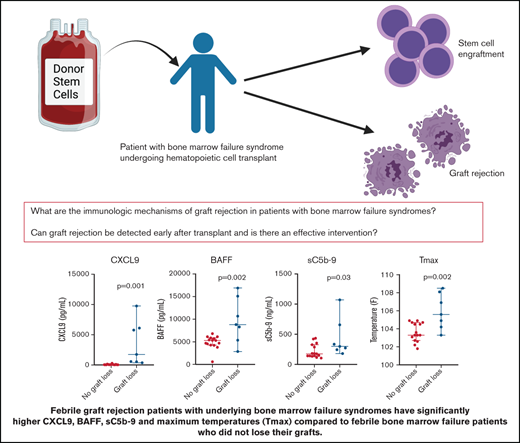
Key Points
CXCL9, BAFF, and sC5b-9 are potential biomarkers and therapeutic targets for graft rejection after transplant.
Fever monitoring is a widely available and informative predictor of graft rejection after transplant.
Abstract
Graft rejection (GR) is a poorly understood complication of hematopoietic cell transplant (HCT). GR risk factors are well published, but there are no reliable biomarkers or therapies known. Fever is the most common symptom of GR, but no study has evaluated fever kinetics as a diagnostic marker of GR. The objectives of this study were to identify mechanisms, biomarkers, and potential therapies for GR after HCT. Chemokine ligand 9 (CXCL9), B-cell activating factor (BAFF), and complement markers (sC5b-9, C3a, and C5a) were measured in 7 patients with GR and compared with 15 HCT controls. All patients had a diagnosis of aplastic anemia, Fanconi anemia, or genetically undefined chromosomal fragility syndrome. All patients with GR were febrile during GR; therefore, control patients who underwent HCT were matched for diagnosis and early fevers after HCT. Patients withh GR had significantly higher CXCL9, BAFF, and sC5b-9 at the time of fever and GR compared with control patients who underwent HCT at the time of fever. The maximum fever was significantly higher and occurred significantly later in the transplant course in patients with GR compared with febrile HCT controls. These data support the use of CXCL9, BAFF, sC5b-9, and fever kinetics as GR markers. Two patients with GR underwent a second HCT that was complicated by high fevers. Both patients received interferon and complement blockers during their second HCT, and both preserved their graft. These laboratory and clinical findings support larger studies to evaluate the safety and efficacy of interferon, complement, and BAFF inhibitors for the prevention and treatment of GR after HCT.
Introduction
Graft failure is a deleterious complication of hematopoietic cell transplant (HCT) and complicates 3.5% of all pediatric HCTs.1 The immune-mediated expulsion of an allogeneic graft is termed graft rejection (GR) and is an important cause of both primary and secondary graft failure.2 Risk factors for GR include HLA or ABO mismatch, ex vivo T-cell depletion, reduced intensity conditioning, and HCT for nonmalignant diseases.2-4 These risk factors make GR highly relevant to HCT candidates with bone marrow failure (BMF) syndromes. Few treatment strategies for GR have been studied outside of retransplantation or donor stem cell boost, and outcomes remain poor for these patients.1,5-7 Successful retransplantation after GR can occur but is limited by donor availability and the clinical condition of the patient (eg, morbidity from first HCT). Patients with congenital BMF syndromes such as Fanconi anemia (FA) are at particular risk for treatment-related toxicity because of underlying defects in DNA repair. Ayas et al6 reported a 5-year overall survival of merely 16% to 45% (depending on the timing of second HCT) in patients with FA who had graft failure and underwent a second HCT. This means there is a tremendous clinical need for timely markers and effective interventions to prevent GR after HCT.
