Key Points
Intermediate intensity double unit CBT is associated with high progression-free survival in adults, including those with MRD.
Use of highly HLA mismatched and unmanipulated grafts permits wide application of this therapy.
Abstract
Cord blood transplantation (CBT) after high intensity or nonmyeloablative conditioning has limitations. We investigated cyclosporine-A/mycophenolate mofetil–based intermediate intensity (cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 10 mg/kg, total body irradiation 400 cGy) unmanipulated double-unit CBT (dCBT) with prioritization of unit quality and CD34+ cell dose in graft selection. Ninety adults (median age, 47 years [range, 21-63]; median hematopoietic cell transplantation comorbidity index, 2 [range, 0-8]; 61 [68%] acute leukemia) received double-unit grafts (median CD34+ cell dose, 1.3 × 105/kg per unit [range, 0.2-8.3]; median donor-recipient human leukocyte antigen (HLA) match, 5/8 [range 3-7/8]). The cumulative incidences of sustained CB engraftment, day 180 grade III-IV acute, and 3-year chronic graft-versus-host disease were 99%, 24%, and 7%, respectively. Three-year transplant-related mortality (TRM) and relapse incidences were 15% and 9%, respectively. Three-year overall survival (OS) is 82%, and progression-free survival (PFS) is 76%. Younger age and higher engrafting unit CD34+ cell dose both improved TRM and OS, although neither impacted PFS. Engrafting unit-recipient HLA match was not associated with any outcome with a 3-year PFS of 79% in 39 patients engrafting with 3-4/8 HLA-matched units. In 52 remission acute leukemia patients, there was no association between minimal residual disease (MRD) and 3-year PFS: MRD negative of 88% vs MRD positive of 77% (P = .375). Intermediate intensity dCBT is associated with high PFS. Use of highly HLA mismatched and unmanipulated grafts permits wide application of this therapy, and the low relapse rates support robust graft-versus-leukemia effects even in patients with MRD.
Introduction
Double unit cord blood transplantation (dCBT) can be efficacious in adults with high-risk hematologic malignancies with comparable progression-free survival (PFS) to that of adult donor transplantation in many series.1-4 However, dCBT after high-intensity or nonmyeloablative (NMA) conditioning has limitations. High-intensity regimens are toxic and have been associated with excessive transplant-related mortality (TRM).5,6 NMA regimens can be complicated by graft rejection and a high relapse risk.7-10 We developed an alternative novel intermediate-intensity conditioning regimen. A cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, total body irradiation (TBI), 200 cGy-based platform was chosen.7,8 However, to augment recipient immunosuppression and antitumor potency, the TBI was increased to 400 cGy, and thiotepa 10 mg/kg was added.11 Our aim was to reduce organ toxicity compared with high-intensity conditioning while reducing the rejection risk and improving disease control compared with NMA conditioning. Promising early experience with this regimen was reported in 2013 with a 97% engraftment rate and 60% 2-year PFS in adults with acute leukemia or myelodysplasia (MDS).11
Novel conditioning alone, however, is insufficient to guarantee CB engraftment. Although a major focus in the field has been on ex vivo expansion, we investigated refining unit selection criteria to augment engraftment of unmanipulated units. We prioritize unit quality (as predicted by unit characteristics and bank practices) and since 2014 have incorporated consideration of CD34+ cell dose12 and 8-allele HLA match. Herein, we report for the first time determinants of transplant outcomes in a recent cohort of adult intermediate-intensity dCBT recipients transplanted with grafts that were selected with optimized unit selection criteria.12,13 Our hypothesis was that intermediate-intensity conditioning combined with optimized graft selection would be associated with high rates of engraftment and PFS.
Methods
Patients
This retrospective analysis includes all consecutive recent (January 2014 to December 2017) intermediate-intensity dCBT recipients (n = 90) who were first allograft recipients, 21 to 65 years of age, and had a hematologic malignancy thought to be otherwise incurable. The 2014 cutoff was chosen because this was when cryopreserved CD34+ cell dose and 8-allele donor-recipient HLA match were incorporated in unit selection.
Intermediate-intensity dCBT was performed in 2 clinical trials. The priority (clinicaltrials.gov, #NCT01682226) investigated adding mobilized haplo-identical CD34+ cells to dCB grafts (haplo-dCB) aiming to achieve a haplo-identical donor-derived myeloid bridge before CB engraftment. The disease burden criteria for the haplo-dCB transplantation (haplo-dCBT) trial for patients with acute leukemia, MDS, or myeloproliferative disorders (MPD) was <10% blasts; criteria varied by lymphoma subtype, but aggressive lymphomas were required to be in remission. Forty-three patients were transplanted on this protocol. For patients without a haplo-identical donor, in whom haplo-donor evaluation would delay transplant, or who elected dCBT alone, an intermediate-intensity dCBT trial (clinicaltrials.gov, #NCT00739141) was offered to eligible patients. The dCBT alone trial required acute leukemia patients to be in first or second morphologic remission and <5% blasts for MDS patients. Thirty-two patients were transplanted in the dCBT trial. Performance status and organ function eligibility for both clinical trials included Karnofsky score ≥70, left ventricular ejection fraction ≥50%, spirometry/adjusted diffusing capacity for carbon monoxide ≥50% predicted, alanine aminotransferase ≤3× upper limit of normal, bilirubin <1.5 mg/dL (unless benign congenital hyperbilirubinemia), calculated creatinine clearance ≥60 mL per minute, and albumin ≥3.0 gm/dL.
For the purposes of increasing the sample size, 15 patients transplanted during the study period who fulfilled inclusion criteria (ie, 21-65 years, first allograft, and otherwise incurable hematologic malignancy) but not transplanted on protocol were included. The reason these patients were transplanted off protocol included diagnosis/disease status rendering them ineligible (myeloproliferative disease with aplasia and marrow fibrosis, acute myeloid leukemia [AML] 5% to 9% blasts without haplo-identical donors, MDS with GATA2 deficiency, human T-cell lymphotropic virus type 1–associated adult T-cell leukemia/lymphoma, or aggressive T-cell lymphoma not in remission [n = 8], borderline pulmonary or hepatic function just below protocol eligibility [n = 3], or clinical trial insurance denial [n = 4]). Transplantation was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review/Privacy Board, and all patients gave informed consent.
