Abstract
Venous thromboembolism (VTE) is a relatively frequent complication in hospitalized patients, especially in those with risk factors. The benefit of using direct oral anticoagulants (DOACs) for prevention is controversial. This systematic review was performed as part of the American Society of Hematology (ASH) guidelines on VTE, developed in partnership with McMaster University. MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Epistemonikos were used as data sources from date of inception to November 2019. We included randomized trials in patients hospitalized for an acute medical disease, evaluating any DOACs vs other pharmacological prophylaxis, and included 3 trials with low risk of bias. We analyzed the effects of DOACs vs low-molecular-weight heparins (LMWHs) at 2 different time points: at the end of the short-term treatment phase (both drugs given for the same period of time) and at the end of the extended prophylaxis period (extended DOACs vs a shorter course of LMWHs). We observed that the use of DOACs did not reduce the risk of pulmonary embolism or symptomatic deep venous thrombosis (DVT) in comparison with LMWHs. However, the risk of major bleeding was slightly increased. Additionally, we observed that the benefit of DOACs previously reported was largely based on the reduction of asymptomatic DVT and was not apparent when only symptomatic events were considered. The use of DOACs in hospitalized medical patients slightly increases the risk of major bleeding with no appreciable benefit over LMWHs.
Introduction
Venous thromboembolism (VTE) is a relatively frequent complication in hospitalized patients, especially in those with risk factors.1 Low-molecular-weight heparins (LMWHs) are effective for preventing thrombosis during hospitalization,2 and have been the treatment of choice during many years in patients with high risk of VTE. Currently, in the United States, betrixaban and rivaroxaban have been licensed for prophylaxis in acute medically ill patients, although their relative benefit over LMWHs is controversial. The US Food and Drug Administration (FDA) approval of betrixaban was based on a multicenter trial that found a small benefit in a composite outcome of thrombotic and bleeding events.3 The approval of rivaroxaban was based on a post hoc analysis of a multicenter trial that overall showed a higher risk of bleeding with rivaroxaban in comparison with LMWHs.4
In the context of the development of prophylaxis recommendations for hospitalized medical patients for the American Society of Hematology (ASH), we closely examined the evidence regarding the effect of extended and short-term prophylaxis with direct oral anticoagulants (DOACs) vs short-term LMWHs in hospitalized medical patients. This analysis informed a strong recommendation against DOACs.5 In this review, we expand on what was already published along with the guidelines.
Methods
This systematic review was performed as part of the ASH guidelines on VTE, developed in partnership with McMaster University’s Grading of Recommendations Assessment, Development and Evaluation (GRADE) Centre, and investigates 1 of the prioritized questions. Review and meta-analysis methodology followed the Cochrane Handbook with reporting according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources
We electronically searched in MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials from their date of inception to November 2019. We also conducted a search in the Epistemonikos database (see supplemental Appendix 1 for detailed strategy). Additionally, we asked the panelists from the ASH guideline if they were aware of additional trials not identified in the electronic search. Finally, investigators of the APEX trial3 provided additional unpublished data.
Study selection
Two reviewers independently applied the selection criteria to the search results. Disagreements were resolved by consensus. We used the following inclusion criteria: (1) study design: randomized clinical trials evaluating VTE prophylaxis; (2) population: patient hospitalized for an acute medical disease; (3) intervention: any DOACs, including rivaroxaban, apixaban, or betrixaban, used for any period of time; and (4) comparison: any other pharmacological anticoagulant. We excluded trials conducted in patients who required surgery and studies conducted on neurological patients.
Data extraction
Two independent investigators conducted a duplicate data abstraction. For each included trial, we collected the following population characteristics: age, sex, reason for hospitalization, and presence of cancer. We also extracted the number of events and the total number of individuals analyzed in each group for the outcomes of this review (see "Data analysis").
