Key Points
Prevalence of PJP in BTKi-monotherapy patients not on prophylaxis was low at 2.4% (2 of 85).
All PJP cases were successfully treated and patients resumed BTKi’s following PJP treatment without issue; there were no PJP recurrences.
Abstract
Opportunistic infections (OIs), such as Pneumocystis jirovecii pneumonia (PJP), have been reported in chronic lymphocytic leukemia (CLL) patients treated with ibrutinib, and are an important cause of morbidity and mortality. Currently, there are no international consensus guidelines regarding the use of antimicrobial prophylaxis for OIs, and in particular PJP, in CLL patients treated with Bruton tyrosine kinase inhibitors (BTKi’s). We evaluated the frequency of PJP in CLL patients at our institution who were treated with BTKi’s, and assessed the impact of prophylaxis on reducing the risk of PJP. We identified 217 patients treated with BTKi’s, consisting of 3 cohorts: 143 patients on either BTKi monotherapy with ibrutinib or acalabrutinib, 17 patients receiving ibrutinib combination therapy with umbralisib as part of a clinical trial, and 57 patients receiving ibrutinib in combination with standard chemotherapy, also as part of a clinical trial. Forty-one percent of patients on BTKi monotherapy received prophylaxis, which was given at the discretion of the treating physician. The prevalence of PJP in all patients not on prophylaxis was 3.4% (3 of 87), and, specifically in BTKi-monotherapy patients not on prophylaxis, the PJP prevalence was 2.4% (2 of 85). PJP prophylaxis was effective, as there were no cases of PJP in patients on prophylaxis (0 of 130). The relatively low prevalence of PJP in our study population suggests that routine prophylaxis may not be indicated in CLL patients on BTKi therapy.
Introduction
Although novel agent therapies have recently transformed the management of chronic lymphocytic leukemia (CLL),1-8 infectious complications continue to be an important cause of morbidity and mortality for CLL patients. Recently, growing numbers of opportunistic infections including Pneumocystis jirovecii pneumonia (PJP) and other invasive fungal infections have been reported in CLL patients treated with Bruton tyrosine kinase inhibitors (BTKi’s).9-12 Some studies have specifically described PJP in ibrutinib-treated CLL patients,13 including a retrospective study of 96 ibrutinib-monotherapy patients in which 5 PJP cases occurred, with an estimated cumulative incidence of 5.6% at 2 years.14 In another recent large single-center, retrospective study of 566 CLL patients treated with ibrutinib, there were no cases of PJP reported in any patients, even among those not on prophylaxis. Prophylaxis practices were quite varied in the cohort, with just under one-half of the patients (44.9%) receiving prophylaxis.15
Currently, PJP prophylaxis is often implemented for CLL patients receiving fludarabine-based chemoimmunotherapy regimens.16 These patients are at increased risk of developing PJP,17 likely due to the reduction in CD4+ T-cell immunity following fludarabine. However, there are insufficient data to recommend the use of routine PJP prophylaxis for CLL patients receiving BTKi’s and there are no international consensus guidelines regarding PJP prophylaxis in these patients. The 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines do not address this issue,18 whereas the 2018 British Society for Haematology guidelines recommend PJP prophylaxis for all relapsed/refractory patients regardless of therapeutic agent.19 The paucity of data on this question has led to varying practices across institutions and even within the same institution. To address this knowledge gap, we sought to determine both the frequency of PJP in CLL patients treated with BTKi’s at our institution and the impact of prophylaxis on reducing the risk of PJP.
Methods
We performed a retrospective, institutional review board–approved study of CLL patients treated with BTKi’s at Dana-Farber Cancer Institute (DFCI). Patients were included if they: had received at least 30 days of ibrutinib or acalabrutinib either as monotherapy or as part of a combination-therapy regimen, initiated therapy between 1 January 2010 and 1 January 2019, and received their care at DFCI throughout the duration of BTKi therapy. Patients were excluded if they had undergone an allogeneic hematopoietic stem cell transplant prior to BTKi initiation. Data including patient demographics, CLL disease features, number and type of prior therapies, and BTKi duration were collected. Electronic medical records were reviewed and cross-referenced with pharmacy records to determine whether patients were on PJP prophylaxis. PJP cases were defined by the following criteria: (1) new onset of respiratory symptoms and concomitant computer tomography scans demonstrating findings consistent with PJP infection20 and (2) successful treatment of presumed PJP with PJP-specific treatment. Identified cases were adjudicated by 2 investigators. Patients were followed through 1 February 2019, which was the data cutoff. The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board.