Graft selection
Two units were selected based on unit quality, cell dose, and donor-recipient HLA match.12,13 Selection was restricted to units of optimal quality: red blood cell depleted, cryopreserved in standard cryovolumes of approximately 25 mL per unit (or per bag if 50 mL), and preferably from banks accredited by the Foundation for the Accreditation of Cellular Therapy. Each unit had a total nucleated cell (TNC) dose ≥1.5 × 107/kg recipient body weight and was ≥4/6 HLA-A, -B antigen, -DRB1 allele-matched to the recipient. Within this unit pool, as CD34+ cell dose is a critical determinant of engraftment potential,12,14-16 priority was given to units with the highest cryopreserved CD34+ cell dose. After CD34+ cell dose, the donor-recipient 8-allele HLA match was considered. The same criteria were used to select each unit of the graft. All dCB grafts were unmanipulated and administered after albumin-dextran dilution.17 dCB grafts were given with haplo-identical CD34+ cells (clinicaltrials.gov, #NCT01682226) or alone (clinicaltrials.gov, #NCT00739141). Haplo-identical donors were selected as recently described.18,19
Conditioning, graft-versus-host disease prophylaxis, growth factor support, and other supportive care
Conditioning included cyclophosphamide 50 mg/kg on day −6, fludarabine 150 mg/m2 (30 mg/m2 per day on days −6 to −2), thiotepa 10 mg/kg (5 mg/kg per day on days −5 and −4), and TBI 400 cGy (200 cGy per day on days −2 and −1).11 Dosing was by adjusted body weight if the patient was ≥125% ideal body weight. Graft-versus-host disease (GVHD) prophylaxis was with cyclosporine-A (CSA) and mycophenolate mofetil (15 mg/kg every 8 hours [minimum 1 g per dose and capped at 1.5 g per dose]) starting intravenously on day −3 as previously described.20,21 To optimize immune recovery, no patient received anti-thymocyte globulin.22-26 Patients received granulocyte colony-stimulating factor 5 μg/kg per day from day +7 until neutrophil recovery. Other supportive care was as previously described.1
Study definitions
The Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI) and revised disease risk index (rDRI) definitions of Sorror27 and Armand28 were used to classify comorbidities and disease risk, respectively. MRD in acute leukemia patients in complete remission (CR) was defined as the presence of any abnormal blast population by 10-color multicolor flow cytometry in the most recent marrow aspirate within 30 days of starting conditioning as described by Getta et al.29 AML genetic risk was stratified by 2010 European Leukemia Network (ELN) criteria.30 Acute GVHD (aGVHD) was diagnosed clinically with histologic confirmation only if clinically indicated and was graded according to International Bone Marrow Transplant Registry criteria.31 Chronic GVHD (cGVHD) was diagnosed and graded according to National Institutes of Health consensus criteria.32 Standard engraftment definitions were used.12 TRM was death from any cause other than disease relapse or progression.
Statistical methods
Cumulative incidence estimates considered competing risks as previously described,1 whereas overall survival (OS), progression-free survival (PFS), and GVHD-free relapse-free survival (GRFS)33 were calculated using Kaplan-Meier methodology. Cox proportional hazards regression was used to evaluate the association between transplant variables and dCBT outcomes; cause-specific Cox models were used in the presence of competing events. The association between the infused cell dose or HLA match and transplant outcomes considered the engrafting unit characteristic only. Two-sided P < .05 was considered significant. All analyses used R statistical software, version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
The haplo-dCBT recipients in this manuscript have been included in a recent engraftment kinetics and chimerism analysis of a larger haplo-dCBT cohort.18 Otherwise, the analysis of the determinants of engraftment (dCBT recipients only), and aGVHD, cGVHD, TRM, relapse, OS and PFS in all patients, has not previously been reported.
Results
Patient and graft characteristics
Ninety patients were transplanted (Table 1). The median age was 47 years (range, 21-63). Only 4 patients were ≥60 years. The median weight was 80 kg (range, 47-138). More than half the patients (n = 49, 54%) had non-European ancestry. The predominant diagnosis was acute leukemia (n = 61, 68%), including 44 AML, 13 acute lymphoblastic leukemia (ALL), and 4 mixed phenotype acute leukemia (MPAL) patients. Fifty-seven acute leukemia patients were transplanted in morphologic remission, and 4 were transplanted with relapsed/refractory disease. Fifty-two acute leukemia patients in remission were evaluated for MRD, and 18 (35%) were positive (median, 0.28%; range, 0.001-5.22). Of 14 patients with myelodysplasia or myeloproliferative disease, 6 (43%) were transplanted with advanced disease (≥5% blasts or accelerated phase). The median HCT-CI was 2 (range, 0-8), with 32 of 90 (36%) patients having scores of 3 to 8.
Patient and graft characteristics of dCBT recipients (n = 90)
| Variables . | Value . |
|---|---|
| Patient characteristics | |
| Age, median (range), y | 47 (21-63) |
| Weight, median (range), kg | 80 (47-138) |
| Sex, male, n (%) | 47 (52) |
| Ancestry, non-European, n (%) | 49 (54) |
| CMV seropositive, n (%) | 55 (61) |
| Diagnosis and disease status, n or n (%) | |
| Acute leukemia | 61 (68) |
| CR1 | 46 |
| CR2 | 11 |
| Relapsed/refractory | 4 |
| MDS | 8 (9) |
| <5% blasts | 4 |
| ≥5% blasts | 4 |
| CML | 3 (3) |
| Chronic phase | 2 |
| Accelerated phase | 1 |
| MPD | 3 (3) |
| <5% blasts | 2 |
| ≥5% blasts | 1 |
| NHL | 14 (16) |
| Large cell B cell | 2 (2) |
| CR | 1 |
| PR | 1 |
| Low-grade B-cell | 3 (3) |
| CR | 2 |
| PR | 1 |
| T cell | 9 (10) |
| CR | 4 |
| PR | 4 |
| Progressive disease | 1 |
| Blastic plasmacytoid dendritic cell neoplasm | 1 (1) |
| CR | 1 |
| HCT-CI, n (%) | |
| 0 | 18 (20) |
| 1-2 | 40 (44) |
| 3-4 | 26 (29) |
| 5-8 | 6 (7) |
| Median (range) | 2 (0-8) |
| rDRI, n (%) | |
| Low | 6 (7) |
| Intermediate | 62 (69) |
| High | 21 (23) |
| Very high | 1 (1) |
| Graft characteristics (n = 180 units)* | |
| Infused TNC dose, median (range), × 107/kg per unit | 2.5 (1.0-7.4) |
| Infused viable CD34+ dose, median (range), × 105/kg per unit | 1.3 (0.2-8.3) |
| Unit recipient HLA- A, -B antigen, -DR allele match, n (%) | |
| 4/6 | 162 (90) |
| 5/6 | 16 (9) |
| 6/6 | 2 (1) |
| Unit recipient 8-allele HLA-match, n (%) | |
| 3-4/8 | 80 (44) |
| 5/8 | 76 (42) |
| 6-7/8 | 24 (13) |
| Median (range) | 5/8 (3-7/8) |
| Variables . | Value . |
|---|---|
| Patient characteristics | |
| Age, median (range), y | 47 (21-63) |
| Weight, median (range), kg | 80 (47-138) |
| Sex, male, n (%) | 47 (52) |
| Ancestry, non-European, n (%) | 49 (54) |
| CMV seropositive, n (%) | 55 (61) |
| Diagnosis and disease status, n or n (%) | |
| Acute leukemia | 61 (68) |
| CR1 | 46 |
| CR2 | 11 |
| Relapsed/refractory | 4 |
| MDS | 8 (9) |
| <5% blasts | 4 |
| ≥5% blasts | 4 |
| CML | 3 (3) |
| Chronic phase | 2 |
| Accelerated phase | 1 |
| MPD | 3 (3) |
| <5% blasts | 2 |
| ≥5% blasts | 1 |
| NHL | 14 (16) |
| Large cell B cell | 2 (2) |
| CR | 1 |
| PR | 1 |
| Low-grade B-cell | 3 (3) |
| CR | 2 |
| PR | 1 |
| T cell | 9 (10) |
| CR | 4 |
| PR | 4 |
| Progressive disease | 1 |
| Blastic plasmacytoid dendritic cell neoplasm | 1 (1) |
| CR | 1 |
| HCT-CI, n (%) | |
| 0 | 18 (20) |
| 1-2 | 40 (44) |
| 3-4 | 26 (29) |
| 5-8 | 6 (7) |
| Median (range) | 2 (0-8) |
| rDRI, n (%) | |
| Low | 6 (7) |
| Intermediate | 62 (69) |
| High | 21 (23) |
| Very high | 1 (1) |
| Graft characteristics (n = 180 units)* | |
| Infused TNC dose, median (range), × 107/kg per unit | 2.5 (1.0-7.4) |
| Infused viable CD34+ dose, median (range), × 105/kg per unit | 1.3 (0.2-8.3) |
| Unit recipient HLA- A, -B antigen, -DR allele match, n (%) | |
| 4/6 | 162 (90) |
| 5/6 | 16 (9) |
| 6/6 | 2 (1) |
| Unit recipient 8-allele HLA-match, n (%) | |
| 3-4/8 | 80 (44) |
| 5/8 | 76 (42) |
| 6-7/8 | 24 (13) |
| Median (range) | 5/8 (3-7/8) |
dCB grafts were supplemented with haplo-identical mobilized peripheral blood CD34+ cells in 43 (48%) patients.18 Haplo-identical donors (median age, 30 y) were siblings (n = 15), children (n = 20), parents (n = 4), or other family members (n = 4).