Data analysis
We conducted a complete case analysis for the following prespecified outcomes: all-cause mortality, pulmonary embolism (PE), symptomatic deep venous thrombosis (DVT) and major bleeding. We analyzed the data at 2 different time points: at the end of the short-term parenteral treatment phase (both drugs given for the same period of time) and at the end of the extended prophylaxis period (extended DOACs [intervention] vs a shorter course of anticoagulant [control]). Additionally, we reanalyzed the composite outcome reported in the included trials under 2 different definitions: the original definition (a composite of asymptomatic proximal DVT plus symptomatic proximal or distal DVT plus PE plus death due to VTE plus major bleeding), and our own definition, limited only to symptomatic events (symptomatic proximal or distal DVT plus PE plus major bleeding).
The influence of study limitations was assessed in duplicate by 2 investigators using the Cochrane Risk of Bias Tool at outcome level.6 To evaluate the influence of missing outcome data on our findings, when a study did not report all participants originally randomized, we conducted a sensitivity analysis including all participants in the denominators using plausible assumptions about the outcome of participants with missing data: we assumed that event risk in those with missing data in the intervention group was 2 and 3 times the event risk of those with available data, and assumed the same event risk in those with missing and available data in control groups.7
We conducted all meta-analyses using RevMan 5.3 (version 5.3; Copenhagen, Denmark). We pooled risk ratios of included trials using the Mantel-Haenszel method with a random-effect model. We assessed heterogeneity with the χ2 test and with the I2 statistic. Publication bias was assessed graphically by evaluating symmetry in the funnel plots. Two investigators assessed the confidence in estimates of treatment effects following the GRADE approach.8 We summarized the findings using GRADEProfiler (version 3.2 for Windows).
To estimate the absolute effect of the intervention, we calculated the risk difference by multiplying the pooled risk ratio by the baseline risk of each outcome. Given that participants of randomized trials might have a lower risk of adverse outcomes than real-life patients, when possible, we used the baseline risk observed in large observational studies. When observational data were not available, we used as baseline risk the median of the risks observed in the control groups of the included trials.
Results
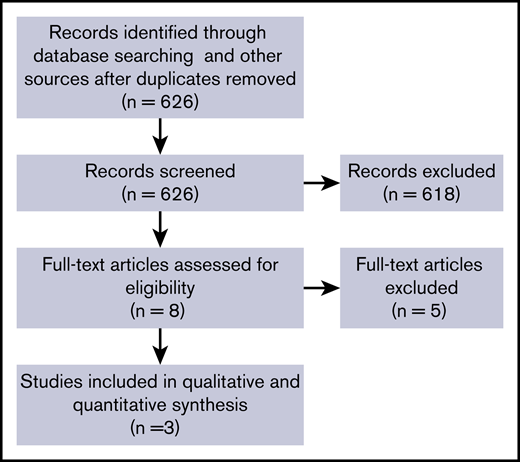
The search strategy identified 626 records (Figure 1). Of 8 studies selected for full text assessment, 3 met eligibility criteria.3,9,10 We did not identify any trial published after the release of the ASH guideline.
All trials compared extended-duration prophylaxis with DOACs vs shorter-duration prophylaxis with the LMWH enoxaparin in populations deemed to be at increased VTE risk. The ADOPT trial10 compared apixaban 2.5 mg every 12 hours for 30 days vs enoxaparin 40 mg/d for 6 to 14 days, the MAGELLAN trial9 evaluated rivaroxaban 10 mg/d for 35 ± 4 days vs enoxaparin 40 mg/d for 6 to 14 days, and the APEX Trial3 compared betrixaban 80 mg/d for 35 to 42 days vs enoxaparin 40 mg/d for 6 to 14 days. Patients included in the trials had a mean age between 66 and 76 years, and approximately one-half were female. The most common reasons for hospital admission were respiratory failure, heart failure, and infections. Only a small proportion of included patients had active cancer at the time of randomization (Table 1).
