the primary outcome to CVD diagnosed at a hospital or death to conservatively capture the symptomatic and most severe cases.59 This suggests that our study might underestimate the true incidence of cardiotoxicity by not capturing milder forms of CVD events. However, this can avoid misclassification as a result of coding errors and uncertainty in the diagnosis of milder CVD events. Previous studies have demonstrated high sensitivity and specificity in diagnosis and medication code retrieval from CDARS.27,28 It is likely that we would have captured the majority of the CVDs diagnosed at the hospital and death, given our public health care system.34 In addition, the registry database lacks detailed information on lymphoma and its treatment, including the stage at diagnosis, body surface area, and RT site and dose. Similar to other studies of electronic medical record databases, our study also lacked data on important lifestyle factors such as physical activity level and diet, which are known to have an impact on the onset of CVD. Although these limitations tend to bias our estimates toward the null, we still detected important associations between doxorubicin and cardiovascular risk factors and risk of CVD. Despite these limitations, our study has several strengths. We analyzed a reasonably large and homogeneous cohort in Hong Kong. Some studies used claims data or the number of cycles as surrogate estimates for chemotherapy dose.10,60 However, chemotherapy dose reductions are common, especially in elderly patients older than age 75 years.60-63 In a study of US veterans, only 14% of patients age 80 years or older who received doxorubicin completed treatment at ≥85% dose intensity.62 We described the magnitude of CVD risks segregated by actual doxorubicin dose levels among patients with DLBCL who received chemotherapy as part of anticancer treatment. Hequet et al11 reported that subclinical cardiomyopathy was more common than clinically overt cardiomyopathy. Although we used diagnosis codes to identify outcome events, we avoided overdiagnosis by using only inpatient codes that generally indicated greater severity, thereby reducing the risk of misclassification.
This study demonstrated for the first time that doxorubicin contributed to excess cardiotoxicity in a dose-related manner in a Hong Kong population of patients with DLBCL without preexisting CVD. In addition, hypertension, an often under-recognized and undertreated condition rampant among Asian patients,52 was associated with an increased trend of therapy-related CVD. We also found that many incident CVDs in our study occurred soon after lymphoma treatment. Together, these findings highlight the importance of pretreatment screening for CVD risk factors, careful balancing of the risks and benefits of treatment decisions, rigorous monitoring of cardiac function, and early cardiac screening and intervention to minimize the risk of CVD during and after lymphoma treatment and throughout cancer survivorship.
For original data, please contact Shing Fung Lee at leesfm@ha.org.hk.
Acknowledgment
The authors thank K.F. Tsang (Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong) for clerical support and data retrieval.
This work was supported by a grant from National Institutes of Health (NIH), Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences) (UL 1TR002541) and by Harvard University and its affiliated academic health care centers. M.A.L.-F. was supported by a Spanish National Health Institute Carlos III Miguel Servet-I Investigator grant/award (CP17/00206-EU-FEDER). M.H.C. was supported by a grant from the NIH, National Cancer Institute (R01 CA196854).
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the NIH. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: S.F.L. and M.A.L.-F. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; S.F.L., A.K.N., and M.H.C. conceived the idea for and design of the study; S.F.L. collected and assembled the data; S.F.L., M.A.L.-F., Y.H.C., P.J.C., C.L.C., E.Y.-F.W., I.C.-K.W., M.H.C., and A.K.N. analyzed and interpreted the data; and all authors helped prepare the manuscript and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea K. Ng, Department of Radiation Oncology, Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, ASB1-L2, 75 Francis St, Boston, MA 02115; e-mail: ang@lroc.harvard.edu; and Ming Hui Chen, Department of Cardiology, 300 Longwood Ave, Boston, MA 02115; e-mail: minghui.chen@cardio.chboston.org.
References
Author notes
M.H.C. and A.K.N. are joint senior authors.
The full-text version of this article contains a data supplement.