One outstanding challenge is that a thorough understanding of GR mechanisms is lacking. Furthermore, there are likely multiple distinct immunologic pathways working to remove the foreign graft.7,8 Recipient alloreactive T cells have historically been blamed for GR, although more recent data note contributions from B cells, natural killer cells, and other discrete facets of humoral and cellular response pathways.8 Expansive studies of host immune response preceding and during GR are needed to further understand this complication and identify targetable pathways for intervention. Published data on targetable GR pathways in HCT are limited; however, Merli et al9 reported interferon γ is elevated in patients with graft failure compared with control patients who underwent HCT. There are currently no published studies on complement or B-cell activating factor (BAFF) levels in GR after HCT, although these markers are well studied in solid organ transplant recipients with GR.10,11 Importantly, interferon γ, complement, and BAFF are each targetable with clinically available inhibitors, which make them suitable candidates for the study of GR mechanisms and the identification of potential therapies.
Although an enhanced understanding of GR mechanisms and therapies is crucial, this must be supplemented with improvements in GR screening. In-depth immunology studies are not readily available at many transplant centers, and these tests are frequently sent out to other laboratories. Transplant centers that perform these studies are also limited by processing times, which can take several days. These time lags impede the routine clinical use of studies for GR surveillance, and more accessible clinical markers of GR are needed. Fever is the most common clinical sign of graft failure in patients who underwent HCT, and a prior study reported 86.7% of patients with graft failure experienced fevers.9 No study has formally investigated temperature kinetics in GR after HCT. Temperature monitoring is inexpensive, noninvasive, and standard clinical practice in patients undergoing HCT, which makes it an appealing clinical marker for GR surveillance.
The goals of this study were to improve the mechanistic understanding of GR after HCT for BMF syndromes and identify potential biomarkers and therapies for these patients. Based on published literature for GR in HCT and solid organ transplant, we studied chemokine ligand 9 (CXCL9), complement markers (sC5b-9, C3a, C5a), and BAFF in patients with BMF who underwent HCT and compared our findings with those in a control group of febrile BMF transplant recipients who did not develop GR. We then describe our experience with 2 patients who had high fevers and GR after their first HCT and were treated with terminal complement and interferon γ blockers during their second HCT.
Methods
Patient selection and definitions
Pediatric and young adult patients who underwent HCT at our institution between 2015 and 2020 and consented to our HCT tissue repository and/or BMF repository were included in this study. Institutional Review Board approval was obtained before the start of the study from the Cincinnati Children's Hospital Medical Center (IRB ID: 2012-1156). Patients had a diagnosis of severe aplastic anemia (SAA), FA, or genetically undefined chromosomal fragility syndrome. All patients with GR were febrile during GR. Fever was defined as any charted temperature greater than or equal to 100.4°F. Febrile HCT controls were patients with similar diagnoses who were transplanted during this time frame and experienced fevers during the first 8 weeks after HCT but did not lose their graft. Eight weeks was chosen as a cutoff based on the timing of the latest fever spike in the GR cohort, which occurred 48 days after HCT (patient 16). Of note, although 8 weeks was the screening cutoff, all febrile controls had fevers within the first 3 weeks of HCT. All fever-associated blood samples were obtained at a time point closest to when patient temperatures were greater than 102.2°F (39°C) or closest to the maximum temperature (Tmax) if the Tmax was less than 102.2°F. Patient 13 (febrile HCT control) was the only patient in the study whose Tmax did not reach 102.2°F (Tmax was 101.8°F). Graft rejection was defined as (1) the absence of donor engraftment and/or failure to achieve an absolute neutrophil count (ANC) ≥ 0.5 × 109/L or platelet count ≥ 30 × 109/L for 3 consecutive days (primary graft failure) and (2) the loss of donor chimerism and/or development of cytopenias (ANC < 0.5 × 109/L and/or platelets < 30 × 109/L) after initial donor engraftment (secondary graft failure).3,8 Engraftment syndrome was defined using previously published major and minor criteria.12 Two patients were followed prospectively during the study and received a second HCT after initial GR. All real-time laboratory testing and clinical interventions in these patients were performed at the clinician’s discretion. Clinical data were obtained through chart reviews.