Units had a median infused viable CD34+ cell dose of 1.3 × 105/kg per unit (range, 0.2-8.3). The median unit-recipient HLA allele match of the 180 units was 5/8 (range, 3-7/8), with 80 (44%) being 3-4/8 HLA matched. In 43 (48%) patients, the dCB grafts were supplemented with haplo-identical CD34+ cells (median dose, 5.7 × 106/kg; range, 1.4-16.8) as recently described.18
Conditioning regimen toxicity
The preparative regimen while myeloablative was relatively well tolerated. The main toxicities were fatigue and gastrointestinal (GI) issues. No patient developed severe oral mucosal toxicity. However, 50 patients (56%) required total parenteral nutrition, which was started at a median of 3 days (range, 1-22) after transplant. No patient developed diffuse alveolar hemorrhage; 1 developed sinusoidal obstruction syndrome.
Hematopoietic engraftment
All 90 patients were evaluable for engraftment. Overall, 89 engrafted with CB. In all 89 engrafting patients (46 dCBT, 43 haplo-dCBT), sustained engraftment was mediated by a dominant CB unit. For the entire cohort, the day 100 cumulative incidence of sustained CB-derived neutrophil engraftment was 99% (95% confidence interval [CI], 87-99), and platelet engraftment to ≥20 × 109/L was 93% (95% CI, 85-97). There were no secondary graft failures.
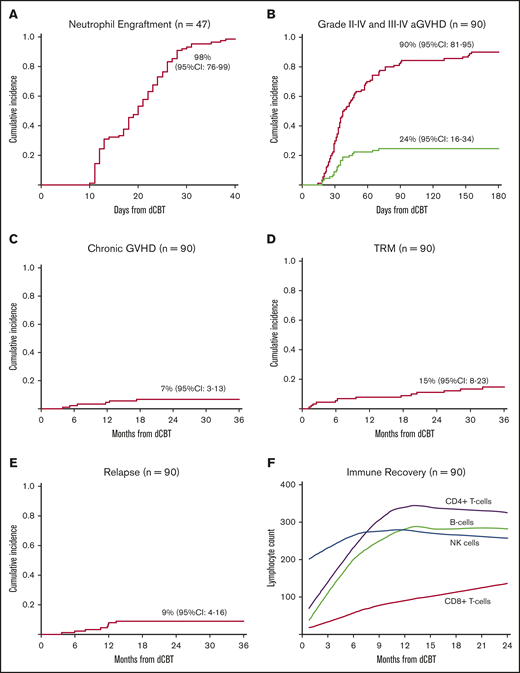
In the 47 dCBT alone recipients, the day 45 incidence of neutrophil engraftment was 98% (95% CI, 76-99; median, 22 days; range, 13-37 days; Figure 1A). Day 100 platelet engraftment was 91% (95% CI, 77-97; median, 40 days; range, 24-60 days), with 1 patient recovering on day 130. In these dCBT-only recipients, higher engrafting unit infused TNC and infused viable CD34+ cell doses were associated with faster neutrophil engraftment (Table 2).
Transplant outcomes after intermediate intensity dCBT. (A) Cumulative incidence of neutrophil engraftment in patients who received dCB grafts only (n = 47). Patients who received dCB grafts combined with haplo-identical CD34+ cells were excluded because of transient haploidentical donor-derived myeloid bridging before sustained CB engraftment. (B) Cumulative incidence of acute GVHD by 180 days after transplant (n = 90). (C) Three-year cumulative incidence of chronic GVHD (n = 90). (D) Three-year cumulative incidence of TRM (n = 90). (E) Three-year cumulative incidence of relapse (n = 90). (F) Loess-smoothed averages of lymphocyte subset recovery including CD4+-, CD8+-, NK-, and B-cell subsets. As there were no differences in recovery after dCBT and dCBT combined with haplo-identical cells, these patients were combined (n = 90).
Transplant outcomes after intermediate intensity dCBT. (A) Cumulative incidence of neutrophil engraftment in patients who received dCB grafts only (n = 47). Patients who received dCB grafts combined with haplo-identical CD34+ cells were excluded because of transient haploidentical donor-derived myeloid bridging before sustained CB engraftment. (B) Cumulative incidence of acute GVHD by 180 days after transplant (n = 90). (C) Three-year cumulative incidence of chronic GVHD (n = 90). (D) Three-year cumulative incidence of TRM (n = 90). (E) Three-year cumulative incidence of relapse (n = 90). (F) Loess-smoothed averages of lymphocyte subset recovery including CD4+-, CD8+-, NK-, and B-cell subsets. As there were no differences in recovery after dCBT and dCBT combined with haplo-identical cells, these patients were combined (n = 90).