Characteristics of participants
| . | ADOPT . | MAGELLAN . | APEX . |
|---|---|---|---|
| Participants, n | 6528 | 8101 | 7513 |
| Age, mean, y | 66.7 | 71* | 76.4 |
| Female, % | 50.9 | 45.9 | 54.4 |
| Respiratory failure, % | 37.1 | 28.0 | 12.3 |
| Heart failure, % | 38.5 | 32.4 | 44.5 |
| Infection, % | 22.2 | 45.4 | 28.9 |
| Stroke, % | NR | 17.3 | 11.2 |
| Active cancer, % | 3.2 | 7.3 | NR |
| . | ADOPT . | MAGELLAN . | APEX . |
|---|---|---|---|
| Participants, n | 6528 | 8101 | 7513 |
| Age, mean, y | 66.7 | 71* | 76.4 |
| Female, % | 50.9 | 45.9 | 54.4 |
| Respiratory failure, % | 37.1 | 28.0 | 12.3 |
| Heart failure, % | 38.5 | 32.4 | 44.5 |
| Infection, % | 22.2 | 45.4 | 28.9 |
| Stroke, % | NR | 17.3 | 11.2 |
| Active cancer, % | 3.2 | 7.3 | NR |
NR, not reported.
The mean was not reported. This value corresponds to the median age.
The 3 included trials reported as primary outcome a composite of symptomatic and asymptomatic events. Figure 2 depicts the relevant time points for outcome assessments.
All included trials were double-blinded and had an adequate generation and concealment of the sequence of randomization. In 2 of the trials (MAGELLAN and APEX), there was a significant proportion of patients originally randomized with missing outcome data for PE and DVT. However, our sensitivity analysis assuming plausible scenarios for the patients with missing outcome data did not substantially change the results. Given this, we considered the risk of bias for the whole body of evidence as low.
Effect of DOACs vs LMWHs at the end of 6- to 10-day prophylaxis
At the end of the parenteral treatment (ie, both drugs used for the same duration, 6-14 days), the use of DOACs, in comparison with LMWHs, did not reduce the risk of PE (relative risk [RR], 1.01; 95% confidence interval [CI], 0.29-3.53; I2 = 46%; moderate-certainty evidence) or symptomatic DVT (RR, 1.03; 95% CI, 0.34-3.08; I2 = 21%; moderate-certainty evidence). However, we did observe a significant increase of major bleeding with DOACs (RR, 1.70; 95% CI, 1.02-2.82; I2 = 0%; high-certainty evidence) (Table 2). In absolute terms, the use of DOACs for the same period of time as LMWHs would lead to 2 more major bleeding episodes per 1000 (95% CI from 0 to 4 more; high-certainty evidence) on the studied populations. However, assuming the baseline risk observed in a high-bleeding-risk population,11 the use of DOACs would lead to an excess of 12 major bleeding episodes per 1000 (95% CI from 0 to 22 more; high-certainty evidence).
Effect of DOACs vs LMWHs on patient-important outcomes at the end of 6- to 10-day prophylaxis
| Outcomes . | No. of participants (studies) followed up . | GRADE certainty in the evidence . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with LMWHs . | Risk difference with any DOACs . | ||||
| Mortality | 19 900 (3 RCTs) | ⊕⊕⊕⊕ High | RR 0.64 (0.21-1.98) | 1 per 1000 | 0 fewer per 1000 (1 fewer to 1 more) |
| PE | 19 895 (3 RCTs) | ⊕⊕⊕◯ Moderate* | RR 1.01 (0.29-3.53) | Study population: 1 per 1000 Moderate: 4 per 1000† | Study population: 0 fewer per 1000 (1 fewer to 3 more) Moderate: 0 fewer per 1000 (3 fewer to 10 more) |
| Symptomatic DVT | 19 900 (3 RCTs) | ⊕⊕⊕◯ Moderate* | RR 1.03 (0.34-3.08) | Study population: 1 per 1000 Moderate: 2 per 1000‡,§ | Study population: 0 fewer per 1000 (1 fewer to 2 more) Moderate: 0 fewer per 1000 (1 fewer to 4 more) |