Laboratory testing
Inflammatory marker studies were performed on frozen plasma samples stored in the HCT and/or BMF repository at Cincinnati Children’s Hospital Medical Center. CXCL9 and BAFF testing were performed by the Diagnostic Immunology Laboratory at Cincinnati Children’s Hospital Medical Center. Complement testing (sC5b-9, C3a, C5a) was performed by the Hemostasis and Thrombosis Laboratory at Cincinnati Children’s Hospital Medical Center. All laboratory testing was Clinical Laboratory Improvement Amendments (CLIA) certified. These studies were performed at baseline (before conditioning therapy), day +7 (±3 days) after transplant, and at a time point during early clinical fever as described above. Baseline and day +7 studies were obtained for the purpose of studying early markers of impending GR.
Statistical analysis
Variables were presented as medians and examined with a 2-sample t test. Receiver operating characteristic (ROC) curves and optimal sensitivity and specificity cutoffs were generated using Prism GraphPad statistical software.
Emapalumab and eculizumab
Emapalumab (interferon γ blocker) dosing was based on previously published data in hemophagocytic lymphohistiocytosis (HLH).13 Patients received approximately 10 mg/kg emapalumab as a single infusion, rounded to the nearest vial size. Eculizumab (C5 blocker) dosing was based on previously published data in transplant-associated thrombotic microangiopathy.14 Patients received either 600 or 900 mg eculizumab as a 1-time infusion. Standard antibiotic prophylaxis regimens (eg, meningococcal) were used with these medications.
Results
Patient demographics
We studied 22 patients who underwent HCT with SAA (n = 11), FA (n = 10), or genetically undefined chromosomal fragility syndrome (n = 1; Table 1). The median age at HCT was 10.2 years (range, 2.1-23.4 years). Thirteen patients were clinically diagnosed with engraftment syndrome. Ten of these patients were febrile HCT controls and the remaining 3 patients eventually rejected their graft. Seven total patients (4 males, 3 females) rejected their graft at a median of 21 days from HCT (range, 13-47 days). Four patients with GR had SAA, 2 had FA, and 1 had a genetically undefined chromosomal fragility syndrome. All patients with GR received ex vivo T cell–depleted grafts processed using CD34 selection (Miltenyi; peripheral blood stem cell [PBSC] = 6, bone marrow = 1). None of these grafts were α/β T cell–depleted. Patients with GR received alemtuzumab, fludarabine, and melphalan conditioning (patients with SAA, n = 4) or busulfan, cyclophosphamide, fludarabine, and antithymocyte globulin conditioning (all other patients with GR, n = 3). Donors of rejected grafts were mismatched unrelated (9/10, n = 3), matched unrelated (10/10, n = 1), or mismatched related (5-7/10, n = 3). Donors of febrile HCT controls were 9/10 or 10/10 unrelated donor matches in all but 1 patient (patient 3, 8/10). Laboratory test findings and fever data are shown with relevant clinical demographics in Table 2.