Variables associated with outcomes after intermediate intensity dCBT
| Variables . | Value . | n . | Univariate analysis . | |
|---|---|---|---|---|
| HR (95% CI) . | P . | |||
| Neutrophil engraftment: dCBT recipients only (n = 47) | ||||
| TNC cell dose per kilogram | <2.6 × 107 | 21 | Reference | .286 |
| ≥2.6 × 107 | 26 | 1.38 (0.76-2.51) | ||
| TNC cell dose per kilogram | Continuous | 47 | 3.81 (1.31-11.01) | .014 |
| CD34+ cell dose per kilogram | <1.4 × 105 | 21 | Reference | .001 |
| ≥1.4 × 105 | 26 | 2.77 (1.51-5.09) | ||
| CD34+ cell dose per kilogram | Continuous | 47 | 2.53 (1.55-4.12) | <.001 |
| Donor-recipient 8-allele HLA-match | 3-4/8 | 20 | Reference | |
| 5/8 | 16 | 1.11 (0.56-2.19) | .764 | |
| 6-7/8 | 11 | 0.87 (0.41-1.86) | .723 | |
| Nonsignificant variables included engrafting unit CD3+ cell dose. Infused CD34+ dose represented viable cells. Haploidentical CD34+ cell recipients were excluded due to the transient engraftment of these cells in some patients. | ||||
| Grade III-IV aGVHD (n = 90) | ||||
| Nonsignificant variables included recipient age, sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, HCT-CI, rDRI and engrafting unit TNC, CD34+ and CD3+ cell doses, and 8-allele HLA match. | ||||
| TRM (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .03 |
| ≥50 | 42 | 4.19 (1.15-15.22) | ||
| Age, y | Continuous | 90 | 1.1 (1.0-1.20) | .016 |
| HCT-CI | 0-2 | 58 | Reference | .79 |
| 3-8 | 32 | 1.16 (0.38-3.56) | ||
| CD34+ cell dose per kilogram | <1.4 × 105 | 45 | Reference | .145 |
| ≥1.4 × 105 | 45 | 0.42 (0.13-1.35) | ||
| CD34+ cell dose per kilogram | Continuous | 90 | 0.30 (0.11-0.82) | .017 |
| Nonsignificant variables included recipient sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, rDRI and engrafting unit TNC, CD3+ cell doses, and 8-allele HLA-match. | ||||
| Relapse (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .261 |
| ≥50 | 42 | 0.46 (0.12-1.79) | ||
| Age, y | Continuous | 90 | 0.97 (0.92-1.03) | .330 |
| rDRI | Low-intermediate | 68 | Reference | .313 |
| High-very high | 22 | 1.92 (0.54-6.84) | ||
| Nonsignificant variables included recipient xer, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, HCT-CI and engrafting unit TNC and CD3+ cell doses, and 8-allele HLA match. | ||||
| OS (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .057 |
| ≥50 | 42 | 2.79 (0.97-8.03) | ||
| Age, y | Continuous | 90 | 1.09 (1.02-1.16) | .013 |
| HCT-CI | 0-2 | 58 | Reference | .446 |
| 3-8 | 32 | 1.47 (0.55-3.94) | ||
| CD34+ cell dose per kilogram | Continuous | 90 | 0.38 (0.14-0.99) | .047 |
| Other nonsignificant variables included recipient sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, rDRI and engrafting unit TNC and CD3+ cell doses, and 8-allele HLA match. | ||||
| PFS (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .364 |
| ≥50 | 42 | 1.47 (0.64-3.35) | ||
| Age, y | Continuous | 90 | 1.03 (0.98-1.07) | .218 |
| HCT-CI | 0-2 | 58 | Reference | .439 |
| 3-8 | 32 | 1.39 (0.61-3.16) | ||
| CD34+ cell dose per kilogram | Continuous | 90 | 0.57(0.26-1.27) | .171 |
| Other nonsignificant variables included recipient sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, rDRI and engrafting unit TNC and CD3+ cell doses, and 8-allele HLA match. | ||||
| Variables . | Value . | n . | Univariate analysis . | |
|---|---|---|---|---|
| HR (95% CI) . | P . | |||
| Neutrophil engraftment: dCBT recipients only (n = 47) | ||||
| TNC cell dose per kilogram | <2.6 × 107 | 21 | Reference | .286 |
| ≥2.6 × 107 | 26 | 1.38 (0.76-2.51) | ||
| TNC cell dose per kilogram | Continuous | 47 | 3.81 (1.31-11.01) | .014 |
| CD34+ cell dose per kilogram | <1.4 × 105 | 21 | Reference | .001 |
| ≥1.4 × 105 | 26 | 2.77 (1.51-5.09) | ||
| CD34+ cell dose per kilogram | Continuous | 47 | 2.53 (1.55-4.12) | <.001 |
| Donor-recipient 8-allele HLA-match | 3-4/8 | 20 | Reference | |
| 5/8 | 16 | 1.11 (0.56-2.19) | .764 | |
| 6-7/8 | 11 | 0.87 (0.41-1.86) | .723 | |
| Nonsignificant variables included engrafting unit CD3+ cell dose. Infused CD34+ dose represented viable cells. Haploidentical CD34+ cell recipients were excluded due to the transient engraftment of these cells in some patients. | ||||
| Grade III-IV aGVHD (n = 90) | ||||
| Nonsignificant variables included recipient age, sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, HCT-CI, rDRI and engrafting unit TNC, CD34+ and CD3+ cell doses, and 8-allele HLA match. | ||||
| TRM (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .03 |
| ≥50 | 42 | 4.19 (1.15-15.22) | ||
| Age, y | Continuous | 90 | 1.1 (1.0-1.20) | .016 |
| HCT-CI | 0-2 | 58 | Reference | .79 |
| 3-8 | 32 | 1.16 (0.38-3.56) | ||
| CD34+ cell dose per kilogram | <1.4 × 105 | 45 | Reference | .145 |
| ≥1.4 × 105 | 45 | 0.42 (0.13-1.35) | ||
| CD34+ cell dose per kilogram | Continuous | 90 | 0.30 (0.11-0.82) | .017 |
| Nonsignificant variables included recipient sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, rDRI and engrafting unit TNC, CD3+ cell doses, and 8-allele HLA-match. | ||||
| Relapse (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .261 |
| ≥50 | 42 | 0.46 (0.12-1.79) | ||
| Age, y | Continuous | 90 | 0.97 (0.92-1.03) | .330 |
| rDRI | Low-intermediate | 68 | Reference | .313 |
| High-very high | 22 | 1.92 (0.54-6.84) | ||
| Nonsignificant variables included recipient xer, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, HCT-CI and engrafting unit TNC and CD3+ cell doses, and 8-allele HLA match. | ||||
| OS (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .057 |
| ≥50 | 42 | 2.79 (0.97-8.03) | ||
| Age, y | Continuous | 90 | 1.09 (1.02-1.16) | .013 |
| HCT-CI | 0-2 | 58 | Reference | .446 |
| 3-8 | 32 | 1.47 (0.55-3.94) | ||
| CD34+ cell dose per kilogram | Continuous | 90 | 0.38 (0.14-0.99) | .047 |
| Other nonsignificant variables included recipient sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, rDRI and engrafting unit TNC and CD3+ cell doses, and 8-allele HLA match. | ||||
| PFS (n = 90) | ||||
| Age, y | <50 | 48 | Reference | .364 |
| ≥50 | 42 | 1.47 (0.64-3.35) | ||
| Age, y | Continuous | 90 | 1.03 (0.98-1.07) | .218 |
| HCT-CI | 0-2 | 58 | Reference | .439 |
| 3-8 | 32 | 1.39 (0.61-3.16) | ||
| CD34+ cell dose per kilogram | Continuous | 90 | 0.57(0.26-1.27) | .171 |
| Other nonsignificant variables included recipient sex, ancestry (European vs non-European), CMV serostatus, addition of haploidentical CD34+ cells, rDRI and engrafting unit TNC and CD3+ cell doses, and 8-allele HLA match. | ||||
Infused cell dose and HLA-match variables are those of the engrafting unit. Infused TNC, infused viable CD34+, and infused viable CD3+ cell doses were naturally log transformed when examined as continuous variables because of skewness. Values of P < .05 are in bold.
The detailed engraftment kinetics in a larger cohort of haplo-dCBT recipients have recently been described.18 In brief, of the 43 haplo-dCBT recipients in this analysis, 17 (39%) had an early haplo-derived myeloid bridge with a median sustained neutrophil recovery of 11 days (range, 10-13) before converting to CB-derived hematopoiesis. The other 26 patients had either a transient haplo-derived bridge with second neutrophil nadir (n = 13), or no bridge (n = 13), before CB engraftment. This was because of rapid haplo-identical graft rejection by the engrafting CB unit18 . Taken together these 26 patients had a median day to CB-derived engraftment of 27 days (range, 18-46).