| Major bleeding | 21 821 (3 RCTs) | ⊕⊕⊕⊕ High | RR 1.70 (1.02-2.82) | Study population: 2 per 1000 Moderate: 12 per 1000|| | Study population: 2 more per 1000 (0 fewer to 4 more) Moderate: 8 more per 1000 (0 fewer to 22 more) |
| Outcomes . | No. of participants (studies) followed up . | GRADE certainty in the evidence . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with LMWHs . | Risk difference with any DOACs . | ||||
| Mortality | 19 900 (3 RCTs) | ⊕⊕⊕⊕ High | RR 0.64 (0.21-1.98) | 1 per 1000 | 0 fewer per 1000 (1 fewer to 1 more) |
| PE | 19 895 (3 RCTs) | ⊕⊕⊕◯ Moderate* | RR 1.01 (0.29-3.53) | Study population: 1 per 1000 Moderate: 4 per 1000† | Study population: 0 fewer per 1000 (1 fewer to 3 more) Moderate: 0 fewer per 1000 (3 fewer to 10 more) |
| Symptomatic DVT | 19 900 (3 RCTs) | ⊕⊕⊕◯ Moderate* | RR 1.03 (0.34-3.08) | Study population: 1 per 1000 Moderate: 2 per 1000‡,§ | Study population: 0 fewer per 1000 (1 fewer to 2 more) Moderate: 0 fewer per 1000 (1 fewer to 4 more) |
| Major bleeding | 21 821 (3 RCTs) | ⊕⊕⊕⊕ High | RR 1.70 (1.02-2.82) | Study population: 2 per 1000 Moderate: 12 per 1000|| | Study population: 2 more per 1000 (0 fewer to 4 more) Moderate: 8 more per 1000 (0 fewer to 22 more) |
RCT, randomized controlled trial; RR, relative risk.
Serious imprecision. The relative estimate of effect is compatible with important harm and important benefit for the intervention that probably crosses the relevant decision threshold.
Guijarro17 reports on the incidence of PE in acutely ill hospitalized medical patients (n = 1 148 301) based on findings from the Spanish National Discharge Database from October 2005 to September 2006 (retrospective database study).
Guijarro17 reports on the incidence of DVT in acutely ill hospitalized medical patients (n = 1 148 301) based on findings from the Spanish National Discharge Database from October 2005 to September 2006 (retrospective database study).
We applied the assumption that ∼20% of symptomatic DVTs are proximal, 80% are distal, and 100% of each is of moderate severity.
Spencer et al11 reported on incidence rates of major bleeding in older adults based on a community-based study (n = 1223) (prospective and retrospective).
Effect of DOACs vs LMWHs at the end of 30- to 42-day prophylaxis
We observed a trend to a reduction of the risk of PE (RR, 0.67; 95% CI, 0.41-1.09; I2 = 0%) and symptomatic DVT (RR, 0.62; 95% CI, 0.36-1.05; I2 = 26%) with extended-term DOACs compared with short-term LMWHs, although the differences were not statistically significant. Furthermore, the absolute effects of DOACs vs LMWHs suggested that the potential differences are likely small: 1 fewer PE case per 1000 treated patients (95% CI from 0 to 2 fewer; moderate-certainty evidence) and 2 fewer DVTs per 1000 treated patients (95% CI from 0 to 4 fewer; moderate-certainty evidence).
The use of extended DOAC prophylaxis compared with short-term LMWHs significantly increased the risk of major bleeding (RR, 1.99; 95% CI, 1.08-3.65; I2 = 58%). In absolute terms, the use of extended DOAC prophylaxis would lead to an excess of 4 major bleeding episodes per 1000 patients treated (95% CI from 0 to 10 more; high-certainty evidence) in the studied population and to 12 more major bleeds per 1000 in a high-bleeding-risk population11 (95% CI from 1 to 32 more; high-certainty evidence) (Table 3).