Results
A total of 217 CLL patients were identified (Table 1). The majority of patients received either ibrutinib monotherapy (n = 122; 56%) or acalabrutinib monotherapy (n = 21; 10%). Seventy-four patients (34%) received ibrutinib in combination with other agents as part of clinical trials, including 57 patients who received ibrutinib and fludarabine/cyclophosphamide/rituximab (iFCR) as frontline therapy,21 and 17 patients who received ibrutinib and the phosphoinositide-3-kinase-δ inhibitor umbralisib for relapsed/refractory disease.22 Sixty-three percent of patients were male, and the median age was 65.1 years (range, 38.2-85.9 years). Notably, this age was similar across all subgroups except for those receiving frontline iFCR, who were considerably younger with a median age of 55.0 years, consistent with the younger, fit population typically treated with this regimen. Over one-half of patients were previously untreated (n = 127; 59%), whereas some had received up to 6 prior lines of therapy. Nearly all previously treated patients had received a CD20 monoclonal antibody (82 of 90; 91%) and the majority had received an alkylating agent (70 of 90; 78%). Twenty-four percent of patients had high-risk disease with del(17p), and 70% had an unmutated variable region of the immunoglobulin heavy chain (IGHV). The median duration of BTKi therapy was 22.7 months (range, 1.0-69.8 months), and 57% of all patients (124 of 217) had ongoing BTKi therapy at the time of data cutoff.
Patient characteristics
| . | All patients . | Treatment . | |||
|---|---|---|---|---|---|
| Ibrutinib . | Acalabrutinib . | iFCR . | Ibrutinib + umbralisib . | ||
| No. of patients | 217 | 122 | 21 | 57 | 17 |
| Age at initiation of BTKi, median (range), y | 65.1 (38.2-85.9) | 69.3 (43.5-83.2) | 68.4 (49.1-83.7) | 55.0 (38.2-66.0) | 67.9 (48.4-85.9) |
| Sex, n (%) | |||||
| Male | 137 (63) | 78 (64) | 14 (67) | 34 (60) | 11 (65) |
| FISH cytogenetic abnormality (hierarchical), n (%) | |||||
| 17p13.1 deletion | 52 (24) | 39 (32) | 7 (33) | 3 (5) | 3 (18) |
| 11q22.3 deletion | 46 (21) | 24 (20) | 4 (19) | 11 (19) | 7 (41) |
| 13q14 deletion | 101 (47) | 44 (36) | 13 (62) | 33 (58) | 11 (65) |
| Trisomy 12 | 55 (25) | 38 (31) | 4 (19) | 8 (14) | 5 (29) |
| None | 76 (35) | 29 (24) | 5 (24) | 38 (67) | 4 (24) |
| Complex karyotype, n (%) | 47 (22) | 30 (25) | 5 (24) | 5 (9) | 7 (41) |
| TP53 mutated, n (%) | 41 (22) | 30 (30) | 6 (38) | 1 (2) | 4 (24) |
| No. evaluable | 188 | 100 | 16 | 55 | 17 |
| Unmutated IGHV, n (%) | 139 (70) | 78 (72) | 17 (81) | 31 (58) | 13 (87) |
| No. evaluable | 198 | 109 | 21 | 53 | 15 |
| Total no. of prior therapies before BTKi initiation, n (%) | |||||
| Median (range) | 0 (0-6) | 1 (0-6) | 1 (0-3) | 0 (NA) | 1 (1-5) |
| 0 | 127 (59) | 60 (49) | 10 (48) | 57 (100) | 0 (0) |
| 1 | 40 (18) | 25 (20) | 5 (24) | 0 (0) | 10 (59) |
| 2 | 21 (10) | 12 (10) | 3 (14) | 0 (0) | 6 (35) |
| 3 | 14 (6) | 11 (9) | 3 (14) | 0 (0) | 0 (0) |
| 4-6 | 15 (7) | 14 (11) | 0 (0) | 0 (0) | 1 (6) |
| Type of prior therapy before BTKi initiation, n = 90, n (%) | |||||
| Purine analog | 57 (63) | 39 (63) | 9 (82) | NA | 9 (53) |
| Alkylator | 70 (78) | 49 (79) | 9 (82) | NA | 12 (71) |