The single patient with graft failure received a dCB-only graft, had relapsed B-ALL that was treated with blinatumomab (and no T-cell suppressive therapy) in the preceding 6 months, and received 2 units with low CD34+ cell doses (0.65 × 105/kg and 0.89 × 105/kg). The patient was successfully retransplanted with a single CB unit.
Acute and chronic GVHD
The incidences of day 180 grade II-IV and III-IV aGVHD were 90% (95% CI, 81-95) and 24% (95% CI, 16-34), respectively (Figure 1B). The median onset of grade II-IV aGVHD was 36 days (range, 14-155). The organ involvement by maximum day 180 aGVHD grade is shown in Table 3. The organ most commonly involved was the GI tract. In the 59 grade II patients, the upper GI was the most common (48 of 59, 81%) either alone (n = 17) or in combination with the lower GI (almost exclusively stage 1; n = 13), stage 1-2 skin (n = 11), or both (n = 7). Stage 2 skin accounted for the overall grade in only 7 patients, whereas stage 1-2 lower GI only involvement was the least common in 4. In the majority (12 of 15, 80%) of grade III patients, the grade was determined by stage 3 skin either alone or in combination with other organs. Only 3 grade III aGVHD patients had stage 3 lower GI involvement. All 7 patients with grade IV aGVHD had upper and stage 4 lower GI disease with 5 also having skin and/or liver involvement.
Acute GVHD organ involvement in the first 180 days after transplant
| Day 180 maximum grade, N . | Organ stage* . |
|---|---|
| Grade I (n = 4) | Skin stage 1 (n = 4) |
| Grade II (n = 59) | Upper GI only (n = 17) |
| Upper GI and lower GI (n = 13) | |
| • 12 stage 1 | |
| • 1 stage 2 | |
| Upper GI and skin stage 1-2 (n = 11) | |
| Upper GI, lower GI stage 1-2 and skin stage 1-2 (n = 7): | |
| • 4 lower GI stage 2 and skin stage 2 | |
| • 3 lower GI stage 1 and skin stage 1-2 | |
| Skin only, stage 2 (n = 7) | |
| Lower GI only, stage 1-2 (n = 4) | |
| • 3 stage 1 | |
| • 1 stage 2 | |
| Grade III (n = 15) | Skin stage 3, upper GI and lower GI stage 1-2 (n = 5) |
| • 4 skin stage 3, upper GI and lower GI stage 1 | |
| • 1 skin stage 3, upper GI and lower GI stage 2 | |
| Skin stage 3 and upper GI (n = 4) | |
| Skin stage 3 (n = 2) | |
| Skin stage 3 and liver stage 1 (n = 1) | |
| Lower GI stage 3 and skin stage 1 (n = 1) | |
| Lower GI stage 3 and upper GI (n = 1) | |
| Lower GI stage 3 and upper GI & skin stage 1 (n = 1) | |
| Grade IV (n = 7) | Lower GI stage 4 and upper GI (n = 2) |
| Lower GI stage 4, upper GI, and liver stage 1 or 3 (n = 2) | |
| Lower GI stage 4, upper GI, skin stage 2-3, and liver stage 2-3 (n = 2) | |
| Lower GI stage 4, upper GI, and skin stage 2 (n = 1) |
| Day 180 maximum grade, N . | Organ stage* . |
|---|---|
| Grade I (n = 4) | Skin stage 1 (n = 4) |
| Grade II (n = 59) | Upper GI only (n = 17) |
| Upper GI and lower GI (n = 13) | |
| • 12 stage 1 | |
| • 1 stage 2 | |
| Upper GI and skin stage 1-2 (n = 11) | |
| Upper GI, lower GI stage 1-2 and skin stage 1-2 (n = 7): | |
| • 4 lower GI stage 2 and skin stage 2 | |
| • 3 lower GI stage 1 and skin stage 1-2 | |
| Skin only, stage 2 (n = 7) | |
| Lower GI only, stage 1-2 (n = 4) | |
| • 3 stage 1 | |
| • 1 stage 2 | |
| Grade III (n = 15) | Skin stage 3, upper GI and lower GI stage 1-2 (n = 5) |
| • 4 skin stage 3, upper GI and lower GI stage 1 | |
| • 1 skin stage 3, upper GI and lower GI stage 2 | |
| Skin stage 3 and upper GI (n = 4) | |
| Skin stage 3 (n = 2) | |
| Skin stage 3 and liver stage 1 (n = 1) | |
| Lower GI stage 3 and skin stage 1 (n = 1) | |
| Lower GI stage 3 and upper GI (n = 1) | |
| Lower GI stage 3 and upper GI & skin stage 1 (n = 1) | |
| Grade IV (n = 7) | Lower GI stage 4 and upper GI (n = 2) |
| Lower GI stage 4, upper GI, and liver stage 1 or 3 (n = 2) | |
| Lower GI stage 4, upper GI, skin stage 2-3, and liver stage 2-3 (n = 2) | |
| Lower GI stage 4, upper GI, and skin stage 2 (n = 1) |
All upper GI involvement is stage 1 by definition.
TRM and relapse
TRM was 6% (95% CI, 2-12) by day 180 and increased to 15% (95% CI, 8-23) by 3 years after transplant (Figure 1D). Age ≥50 years was associated with a higher likelihood of TRM. A higher engrafting unit infused viable CD34+ cell dose was protective, whereas adding haplo-CD34+ cells had no effect (Table 2). The significance of age and CD34+ cell dose was retained in multivariate analysis: older age was associated with higher TRM (hazard ratio [HR], 1.11; 95% CI, 1.0-1.2; P = .016), whereas a higher engrafting unit CD34+ cell dose was protective (HR, 0.30; 95% CI, 0.11-0.82; P = .017).
Immune reconstitution
Recovery of lymphocyte subsets (CD4+ T cells, CD8+ T cells, CD3−CD56+CD16+ NK cells and CD19+ B cells) is shown in Figure 1F. The median CD4+ T-cell counts at 2, 4, and 6 months after dCBT were 109 cells/µL (range, 1-1584), 152 cells/µL (range, 19-912), and 226 cells/µL (range, 6-1069), respectively.
OS and PFS
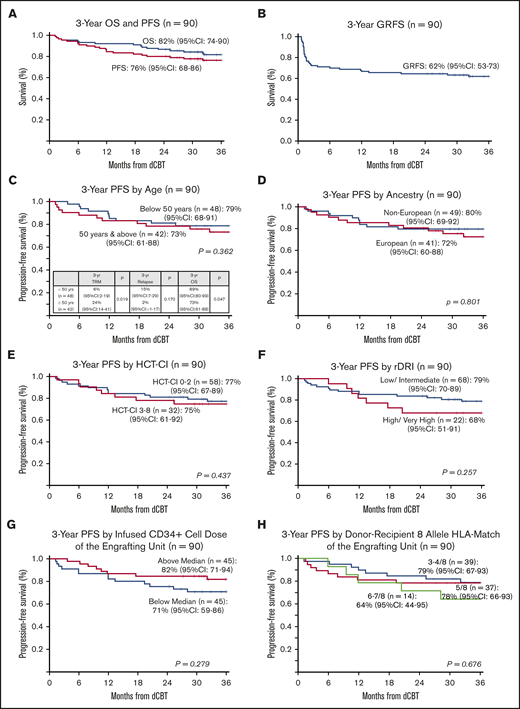
With a median survivor follow-up of 3 years and 8 months (range, 2-5.6 years), OS was 92% (95% CI, 87-98) at 1 year and 82% (95% CI, 74-90) at 3 years (Figure 2A). Causes of death were relapse in 3 patients, whereas 13 died of transplant-related causes (7 aGVHD, 3 infection [1 bacterial, 1 cytomegalovirus pneumonia, 1 human herpes virus-6 pneumonitis], 2 pulmonary failure, and 1 unknown). When examined as a continuous variable, older age was associated with inferior OS, whereas higher engrafting unit-infused viable CD34+ cell dose improved OS (Table 2). Addition of haplo-identical CD34+ cells had no effect on OS. In multivariable analysis, older age (HR, 1.09; 95% CI,1.02-1.17; P = .014) adversely impacted OS and higher engrafting unit CD34+ cell dose (HR, 0.40; 95% CI, 0.16-0.99; P = .049) improved OS.