Effect of DOACs vs LMWHs on patient-important outcomes at the end of 30- to 42-day prophylaxis
| Outcomes . | No. of participants (studies) followed up . | GRADE certainty in the evidence . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with LMWHs . | Risk difference with DOACs . | ||||
| Mortality | 20 225 (3 RCTs) | ⊕⊕⊕◯ Moderate*,† | RR 1.01 (0.89-1.14) | Study population: 49 per 1000 High-risk patients: 99 per 1000‡ | Study population: 0 fewer per 1000 (5 fewer to 7 more) High-risk patients: 1 more per 1000 (11 fewer to 14 more) |
| PE | 18 827 (3 RCTs) | ⊕⊕⊕◯ Moderate§ | RR 0.67 (0.41-1.09) | Study population: 4 per 1000 Moderate-risk patients: 4 per 1000|| | Study population: 1 fewer per 1000 (2 fewer to 0 fewer) Moderate-risk patients: 1 fewer per 1000 (2 fewer to 0 fewer) |
| Symptomatic DVT | 18 838 (3 RCTs) | ⊕⊕⊕◯ Moderate¶ | RR 0.62 (0.36-1.05) | Study population: 6 per 1000 Low-risk patients: 2 per 1000#,** | Study population: 2 fewer per 1000 (4 fewer to 0 fewer) Low-risk patients: 1 fewer per 1000 (1 fewer to 0 fewer) |
| Major bleeding | 21 831 (3 RCTs)1-3 | ⊕⊕⊕⊕ High | RR 1.99 (1.08-3.65) | Study population: 4 per 1000 High-risk patients: 12 per 1000†† | Study population: 4 more per 1000 (0 fewer to 10 more) High-risk patients: 12 more per 1000 (1 more to 32 more) |
| Outcomes . | No. of participants (studies) followed up . | GRADE certainty in the evidence . | Relative effect (95% CI) . | Anticipated absolute effects . | |
|---|---|---|---|---|---|
| Risk with LMWHs . | Risk difference with DOACs . | ||||
| Mortality | 20 225 (3 RCTs) | ⊕⊕⊕◯ Moderate*,† | RR 1.01 (0.89-1.14) | Study population: 49 per 1000 High-risk patients: 99 per 1000‡ | Study population: 0 fewer per 1000 (5 fewer to 7 more) High-risk patients: 1 more per 1000 (11 fewer to 14 more) |
| PE | 18 827 (3 RCTs) | ⊕⊕⊕◯ Moderate§ | RR 0.67 (0.41-1.09) | Study population: 4 per 1000 Moderate-risk patients: 4 per 1000|| | Study population: 1 fewer per 1000 (2 fewer to 0 fewer) Moderate-risk patients: 1 fewer per 1000 (2 fewer to 0 fewer) |
| Symptomatic DVT | 18 838 (3 RCTs) | ⊕⊕⊕◯ Moderate¶ | RR 0.62 (0.36-1.05) | Study population: 6 per 1000 Low-risk patients: 2 per 1000#,** | Study population: 2 fewer per 1000 (4 fewer to 0 fewer) Low-risk patients: 1 fewer per 1000 (1 fewer to 0 fewer) |
| Major bleeding | 21 831 (3 RCTs)1-3 | ⊕⊕⊕⊕ High | RR 1.99 (1.08-3.65) | Study population: 4 per 1000 High-risk patients: 12 per 1000†† | Study population: 4 more per 1000 (0 fewer to 10 more) High-risk patients: 12 more per 1000 (1 more to 32 more) |
Concern about applying the data to a “real-life” population both with regard to baseline risks in the RCTs but baseline risk estimates from observational studies are realistic.
Serious imprecision. The relative estimate of effect is compatible with important harm and important benefit for the intervention that probably crosses the relevant decision threshold.
Spencer et al11 reported on incidence rates of all-cause mortality in older adults based on a community-based study (n = 1223) prospective and retrospective).
Serious imprecision. Wide CI with only 66 events in total, and important harm or benefit is still likely or cannot be excluded.
Guijarro17 reports on the incidence of PE in acutely ill hospitalized medical patients (n = 1 148 301) based on findings from the Spanish National Discharge Database from October 2005 to September 2006 (retrospective database study).
Serious imprecision. Wide CI with only 85 events in total, and important harm or benefit is still likely or cannot be excluded.
Guijarro17 reports on the incidence of DVT in acutely ill hospitalized medical patients (n=1 148 301) based on findings from the Spanish National Discharge Database from October 2005 to September 2006 (retrospective database study).
We applied the assumption that ∼20% of symptomatic DVTs are proximal, 80% are distal, and 100% of each is of moderate severity.
Spencer et al11 reported on incidence rates of major bleeding in older adults based on a community-based study (n = 1223) (prospective and retrospective).