| CD20 mAb | 82 (91) | 57 (92) | 11 (100) | NA | 14 (82) |
| BCR-pathway inhibitor | 7 (8) | 4 (6) | 0 (0) | NA | 3 (18) |
| BCL-2 inhibitor | 4 (4) | 2 (3) | 1 (9) | NA | 1 (6) |
| Duration of BTKi therapy, median (range), mo | 22.7 (1.0-69.8) | 21.4 (1.0-69.8) | 42.0 (4.3-46.9) | 17.6 (2.9-50.5) | 33.1 (9.2-48.9) |
| Ongoing BTKi therapy at time of data cutoff, n (%) | 124 (57) | 61 (50) | 17 (81) | 34 (60) | 12 (71) |
| PJP prophylaxis, n (%) | |||||
| TMP-SMX | 98 (45) | 37 (30) | 9 (43) | 41 (72) | 11 (65) |
| Atovaquone | 32 (15) | 9 (7) | 3 (14) | 15 (26) | 5 (29) |
| None | 87 (40) | 76 (62) | 9 (43) | 1 (2) | 1 (6) |
| . | All patients . | Treatment . | |||
|---|---|---|---|---|---|
| Ibrutinib . | Acalabrutinib . | iFCR . | Ibrutinib + umbralisib . | ||
| No. of patients | 217 | 122 | 21 | 57 | 17 |
| Age at initiation of BTKi, median (range), y | 65.1 (38.2-85.9) | 69.3 (43.5-83.2) | 68.4 (49.1-83.7) | 55.0 (38.2-66.0) | 67.9 (48.4-85.9) |
| Sex, n (%) | |||||
| Male | 137 (63) | 78 (64) | 14 (67) | 34 (60) | 11 (65) |
| FISH cytogenetic abnormality (hierarchical), n (%) | |||||
| 17p13.1 deletion | 52 (24) | 39 (32) | 7 (33) | 3 (5) | 3 (18) |
| 11q22.3 deletion | 46 (21) | 24 (20) | 4 (19) | 11 (19) | 7 (41) |
| 13q14 deletion | 101 (47) | 44 (36) | 13 (62) | 33 (58) | 11 (65) |
| Trisomy 12 | 55 (25) | 38 (31) | 4 (19) | 8 (14) | 5 (29) |
| None | 76 (35) | 29 (24) | 5 (24) | 38 (67) | 4 (24) |
| Complex karyotype, n (%) | 47 (22) | 30 (25) | 5 (24) | 5 (9) | 7 (41) |
| TP53 mutated, n (%) | 41 (22) | 30 (30) | 6 (38) | 1 (2) | 4 (24) |
| No. evaluable | 188 | 100 | 16 | 55 | 17 |
| Unmutated IGHV, n (%) | 139 (70) | 78 (72) | 17 (81) | 31 (58) | 13 (87) |
| No. evaluable | 198 | 109 | 21 | 53 | 15 |
| Total no. of prior therapies before BTKi initiation, n (%) | |||||
| Median (range) | 0 (0-6) | 1 (0-6) | 1 (0-3) | 0 (NA) | 1 (1-5) |
| 0 | 127 (59) | 60 (49) | 10 (48) | 57 (100) | 0 (0) |
| 1 | 40 (18) | 25 (20) | 5 (24) | 0 (0) | 10 (59) |
| 2 | 21 (10) | 12 (10) | 3 (14) | 0 (0) | 6 (35) |
| 3 | 14 (6) | 11 (9) | 3 (14) | 0 (0) | 0 (0) |
| 4-6 | 15 (7) | 14 (11) | 0 (0) | 0 (0) | 1 (6) |
| Type of prior therapy before BTKi initiation, n = 90, n (%) | |||||
| Purine analog | 57 (63) | 39 (63) | 9 (82) | NA | 9 (53) |
| Alkylator | 70 (78) | 49 (79) | 9 (82) | NA | 12 (71) |
| CD20 mAb | 82 (91) | 57 (92) | 11 (100) | NA | 14 (82) |
| BCR-pathway inhibitor | 7 (8) | 4 (6) | 0 (0) | NA | 3 (18) |
| BCL-2 inhibitor | 4 (4) | 2 (3) | 1 (9) | NA | 1 (6) |
| Duration of BTKi therapy, median (range), mo | 22.7 (1.0-69.8) | 21.4 (1.0-69.8) | 42.0 (4.3-46.9) | 17.6 (2.9-50.5) | 33.1 (9.2-48.9) |
| Ongoing BTKi therapy at time of data cutoff, n (%) | 124 (57) | 61 (50) | 17 (81) | 34 (60) | 12 (71) |
| PJP prophylaxis, n (%) | |||||
| TMP-SMX | 98 (45) | 37 (30) | 9 (43) | 41 (72) | 11 (65) |
| Atovaquone | 32 (15) | 9 (7) | 3 (14) | 15 (26) | 5 (29) |
| None | 87 (40) | 76 (62) | 9 (43) | 1 (2) | 1 (6) |
BCL2, B-cell leukemia/lymphoma-2; BCR, B-cell receptor; FISH, fluorescence in situ hybridization; iFCR, ibrutinib and fludarabine/cyclophosphamide/rituximab; mAb, monoclonal antibody; NA, not applicable; TMP-SMX, trimethoprim-sulfamethoxazole.