Demographics in patients with graft rejection and febrile controls
| Demographics . | All patients (n = 22) . | Graft rejection (n = 7) . | Febrile controls (n = 15) . | P . |
|---|---|---|---|---|
| Age at HCT, y | .33 | |||
| 0-10 | 45.5% (n = 10) | 28.5% (n = 2) | 53.5% (n = 8) | |
| 10-20 | 50% (n = 11) | 57% (n = 4) | 46.5% (n = 7) | |
| >20 | 4.5% (n = 1) | 14.5% (n = 1) | None | |
| Sex | 1 | |||
| Female | 45.5% (n = 10) | 43% (n = 3) | 46.5% (n = 7) | |
| Male | 54.5% (n = 12) | 57% (n = 4) | 53.5% (n = 8) | |
| Race | .05 | |||
| Caucasian | 73% (n = 16) | 43% (n = 3) | 86.5% (n = 13) | |
| African American | 27% (n = 6) | 57% (n = 4) | 13.5% (n = 2) | |
| Diagnosis | .33 | |||
| Severe aplastic anemia | 50% (n = 11) | 57% (n = 4) | 46.5% (n = 7) | |
| Fanconi anemia | 45.5% (n = 10) | 28.5% (n = 2) | 53.5% (n = 8) | |
| Genetically undefined chromosomal fragility syndrome | 4.5% (n = 1) | 14.5% (n = 1) | None | |
| Conditioning | .82 | |||
| Bu/Cy/Flu/ATG | 50% (n = 11) | 43% (n = 3) | 53.5% (n = 8) | |
| Alem/Flu/Mel | 36.5% (n = 8) | 57% (n = 4) | 26.5% (n = 4) | |
| Bu/Cy/ATG | 4.5% (n = 1) | None | 6.67% (n = 1) | |
| Cy/ATG | 4.5% (n = 1) | None | 6.67% (n = 1) | |
| Flu/Mel | 4.5% (n = 1) | None | 6.67% (n = 1) | |
| Graft | 1 | |||
| PBSC | 82% (n = 18) | 85.5% (n = 6) | 80% (n = 12) | |
| Bone marrow | 18% (n = 4) | 14.5% (n = 1) | 20% (n = 3) | |
| GVHD prophylaxis | .26 | |||
| Ex vivo T-cell depletion | 82% (n = 18) | 100% (n = 7) | 73.5% (n = 11) | |
| CSA-based regimen | 18% (n = 4) | None | 26.5% (n = 4) |
| Demographics . | All patients (n = 22) . | Graft rejection (n = 7) . | Febrile controls (n = 15) . | P . |
|---|---|---|---|---|
| Age at HCT, y | .33 | |||
| 0-10 | 45.5% (n = 10) | 28.5% (n = 2) | 53.5% (n = 8) | |
| 10-20 | 50% (n = 11) | 57% (n = 4) | 46.5% (n = 7) | |
| >20 | 4.5% (n = 1) | 14.5% (n = 1) | None | |
| Sex | 1 | |||
| Female | 45.5% (n = 10) | 43% (n = 3) | 46.5% (n = 7) | |
| Male | 54.5% (n = 12) | 57% (n = 4) | 53.5% (n = 8) | |
| Race | .05 | |||
| Caucasian | 73% (n = 16) | 43% (n = 3) | 86.5% (n = 13) | |
| African American | 27% (n = 6) | 57% (n = 4) | 13.5% (n = 2) | |
| Diagnosis | .33 | |||
| Severe aplastic anemia | 50% (n = 11) | 57% (n = 4) | 46.5% (n = 7) | |
| Fanconi anemia | 45.5% (n = 10) | 28.5% (n = 2) | 53.5% (n = 8) | |
| Genetically undefined chromosomal fragility syndrome | 4.5% (n = 1) | 14.5% (n = 1) | None | |
| Conditioning | .82 | |||
| Bu/Cy/Flu/ATG | 50% (n = 11) | 43% (n = 3) | 53.5% (n = 8) | |
| Alem/Flu/Mel | 36.5% (n = 8) | 57% (n = 4) | 26.5% (n = 4) | |
| Bu/Cy/ATG | 4.5% (n = 1) | None | 6.67% (n = 1) | |
| Cy/ATG | 4.5% (n = 1) | None | 6.67% (n = 1) | |
| Flu/Mel | 4.5% (n = 1) | None | 6.67% (n = 1) | |
| Graft | 1 | |||
| PBSC | 82% (n = 18) | 85.5% (n = 6) | 80% (n = 12) | |
| Bone marrow | 18% (n = 4) | 14.5% (n = 1) | 20% (n = 3) | |
| GVHD prophylaxis | .26 | |||
| Ex vivo T-cell depletion | 82% (n = 18) | 100% (n = 7) | 73.5% (n = 11) | |
| CSA-based regimen | 18% (n = 4) | None | 26.5% (n = 4) |
Percentages were rounded to nearest 0.5 or 0.01 to a sum of 100%. Alem, alemtuzumab; ATG, antithymocyte globulin; Bu, busulfan; CSA, cyclosporine; Cy, cyclophosphamide; Flu, fludarabine; GVHD, graft-versus-host disease; Mel, melphalan.