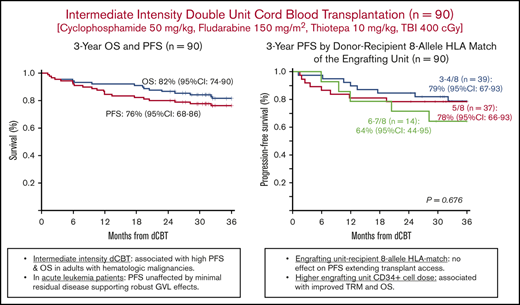
Three-year probabilities of PFS after intermediate intensity dCBT (n = 90). Three-year PFS and OS (A), 3-year GRFS (B), 3-year PFS according to age (split at 50 years) (C), European vs non-European ancestry (D), HCT-CI (low score of 0-2 vs 3-8) (E), rDRI (low-intermediate vs high-very high) (F), engrafting unit infused CD34+ cell dose (split at the median) (G), and engrafting unit-recipient 8-allele HLA match (H).
Three-year probabilities of PFS after intermediate intensity dCBT (n = 90). Three-year PFS and OS (A), 3-year GRFS (B), 3-year PFS according to age (split at 50 years) (C), European vs non-European ancestry (D), HCT-CI (low score of 0-2 vs 3-8) (E), rDRI (low-intermediate vs high-very high) (F), engrafting unit infused CD34+ cell dose (split at the median) (G), and engrafting unit-recipient 8-allele HLA match (H).
PFS was 84% (95% CI, 77-92) at 1 year and 76% (95% CI, 68-86) at 3 years (Figure 2A). The 3-year GRFS was 62% (95% CI, 53-73; Figure 2B). Univariate analyses of factors evaluated for an association with PFS are shown in Table 2 and Figure 2C-H. No recipient characteristic was associated with PFS including age (split at 50 years; Figure 2C), ancestry (European vs non-European; Figure 2D), HCT-CI (0-2 vs 3-8; Figure 2E), and rDRI (low-intermediate vs high-very high; Figure 2F). Engrafting unit-infused CD34+ cell dose (split at the median; Figure 2G) and 8-allele HLA match (Figure 2H) had no discernible associations with PFS. Addition of haplo-identical CD34+ cells also had no effect on PFS.
Survival of patients with acute leukemia
The 61 patients with AML, ALL, or MPAL had a 1-year OS of 92% (95% CI, 85-99) and 3-year OS of 81% (95% CI, 72-92). One-year PFS was 87% (95% CI, 79-96) and 3-year PFS was 76% (95% CI, 66-88). Details of their diagnoses and survival outcomes are shown in Table 4. Three-year PFS in the 17 fms-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD) AML patients (16 CR, 1 refractory), a common allograft indication, was 82% (95% CI, 66-99).
Characteristics and survival of patients with acute leukemia (n = 61)
| Disease risk and remission status . | n . | Alive in remission . | Relapsed and alive . | Relapse-related mortality . | TRM . |
|---|---|---|---|---|---|
| AML in complete remission (n = 40) | |||||
| ELN favorable* | 6 | 6 | |||
| CR1 MRD− | 5 | 5 | |||
| CR2 MRD− | 1 | 1 | |||
| ELN intermediate 1/2† | 25 | 20 | 1 | — | 4 |
| CR1 MRD− | 14 | 12 | 1 (NPM1+ and FLT3-ITD) | 1 | |
| CR1 MRD+ | 7 | 6 | 1 | ||
| CR1 MRD unknown | 2 | 1 | 2 | ||
| CR2 MRD+ | 1 | 1 | |||
| CR2 MRD unknown | 1 | ||||
| ELN adverse‡ | 9 | 6 | 1 | 1 | 1 |
| CR1 MRD− | 3 | 3 | |||
| CR1 MRD+ | 3 | 2 | 1 | ||
| CR1 MRD unknown | 2 | 1 | 1 [del(5q)] | 1 (MLL rearrangement) | |
| CR2 MRD− | 1 | ||||
| AML, relapsed/refractory (n = 4) | |||||
| ELN intermediate 1/2§ | 2 | 2 | |||
| ELN adverse|| | 2 | 1 | 1 | ||
| ALL in complete remission (n = 13)¶ | |||||
| CR1 MRD− | 3 | 2 | 1 | ||
| CR1 MRD+ | 3 | 2 | 1 | ||
| CR2 MRD+ | 2 | 2 | |||
| CR2 MRD− | 5 | 4 | 1 | ||
| MPAL in complete remission (n = 4)# | |||||
| CR1 MRD− | 2 | 2 | |||
| CR1 MRD+ | 2 | 1 | 1 | ||
| Disease risk and remission status . | n . | Alive in remission . | Relapsed and alive . | Relapse-related mortality . | TRM . |
|---|---|---|---|---|---|
| AML in complete remission (n = 40) | |||||
| ELN favorable* | 6 | 6 | |||
| CR1 MRD− | 5 | 5 | |||
| CR2 MRD− | 1 | 1 | |||
| ELN intermediate 1/2† | 25 | 20 | 1 | — | 4 |
| CR1 MRD− | 14 | 12 | 1 (NPM1+ and FLT3-ITD) | 1 | |
| CR1 MRD+ | 7 | 6 | 1 | ||
| CR1 MRD unknown | 2 | 1 | 2 | ||
| CR2 MRD+ | 1 | 1 | |||
| CR2 MRD unknown | 1 | ||||
| ELN adverse‡ | 9 | 6 | 1 | 1 | 1 |
| CR1 MRD− | 3 | 3 | |||
| CR1 MRD+ | 3 | 2 | 1 | ||
| CR1 MRD unknown | 2 | 1 | 1 [del(5q)] | 1 (MLL rearrangement) | |
| CR2 MRD− | 1 | ||||
| AML, relapsed/refractory (n = 4) | |||||
| ELN intermediate 1/2§ | 2 | 2 | |||
| ELN adverse|| | 2 | 1 | 1 | ||
| ALL in complete remission (n = 13)¶ | |||||
| CR1 MRD− | 3 | 2 | 1 | ||
| CR1 MRD+ | 3 | 2 | 1 | ||
| CR2 MRD+ | 2 | 2 | |||
| CR2 MRD− | 5 | 4 | 1 | ||
| MPAL in complete remission (n = 4)# | |||||
| CR1 MRD− | 2 | 2 | |||
| CR1 MRD+ | 2 | 1 | 1 | ||
AML favorable: inv(16) (n = 2); t(8;21) (n = 1); normal karyotype and NPM1+, FLT3 WT (n = 3).