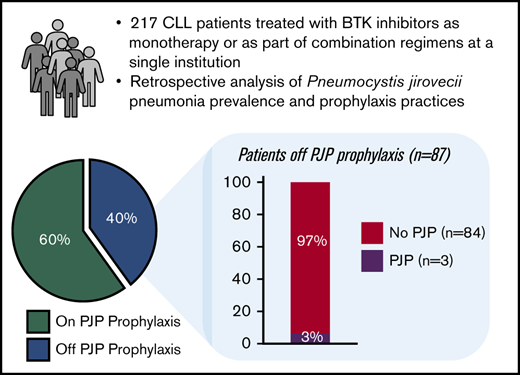
Sixty percent of all patients (130 of 217) were on prophylaxis; 75% of these patients received trimethoprim-sulfamethoxazole (n = 98) and 25% received atovaquone (n = 32). Notably, more than three-quarters of the patients receiving atovaquone (25 of 32; 78%) had a drug allergy or intolerance to sulfonamide drugs and/or specifically to trimethoprim-sulfamethoxazole. For nearly all of these patients (23 of 25), their allergic reaction was listed as a rash. Forty-one percent of BTKi-monotherapy patients (58 of 143) received prophylaxis (38% of ibrutinib-monotherapy and 57% of acalabrutinib-monotherapy patients), which was prescribed at the discretion of the treating provider. All patients enrolled in the combination clinical trials were prescribed PJP prophylaxis as a protocol requirement. Patients on prophylaxis received it for the duration of their BTKi course. The median duration of prophylaxis medication was 27.2 months (range, 2.0-69.8 months).
Three cases of PJP occurred among the 217 patients. Two of these cases occurred in the 122 patients on ibrutinib monotherapy: 1 in a patient with previously untreated CLL and 1 in a patient with relapsed/refractory disease (Table 2). Neither patient was on PJP prophylaxis at the time of infection. The third PJP case occurred in a patient receiving frontline iFCR who had been prescribed PJP prophylaxis but had self-discontinued the prophylactic medication 2 months prior to infection. The 3 cases of PJP were treated successfully with trimethoprim-sulfamethoxazole and prednisone for 21 days. Ibrutinib was held during this period. Both ibrutinib and PJP prophylaxis were then resumed thereafter and there were no recurrences of PJP in these patients. No PJP cases occurred in the 21 patients on acalabrutinib or the 17 patients receiving ibrutinib plus umbralisib.