Temperature and inflammatory marker analysis at baseline, day 7 and at time of fever
| . | Diagnosis . | Conditioning . | Donor, match . | Graft . | Tmax (°F) . | Tmax day after HCT . | Fever sample day after HCT . | Baseline CXCL9 (RR: ≤ 121 pg/mL) . | Day 7 CXCL9 . | Fever CXCL9 . | Baseline BAFF (RR: 241-1748 pg/mL) . | Day 7 BAFF . | Fever BAFF . | Baseline sC5b9 (RR: ≤ 244 ng/mL) . | Day 7 sC5b9 . | Fever sC5b9 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No graft rejection | ||||||||||||||||
| Patient 1 | FA | Bu/Cy/Flu/ATG | URD, 12/12 | T-depleted PBSC | 104.7 | 6 | 8 | <31 | <31* | <31 | 1547 | 4708* | 4708 | 121 | 141* | 141 |
| Patient 2 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 103.1 | 7 | 6 | <31 | 33* | 33 | 1053 | 5861* | 5861 | 112 | 142* | 142 |
| Patient 3 | FA | Bu/Cy/Flu/ATG | URD, 8/10 | T-depleted PBSC | 104.9 | 6 | 7 | 250 | 44* | 44 | 2290 | 6119* | 6119 | 312 | 115* | 115 |
| Patient 4 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 104.5 | 4 | 4 | 40 | 85 | 105 | 824 | 6198 | 5730 | 90 | 234 | 181 |
| Patient 5 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 104.5 | 6 | 10 | 32 | <31 | 45 | 1312 | 2419 | 6120 | 150 | N/A | 354 |
| Patient 6 | FA | Bu/Cy/Flu/ATG | URD, 9/10 | T-depleted PBSC | 103.3 | 6 | 5 | <31 | 45* | 45 | 2363 | 4687* | 4687 | 131 | 143* | 143 |
| Patient 7 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 104.9 | 6 | 5 | 68 | 33 | 88 | 1329 | 4421 | 4217 | 198 | 288 | 422 |
| Patient 8 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 102.7 | 7 | 4 | <31 | <31* | <31 | 1454 | 3785* | 3785 | 120 | 176* | 176 |
| Patient 9 | SAA | Bu/Cy/ATG | URD, 9/10 | Bone Marrow | 103.6 | 6 | 7 | 78 | 145* | 145 | 909 | 5321* | 5321 | 140 | 327* | 327 |
| Patient 10 | SAA | Flu/Mel | URD, 9/10 | PBSC | 102.2 | 5 | 7 | 78 | 70* | 70 | 990 | 4274* | 4274 | 244 | 288* | 288 |
| Patient 11 | SAA | Alem/Flu/Mel | URD, 9/10 | T-depleted PBSC | 102.6 | 8 | 8 | 56 | 45* | 45 | 853 | 5400* | 5400 | 143 | 149* | 149 |
| Patient 12 | SAA | Alem/Flu/Mel | URD, 9/10 | PBSC | 103.3 | 5 | 9 | 233 | 276* | 276 | 4500 | 6816* | 6816 | 138 | 182* | 182 |
| Patient 13 | SAA | Cy/ATG | Sibling, 10/10 | Bone Marrow | 101.8 | 14 | 16 | 72 | <31 | 71 | 719 | 581 | 586 | 251 | 322 | 434 |
| Patient 14 | SAA | Alem/Flu/Mel | URD, 10/10 | T-depleted PBSC | 103.3 | 5 | 5 | <31 | 39* | 39 | 736 | 4560* | 4560 | 162 | 134* | 134 |
| Patient 15 | SAA | Alem/Flu/Mel | Mother, 10/10 | T-depleted PBSC | 104.7 | 4 | 6 | 616 | 59* | 59 | 4816 | 5288* | 5288 | 171 | 105* | 105 |