AML intermediate 1 (normal karyotype) (n = 14): NPM1+ and FLT3-ITD (n = 7), WT NPM1 and FLT3-ITD (n = 2), and WT NPM1 without FTL3-ITD (n = 5). AML intermediate 2 (n = 11): t(9;11) (n = 1), cytogenetic abnormalities not defined as favorable or adverse (n = 10). Three intermediate 2 risk patients were FLT3-ITD–positive.
AML adverse: complex karyotype (n = 5), −7 (n = 1), del(5q) (n = 1), MLL rearranged (n = 2). Three adverse risk patients were FLT3-ITD–positive.
AML relapsed/ refractory: intermediate 1, WT NPM1 without FTL3-ITD (n = 1); intermediate 2, cytogenetic abnormalities not defined as favorable or adverse and FLT3-ITD (n = 1).
AML relapsed/refractory: complex karyotype (n = 1), −7 (n = 1).
ALL: Ph+ (n = 2) and Ph− (n = 11).
MPAL: Ph+ (n = 1); other (n = 3).
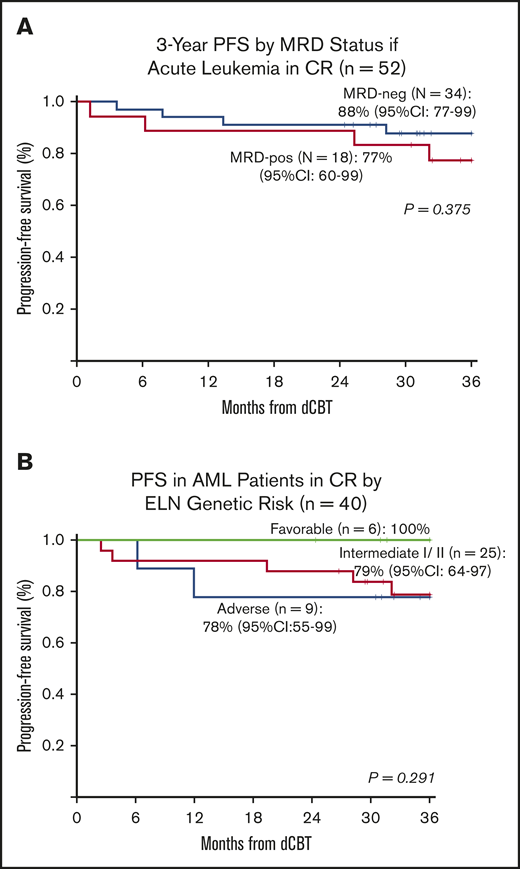
The 3-year PFS in the 52 CR patients (35 AML, 13 ALL, 4 MPAL) who were evaluated pretransplant for MRD is shown in Figure 3A. In MRD-negative patients, 3-year PFS was 88% (95% CI, 77-99) compared with 77% (95% CI, 60-99) in MRD-positive patients (P = .375). Treatment failure in MRD-positive patients was caused by TRM and not relapse.
Three-year probabilities of PFS in patients with acute leukemia after intermediate-intensity dCBT. (A) Three-year PFS according to pretransplant MRD status in the 52 patients with acute leukemia in complete remission. (B) Three-year PFS in 40 AML patients transplanted in CR according to their ELN 2010 genetic risk classification at diagnosis.
Three-year probabilities of PFS in patients with acute leukemia after intermediate-intensity dCBT. (A) Three-year PFS according to pretransplant MRD status in the 52 patients with acute leukemia in complete remission. (B) Three-year PFS in 40 AML patients transplanted in CR according to their ELN 2010 genetic risk classification at diagnosis.
Overall, patients with AML transplanted in CR1/2 (n = 40) had a high 3-year PFS of 82% (95% CI, 70-95), and no association between the diagnosis ELN risk and PFS was observed (Figure 3B). However, the 4 patients with AML transplanted with morphologic disease (induction failure or refractory relapse) did poorly (3 TRM, 1 relapse).
Survival of patients with other diagnoses
Of 8 MDS patients, 6 are alive disease free, whereas 1 patient relapsed and is now disease free after a second dCBT, and 1 died of relapse. Both patients with MDS who relapsed had >5% blasts at transplant. Of 6 patients with CML or another MPD, 5 are alive disease free, whereas 1 died of TRM. Of 14 lymphoma patients, 3 of 5 with B-cell disease are alive disease free, 1 relapsed and is disease free after immunosuppression taper, and 1 died of relapse. Seven of 9 T-cell lymphoma patients are alive disease free and 2 had TRM. The single patient with blastic plasmacytoid dendritic cell neoplasm is alive disease free.
Use of targeted maintenance therapy in patients with acute leukemia or CML
Of 61 acute leukemia patients, none received nontargeted maintenance. However, 4 of 61 patients received targeted maintenance therapy. These included 1 of 17 patients with FLT3 ITD AML who was transplanted in CR2 with MRD and received quizartinib starting 2 months after transplant. This is ongoing at 47-month follow-up. Also, of 3 patients with Ph+ acute leukemia, 1 received dasatinib for low-level blood BCR-ABL positivity at 18 months after dCBT; this was continued for 25 months with molecular remission to date 6 months after maintenance discontinuation. A second patient briefly received imatinib for BCR-ABL positivity at 6 months but progressed to morphologic relapse, achieved remission after ALL-directed therapy, and remains on dasatinib 3 years later. A third patient received prophylactic dasatinib approximately 100 days after dCBT (because of the physician’s preference), but this was aborted after 1 month because of pulmonary toxicity.
Of 3 patients with CML, only 1 received maintenance. This was ponatinib given for low-level BCR-ABL detection at 6 months. This was discontinued 1 year later because of vasculopathy. The patient has since remained in molecular remission for more than 2 subsequent years. There was no maintenance therapy of any kind administered to any other patient population such as MDS patients.
Discussion
Herein, we report that intermediate-intensity dCBT with optimized graft selection is associated with multiple promising outcomes in middle-aged adults with acute leukemias and other hematologic malignancies thought to be otherwise incurable. CB engraftment was very high, with only a single graft failure, cGVHD was low, and patients immune reconstituted despite high rates of aGVHD.26 The incidences of both TRM and relapse were low, and the 3-year PFS probability was more than 75%. Moreover, our use of highly HLA-mismatched units enabled a major extension of allograft access,34 with more than 50% of patients having part or full non-European origins and no difference in PFS in Europeans and non-Europeans. Notably, there was no association between engrafting unit donor-recipient HLA allele match and outcomes, with a PFS of more than 75% in those who engrafted with a 3-4/8 HLA-matched unit. The lack of any association between the donor-recipient HLA match and PFS is in contrast to a number of single- and double-unit CBT series35,36 but not others.37,38 Importantly, our observation permits conducting dCBT in patients without well HLA-matched units, especially for many patients of mixed or non-European ancestry.34,39 Additionally, MRD did not adversely impact PFS of leukemia patients supporting a robust CB-mediated graft-versus-leukemia effect as previously reported.3,40 Very promising PFS was also observed in AML patients with high relapse risk according to their ELN classification at diagnosis and those with FLT3 ITD.