PJP cases
| Age, y/sex . | Prior treatments . | Therapy . | Ibrutinib duration at time of PJP, mo . | Steroid use within preceding 30 d . | IgG level at time of infection . | Symptoms . | Diagnostic tests . | PJP therapy . | Outcome . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chest CT scan . | β-d-glucan level . | |||||||||
| Case 1 | ||||||||||
| 75/male | None: randomized to ibrutinib-alone arm of phase 3 trial | Ibrutinib single agent | 1.1 | None | Data not available | Fever, cough, dyspnea, fatigue | Diffuse bilateral patchy ground-glass opacities | 289 | TMP-SMX + prednisone for 21 d; ibrutinib held | Following PJP treatment, ibrutinib and PJP prophylaxis were resumed; symptoms resolved without subsequent recurrence of PJP |
| Case 2 | ||||||||||
| 80/female | F/FR/PCR | Ibrutinib single agent | 2.7 | None | 272 | Fever, dyspnea, hypoxia (oxygen saturation 88% on room air) | Diffuse bilateral patchy ground-glass opacities | 171 | TMP-SMX + prednisone for 21 d; ibrutinib held | Following PJP treatment, ibrutinib and PJP prophylaxis were resumed; symptoms resolved without subsequent recurrence of PJP |
| Case 3 | ||||||||||
| 62/female | None: enrolled in clinical trial of frontline therapy | iFCR | 30.6 | None | 166 | Fever, dyspnea, hypoxia (oxygen saturation 82% on room air) | Diffuse bilateral patchy ground-glass opacities | 394 | TMP-SMX + prednisone for 21 d; ibrutinib held | Following PJP treatment, ibrutinib and PJP prophylaxis were resumed; symptoms resolved without subsequent recurrence of PJP |
| Age, y/sex . | Prior treatments . | Therapy . | Ibrutinib duration at time of PJP, mo . | Steroid use within preceding 30 d . | IgG level at time of infection . | Symptoms . | Diagnostic tests . | PJP therapy . | Outcome . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chest CT scan . | β-d-glucan level . | |||||||||
| Case 1 | ||||||||||
| 75/male | None: randomized to ibrutinib-alone arm of phase 3 trial | Ibrutinib single agent | 1.1 | None | Data not available | Fever, cough, dyspnea, fatigue | Diffuse bilateral patchy ground-glass opacities | 289 | TMP-SMX + prednisone for 21 d; ibrutinib held | Following PJP treatment, ibrutinib and PJP prophylaxis were resumed; symptoms resolved without subsequent recurrence of PJP |
| Case 2 | ||||||||||
| 80/female | F/FR/PCR | Ibrutinib single agent | 2.7 | None | 272 | Fever, dyspnea, hypoxia (oxygen saturation 88% on room air) | Diffuse bilateral patchy ground-glass opacities | 171 | TMP-SMX + prednisone for 21 d; ibrutinib held | Following PJP treatment, ibrutinib and PJP prophylaxis were resumed; symptoms resolved without subsequent recurrence of PJP |
| Case 3 | ||||||||||
| 62/female | None: enrolled in clinical trial of frontline therapy | iFCR | 30.6 | None | 166 | Fever, dyspnea, hypoxia (oxygen saturation 82% on room air) | Diffuse bilateral patchy ground-glass opacities | 394 | TMP-SMX + prednisone for 21 d; ibrutinib held | Following PJP treatment, ibrutinib and PJP prophylaxis were resumed; symptoms resolved without subsequent recurrence of PJP |
CT, computed tomography; F, fludarabine; FCR, fludarabine, cyclophosphamide, rituximab; FR, fludarabine, rituximab; IgG, immunoglobulin G; PCR, pentostatin, cyclophosphamide, rituximab.
The overall prevalence of PJP in patients not on prophylaxis was 3.4% (3 of 87; 95% confidence interval [CI], 0.7% to 9.7%) and the incidence rate was 1.9 per 100 person-years. Specifically, in BTKi-monotherapy patients, the prevalence of PJP in patients not on prophylaxis was 2.4% (2 of 85; 95% CI, 0.3% to 8.2%). Within this cohort, the number needed to treat to prevent 1 case of PJP was 42. Notably, no cases of PJP occurred in the 130 patients on either trimethoprim-sulfamethoxazole or atovaquone prophylaxis (0%; 95% CI, 0.0% to 2.3%), demonstrating that both PJP prophylaxis regimens were effective in CLL patients on BTKi.