| Graft rejection | ||||||||||||||||
| Patient 16 | SAA | Alem/Flu/Mel | URD, 9/10 | T-depleted PBSC | 105.6 | 48 | 48 | <31 | 64 | 563 | 545 | 4364 | 5362 | 153 | 167 | 336 |
| Patient 17 | SAA | Alem/Flu/Mel | URD, 9/10 | T-depleted PBSC | 103.3 | 13 | 13 | 36 | 48 | 530 | 3179 | 5240 | 15 087 | 125 | 174 | 273 |
| Patient 18 | SAA | Alem/Flu/Mel | URD, 10/10 | T-depleted PBSC | 104.2 | 7 | 11 | 74 | 337 | 5772 | 466 | 8702 | 2862 | 141 | 184 | 181 |
| Patient 19 | SAA | Alem/Flu/Mel | Mother, 7/10 | T-depleted PBSC | 108.5 | 11 | 10 | <31 | 41 | 6106 | 2613 | 8176 | 16 893 | 68 | 141 | 1070 |
| Patient 20 | FA | Bu/Cy/Flu/ATG | Mother, 6/10 | T-depleted PBSC | 106.9 | 16 | 19 | 52 | 199 | 9760 | 1743 | 11 494 | 10 683 | 81 | 103 | 657 |
| Patient 21 | FA | Bu/Cy/Flu/ATG | URD, 9/10 | T-depleted Bone Marrow | 108.1 | 24 | 19 | 80 | 94 | 367 | 1093 | 3591 | 8811 | 105 | 149 | 244 |
| Patient 22 | GUCFS | Bu/Cy/Flu/ATG | Father, 5/10 | T-depleted PBSC | 104.9 | 12 | 13 | 36 | 98 | 1744 | 1298 | 4104 | 8295 | 59 | 60 | 299 |
| . | Diagnosis . | Conditioning . | Donor, match . | Graft . | Tmax (°F) . | Tmax day after HCT . | Fever sample day after HCT . | Baseline CXCL9 (RR: ≤ 121 pg/mL) . | Day 7 CXCL9 . | Fever CXCL9 . | Baseline BAFF (RR: 241-1748 pg/mL) . | Day 7 BAFF . | Fever BAFF . | Baseline sC5b9 (RR: ≤ 244 ng/mL) . | Day 7 sC5b9 . | Fever sC5b9 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No graft rejection | ||||||||||||||||
| Patient 1 | FA | Bu/Cy/Flu/ATG | URD, 12/12 | T-depleted PBSC | 104.7 | 6 | 8 | <31 | <31* | <31 | 1547 | 4708* | 4708 | 121 | 141* | 141 |
| Patient 2 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 103.1 | 7 | 6 | <31 | 33* | 33 | 1053 | 5861* | 5861 | 112 | 142* | 142 |
| Patient 3 | FA | Bu/Cy/Flu/ATG | URD, 8/10 | T-depleted PBSC | 104.9 | 6 | 7 | 250 | 44* | 44 | 2290 | 6119* | 6119 | 312 | 115* | 115 |
| Patient 4 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 104.5 | 4 | 4 | 40 | 85 | 105 | 824 | 6198 | 5730 | 90 | 234 | 181 |
| Patient 5 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 104.5 | 6 | 10 | 32 | <31 | 45 | 1312 | 2419 | 6120 | 150 | N/A | 354 |
| Patient 6 | FA | Bu/Cy/Flu/ATG | URD, 9/10 | T-depleted PBSC | 103.3 | 6 | 5 | <31 | 45* | 45 | 2363 | 4687* | 4687 | 131 | 143* | 143 |
| Patient 7 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 104.9 | 6 | 5 | 68 | 33 | 88 | 1329 | 4421 | 4217 | 198 | 288 | 422 |