Effect of long-term DOACs vs short-term LMWHs in a composite of VTE and major bleeding
When analyzing the composite outcome originally reported on trials (asymptomatic proximal DVT plus symptomatic proximal or distal DVT plus PE plus death due to VTE plus major bleeding), we observed that extended DOACs may decrease the risk of the composite outcome by 12% (RR, 0.88; 95% CI, 0.76-1.01; I2 = 0%) compared with short-term LMWHs, although the difference was not statistically significant. However, this risk reduction was largely driven by the effect of DOACs on asymptomatic proximal DVT, which accounted for the large majority of the VTE events (Table 4).
Effect of DOACs vs LMWHs
| Composite outcome . | Relative effect (95% CI) . | Proportion of events, % . |
|---|---|---|
| Including asymptomatic events | 0.88 (0.76-1.01) | |
| Asymptomatic proximal DVT | 0.84 (0.70-1.01) | 64.1 |
| Symptomatic proximal or distal DVT | 0.62 (0.36-1.05) | 8.5 |
| PE | 0.67 (0.41-1.09) | 6.6 |
| Death due to DVT | 0.70 (0.45-1.08) | 8.3 |
| Major bleeding | 1.99 (1.08-3.65) | 12.4 |
| Based only on symptomatic events | 1.04 (0.67-1.60) | |
| Symptomatic DVT | 0.62 (0.36-1.05) | 23.8 |
| PE | 0.67 (0.41-1.09) | 31.0 |
| Major bleeding | 1.99 (1.08-3.65) | 45.1 |
| Composite outcome . | Relative effect (95% CI) . | Proportion of events, % . |
|---|---|---|
| Including asymptomatic events | 0.88 (0.76-1.01) | |
| Asymptomatic proximal DVT | 0.84 (0.70-1.01) | 64.1 |
| Symptomatic proximal or distal DVT | 0.62 (0.36-1.05) | 8.5 |
| PE | 0.67 (0.41-1.09) | 6.6 |
| Death due to DVT | 0.70 (0.45-1.08) | 8.3 |
| Major bleeding | 1.99 (1.08-3.65) | 12.4 |
| Based only on symptomatic events | 1.04 (0.67-1.60) | |
| Symptomatic DVT | 0.62 (0.36-1.05) | 23.8 |
| PE | 0.67 (0.41-1.09) | 31.0 |
| Major bleeding | 1.99 (1.08-3.65) | 45.1 |
When we considered only symptomatic events (symptomatic proximal or distal DVT plus PE plus major bleeding), extended DOACs compared with shorter-term LMWHs were no longer associated with an overall benefit (RR, 1.04; 95% CI, 0.67-1.60; I2 = 69%).
Discussion
This systematic review suggests that the use of DOACs instead of LMWHs to prevent VTE in hospitalized medical patients increases the risk of bleeding. This effect was observed when both alternatives were used for the same short-term period of time (at the end of parenteral treatment) and in the comparison of extended DOACs vs short-term LMWHs. Also, examining the composite outcome of thrombosis and bleeding, we found that any benefit of DOACs was driven by a reduction in asymptomatic VTE events, and was not apparent when only symptomatic events were considered.
The relevance of asymptomatic DVT as a surrogate for symptomatic DVT is controversial. To trust in a surrogate outcome and be able to use it for decision-making, there should be a consistent relationship between the surrogate and the outcomes that are important to patients. However, as shown by a systematic review of 26 randomized trials, there is poor agreement between the effects of anticoagulants on asymptomatic DVT and symptomatic VTE in trials assessing thromboprophylaxis.12 Thus, reduction of the incidence of asymptomatic DVT observed in trials cannot be easily extrapolated to a benefit on patient-important outcomes such as symptomatic DVT or PE.