We acknowledge our intermediate-intensity dCBT approach also has limitations. First, it is not known whether similar results could be achieved with an adequately dosed single-unit graft. Additionally, although graft failure was rare, most patients had received prior immunosuppressive chemotherapy. Whether intermediate-intensity conditioning can always reliably engraft patients without prior immunosuppressive therapy requires further investigation in a larger cohort controlling for infused CD34+ cell dose. Also, although the median neutrophil engraftment of 22 days in dCBT recipients was improved compared with 25 days in our historic controls,12 it remains delayed compared with adult donor allografts. As we recently reported,18 haplo-dCBT does not reliably improve the speed of neutrophil recovery because of rapid haplo-identical graft rejection by the engrafting CB unit, and therefore we have abandoned this strategy. However, importantly, we demonstrate for the first time that, as with single-unit CBT,14,16 adequate CD34+ cell dose is critical to augment not only engraftment but mitigate mortality in dCBT recipients. This is further argument to ensure that CD34+ cell dose is incorporated into unit selection for dCBT. Additionally, for patients without units with adequate CD34+ dose, alternative graft sources should be chosen, or intermediate-intensity CBT could be investigated with expansion.
Another limitation that requires refinement is the high incidence of aGVHD. It is possible that the rate of grade II aGVHD may have been inflated by the lack of requirement for histologic proof. Also, grade II aGVHD has not been associated with an adverse effect on survival after CBT41 (I.P. and J.N.B., unpublished data, 15 October 2020), and a high proportion of our grade II aGVHD patients are treated with nonabsorbable or topical corticosteroids with high response rates.42 Nonetheless, the grade III-IV disease incidence was excessive. Whether this could be mitigated by improving the HLA match requires investigation but is greatly complicated by the fact that prioritizing HLA match may compromise the graft CD34+ cell dose and consequently worsen survival. Increasing CSA trough target early after transplant may be an option in selected patients but can risk nephrotoxicity.20,43 We are now investigating adding a single dose of tocilizumab to CSA/mycophenolate mofetil as an approach to reduce severe aGVHD risk.44 Other strategies of augmented, but nonlymphodepleting, prophylaxis, risk-adapted aGVHD therapy, or new agents warrant investigation.45-48 It is also possible that the incidence of severe aGVHD will be reduced by CMV prophylaxis with letermovir.
We also have little experience with intermediate-intensity dCBT in patients over 60 years of age. Accordingly, as there was an association between age and mortality, the acceptable upper age limit requires further study. The extent of comorbidity and disease burden that could still be associated with acceptable outcomes is also not fully established.
Despite these limitations, intermediate-intensity dCBT can result in a PFS that compares very favorably with dCBT after either high-intensity or NMA conditioning.5,6,8-10 It is considered the standard of care at our institution in adults and has now been adopted by multiple other centers.9,49,50 Importantly, although retrospective, this analysis reflects the outcomes of consecutive patients with characteristics that are commonly encountered in adult transplant centers (ie, age 21-65 years, first allograft, otherwise incurable hematologic malignancy). Moreover, the study included a significant proportion of patients with high HCT-CI and/or advanced disease. In patients with high-risk hematologic malignancies, intermediate-intensity dCBT may be especially attractive. This is because of the low relapse rate (9% at 3 years in this report) compared with those reported in multiple series of adult donor allograft recipients transplanted with posttransplant cyclophosphamide (25%-40% after ablative conditioning51-54 and ≥40% after reduced intensity or NMA regimens10,52,55 ). Also, the reduced stringency of required HLA match permits significant extension of transplant access to patients without suitable adult donors.19,34,39
We also demonstrated that very high engraftment rates can be achieved without expansion, and for some patients with units of good CD34+ cell dose, expansion may not be necessary. This is relevant because expansion can sometimes be logistically challenging. Also, units undergoing ex vivo expansion are CD34+ selected, and the recryopreserved CD34− fraction is rethawed for T-cell add-back infusion. This results in a loss of viable T cells,50,56,57 and the consequent T-cell depletion could potentially compromise the graft’s immunologic properties. Unmanipulated dCBT also has the advantage of rapid availability and flexible scheduling. This is especially relevant for urgent transplants, patients with unpredictable transplant timelines, and at times of adult donor supply chain disruption (eg, the COVID-19 pandemic). Taken together, use of unmanipulated units could facilitate the wider adoption of dCBT.
Our findings support further investigation of intermediate-intensity dCBT in a multicenter setting. These studies should address what is the acceptable upper limit for recipient age, pretransplant disease burden, and comorbidity score. Future analyses should also question whether, with a higher proportion of better HLA-matched units, an association between HLA match and survival can be discerned. Finally, because multiple components of CBT contribute to the high rates of engraftment and PFS, such studies should adhere to optimal CBT practices to ensure favorable outcomes.13 To assist with this, the American Society of Transplantation and Cellular Therapy CB Special Interest Group has developed guidelines outlining current standards of care for unit selection,58 thaw, and infusion,59 and guidelines for multiple other aspects of CBT are in preparation.
For original data, please contact the corresponding author at barkerj@mskcc.org.
Acknowledgments
The authors thank the search coordinators who secured the CB grafts and the clinical and laboratory staff that cared for the patients and contributed to this work.
This work was supported, in part, by National Institute of Health (NIH), National Cancer Institute grants P01 CA23766 and P30 CA008748. J.U.P. acknowledges funding from NIH, National Heart, Lung, and Blood Institute award K08HL143189, and the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center.
Authorship
Contribution: J.N.B. and I.P. designed the study, assembled and analyzed the data, and wrote the manuscript; S.M.D. performed the statistical analysis; K.A.N., K.S., M.A.M., L.F., and T.A. maintained the patient database and provided data; J.N.B., T.A., S.T.A., A.S., C.C., P.B.D., S.A.G., B.G., A.M.H., K.H., A.A.J., E.B.P., J.U.P., M.-A.P., C.S.S., G.L.S., B.C.S., R.T., J.W.Y., M.R., D.M.P., and I.P. provided patient care; and all authors interpreted the data, reviewed and edited the manuscript, and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: J.N.B. has received clinical trial funding from Angiocrine Bioscience and an unrestricted educational grant from Merck. S.T.A. has received honoraria from Abbott Laboratories. A.S. was the Medical Director of the National Cord Blood Program until 1/2020 and serves as a consultant at the Scientific Advisory Board of ExCellThera. S.A.G. has served as a consultant for Amgen, Actinium, Celgene, Johnson & Johnson, Jazz Pharmaceutical, Takeda, Novartis, Kite, and Spectrum Pharma and has received research funding from Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi, and Takeda. J.U.P. reports research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics and consulting fees from DaVolterra. M.-A.P. has received honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Kite (Gilead), Merck, Novartis, Nektar Therapeutics, and Takeda; serves on data and safety monitoring boards for Servier, Cidara Therapeutics and Medigene; serves on the scientific advisory boards of MolMed and NexImmune; and has received research support for clinical trials from Incyte, Kite (Gilead) and Miltenyi Biotec. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene, Gamida Cell, and GSK, and has received research funds for clinical trials from Juno Therapeutics, Celgene, Bristol-Myers Squibb, Precision Biosciences, and Sanofi-Genzyme. R.J.O. receives royalties from Atara Biotherapeutics. I.P. has received research funding from Merck and serves as a member on a data and safety monitoring board for ExcellThera. The remaining authors declare no competing financial interests.
Correspondence: Juliet N. Barker, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org.