Discussion
With the growing use of BTKi’s for CLL, it is important to understand the risk of PJP and whether PJP prophylaxis is needed in this patient population. Given the potential adverse events associated with antimicrobial prophylaxis (eg, allergic reaction, cytopenias), a PJP incidence of 5% in a given potentially susceptible population is a commonly accepted threshold above which routine prophylaxis becomes recommended.23 Our observed prevalence of 2.4% in BTKi-monotherapy patients not on PJP prophylaxis falls below this threshold. It is notable that all 3 PJP cases developed within 3 months of BTKi treatment without concomitant prophylaxis. Similarly, in the phase 1 trials with ibrutinib, there appears to be a peak in overall pneumonia risk earlier in treatment, which declines thereafter,24 and a recent report of invasive fungal infections in ibrutinib-treated patients similarly reported that the majority of cases were early-onset.25 Collectively, these observations suggest that early PJP prophylaxis in the first few months of therapy, as a strategy to address the period of highest risk, could be an area of further exploration. Prophylaxis could then be discontinued when the CLL is under better control and a more effective immune system may mitigate the risk of PJP infection.
Limitations of our retrospective study include being performed at a single institution with a heterogenous population and lacking a dedicated monitoring system to assess patient adherence to prophylactic medications. PJP diagnoses were not confirmed by pathology, nor were induced sputum or bronchoalveolar lavage fluid studies performed, which are additional limitations. However, as described previously, patients were diagnosed based on clinical symptoms and radiographic evidence, with all cases adjudicated by at least 2 clinicians, 1 of whom was a specialist in infectious diseases. Another study limitation is that given the small number of PJP cases observed, it is not possible for us to identify specific risk factors for the development of PJP.
Our study is now the second of 2 large retrospective single-institution analyses of PJP prevalence in CLL patients treated with BTKi’s. In the other large cohort recently reported,15 there were no cases of PJP among 312 patients not receiving prophylaxis. The authors concluded that their data did not support the routine use of PJP prophylaxis. We similarly found an overall low prevalence of PJP in patients in our cohort not on prophylaxis. The congruity of these results at 2 of the institutions with the largest and most longstanding experience treating patients with BTKi’s is notable. Furthermore, in both our cohort and the cohort previously reported by Ahn et al,14 it is notable that all cases of PJP were successfully treated with complete resolution of symptoms. Although both cohorts encompass a relatively small number of overall cases, these results suggest that even if there is a very low prevalence of PJP in patients off prophylaxis, these cases could be successfully treated. Although further study in a large, prospective multicenter trial would more definitively address this question, these retrospective data strongly suggest that routine PJP prophylaxis may not be routinely needed for all CLL patients on BTKi therapy. The accumulation of larger data sets over time will enable elucidation of any specific risk factors for PJP development and, subsequently, potential identification of specific subpopulations that may warrant PJP prophylaxis. Overall, as the worldwide experience with BTKi’s for CLL continues to grow, developing consensus guidelines on PJP prophylaxis indications would be beneficial to guiding hematologists on how to optimally approach this issue.
Data-sharing requests may be e-mailed to the corresponding author, Matthew S. Davids, at matthew_davids@dfci.harvard.edu.
Acknowledgments
The authors thank the patients who participated in this study, as well as the clinical support staff and database administrators at Dana-Farber Cancer Institute.
M.S.D. was supported by the Leukemia & Lymphoma Society Scholar in Clinical Research Award.
Authorship
Contribution: All authors contributed to the design of the study; C.E.R. performed the chart review and data analysis; M.P.C. reviewed selected patient cases; C.E.R. and M.S.D. wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.R.B. served as a consultant for AbbVie, Acerta Pharma, AstraZeneca, Beigene, Catapult, Dynamo Therapeutics, Genentech/Roche, Gilead, Juno/Celgene, Kite, Loxo, Novartis, Octapharma, Pfizer, Pharmacyclics, Sunesis, TG Therapeutics, and Verastem; received honoraria from Janssen and Teva; received research funding from Gilead, Loxo, Sun, and Verastem; and served on data safety monitoring committees for Morphosys and Invectys. M.S.D. served as a consultant for AbbVie, Acerta Pharma, AstraZeneca, Adaptive Biotechnologies, Beigene, Genentech, Janssen, MEI Pharma, Pharmacyclics, Research to Practice, Syros Pharmaceuticals, TG Therapeutics, and Verastem; and received research funding from Acerta Pharma, Ascentage Pharma, Genentech, MEI Pharma, Surface Oncology, TG Therapeutics, and Verastem. The remaining authors declare no competing financial interests.
Correspondence: Matthew S. Davids, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: matthew_davids@dfci.harvard.edu.