As we observed, reporting composite outcomes, which include asymptomatic VTE events, may be misleading. By doing so, the increment in major bleeding with DOACs is concealed by their effect on asymptomatic DVT, which has uncertain clinical significance. Furthermore, composite outcomes do not allow an appropriate tradeoff between the desirable and undesirable consequences of an intervention because it is assumed that patients may place the same value on each component of the composite outcome. The available evidence contradicts this assumption. Additionally, in the context of the development of the ASH clinical guidelines, we conducted a systematic review on patients´ values and preferences with regard to relevant outcomes in VTE prevention and treatment. We found that preferences were highly variable, and likely individual patients place different values on thrombotic and bleeding events. The utility associated with DVT and PE ranged from 0.63 to 0.95, whereas the utility associated with bleeding varied from 0.15 to 0.75 depending of the site of bleeding and its magnitude. Patients in general preferred avoiding a thrombotic event over a bleeding event.13
One potential criticism of this analysis is to what extent different DOACs can be combined into a single pooled estimate. Although a valid concern, our group has conducted several meta-analyses evaluating the effect of DOACs across multiple settings and populations: as prophylaxis in orthopedic patients,14,15 as treatment of VTE (Neumann and Schünemann15,16 ; T. Ortel and I.N., manuscript in preparation; and I.N. and A.I., manuscript in preparation), and as stroke prevention in atrial fibrillation.15,16 In none of these analyses did we observe an interaction between the specific drug used and the effect on thrombosis or bleeding. Thus, the available evidence suggests that DOACs may have a class effect; it is perfectly reasonable to analyze them together in well-defined populations. Additionally, the populations included in the identified trials are similar enough to allow a meaningful comparison (Table 1). Ongoing trials comparing specific DOACs head to head may confirm or refute this assumption in the future.
Although the 3 trials analyzed included a large number of participants, given that individual patient data are not publicly available, all of our analyses were conducted at the trial level, which limits our conclusions. Additionally, we did observe some heterogeneity in the outcome major bleeding. Given the limited sample size (n = 3 trials), exploring this heterogeneity by evaluating potential interactions between the effect estimates and patients’ characteristic, or the specific drug used, was considered prone to be influenced by chance and ultimately unreliable. Individual patient data meta-analysis of the included trials may help further exploration of potential differences in specific subgroups of patients and characterize potential differences between specific DOACs.
The strengths of this review include careful examination of the evidence on the effect of DOACs to prevent VTE in hospitalized medical patients. We analyzed the data available at different time points, differentiating the effect of the drugs studied and the effect of a shorter vs longer duration of treatment. Also, we conducted an evaluation of the assertion of net benefit and we were able to distinguish what factors most influenced this apparent benefit and how it changed when only patient-important outcomes were considered.
In conclusion, when only symptomatic outcomes are considered, the use of DOACs compared with LMWHs in hospitalized medical patients is associated with a higher risk of bleeding, with at most a very small benefit in VTE reduction.
Data-sharing requests may be e-mailed to the corresponding author, Ignacio Neumann, at ignacio.neumann@gmail.com.
Acknowledgments
The authors thank investigators of the APEX trial 3, who provided additional unpublished data.
Development of this review was supported by ASH. S.R.K. was supported by a Tier 1 Canada Research Chair, and is an investigator of the CanVECTOR Network, which receives grant funding from the Canadian Institutes of Health Research (funding reference CDT-142654).
All of the authors participated in the development of the American Society of Hematology 2018 guidelines in Schünemann et al.5
Authorship
Contribution: I.N., A.I., Y.Z., and G.R. conducted the searches and screened potential relevant articles; I.N. and A.I. conducted the data abstraction and analysis; I.N. wrote the first draft of the manuscript and revised the manuscript based on authors’ suggestions; guideline panel members (S.R.K., F.S., S.R., F.D., K.B., M.C., and H.S.) and members of the knowledge synthesis team (W.W., R.N., J.J.Y.-N., G.P.M., L.L., and J.W.) critically reviewed the manuscript and provided suggestions for improvement; and all authors approved the content.
Conflict-of-interest disclosure: Full conflict-of-interest declarations for all of the authors of this review are available in Schünemann et al.5 F.D. and K.B. have received funding from DOAC manufacturers. The remaining authors declare no competing financial interests.
Correspondence: Ignacio Neumann, Department of Internal Medicine, School of Medicine, Pontificia Universidad Catolica de Chile, Av. Libertador Bernardo O’Higgins 340, Santiago, Región Metropolitana, Chile; e-mail: ignacio.neumann@gmail.com.
References
Author notes
The full-text version of this article contains a data supplement.