| Patient 8 | FA | Bu/Cy/Flu/ATG | URD, 10/10 | T-depleted PBSC | 102.7 | 7 | 4 | <31 | <31* | <31 | 1454 | 3785* | 3785 | 120 | 176* | 176 |
| Patient 9 | SAA | Bu/Cy/ATG | URD, 9/10 | Bone Marrow | 103.6 | 6 | 7 | 78 | 145* | 145 | 909 | 5321* | 5321 | 140 | 327* | 327 |
| Patient 10 | SAA | Flu/Mel | URD, 9/10 | PBSC | 102.2 | 5 | 7 | 78 | 70* | 70 | 990 | 4274* | 4274 | 244 | 288* | 288 |
| Patient 11 | SAA | Alem/Flu/Mel | URD, 9/10 | T-depleted PBSC | 102.6 | 8 | 8 | 56 | 45* | 45 | 853 | 5400* | 5400 | 143 | 149* | 149 |
| Patient 12 | SAA | Alem/Flu/Mel | URD, 9/10 | PBSC | 103.3 | 5 | 9 | 233 | 276* | 276 | 4500 | 6816* | 6816 | 138 | 182* | 182 |
| Patient 13 | SAA | Cy/ATG | Sibling, 10/10 | Bone Marrow | 101.8 | 14 | 16 | 72 | <31 | 71 | 719 | 581 | 586 | 251 | 322 | 434 |
| Patient 14 | SAA | Alem/Flu/Mel | URD, 10/10 | T-depleted PBSC | 103.3 | 5 | 5 | <31 | 39* | 39 | 736 | 4560* | 4560 | 162 | 134* | 134 |
| Patient 15 | SAA | Alem/Flu/Mel | Mother, 10/10 | T-depleted PBSC | 104.7 | 4 | 6 | 616 | 59* | 59 | 4816 | 5288* | 5288 | 171 | 105* | 105 |
| Graft rejection | ||||||||||||||||
| Patient 16 | SAA | Alem/Flu/Mel | URD, 9/10 | T-depleted PBSC | 105.6 | 48 | 48 | <31 | 64 | 563 | 545 | 4364 | 5362 | 153 | 167 | 336 |
| Patient 17 | SAA | Alem/Flu/Mel | URD, 9/10 | T-depleted PBSC | 103.3 | 13 | 13 | 36 | 48 | 530 | 3179 | 5240 | 15 087 | 125 | 174 | 273 |
| Patient 18 | SAA | Alem/Flu/Mel | URD, 10/10 | T-depleted PBSC | 104.2 | 7 | 11 | 74 | 337 | 5772 | 466 | 8702 | 2862 | 141 | 184 | 181 |
| Patient 19 | SAA | Alem/Flu/Mel | Mother, 7/10 | T-depleted PBSC | 108.5 | 11 | 10 | <31 | 41 | 6106 | 2613 | 8176 | 16 893 | 68 | 141 | 1070 |
| Patient 20 | FA | Bu/Cy/Flu/ATG | Mother, 6/10 | T-depleted PBSC | 106.9 | 16 | 19 | 52 | 199 | 9760 | 1743 | 11 494 | 10 683 | 81 | 103 | 657 |
| Patient 21 | FA | Bu/Cy/Flu/ATG | URD, 9/10 | T-depleted Bone Marrow | 108.1 | 24 | 19 | 80 | 94 | 367 | 1093 | 3591 | 8811 | 105 | 149 | 244 |
| Patient 22 | GUCFS | Bu/Cy/Flu/ATG | Father, 5/10 | T-depleted PBSC | 104.9 | 12 | 13 | 36 | 98 | 1744 | 1298 | 4104 | 8295 | 59 | 60 | 299 |
CXCL9 (P = .001), BAFF (P = .002), and sC5b-9 (P = .03) were significantly higher in patients with GR at time of fever compared with febrile controls. Fevers in patients with GR were also significantly higher (P = .002) and occurred significantly later (P = .001) after HCT compared with febrile controls.
Alem, alemtuzumab; ATG, antithymocyte globulin; Bu, busulfan; Cy, cyclophosphamide; Flu, fludarabine; GUCFS, genetically undefined chromosomal fragility syndrome; Mel, melphalan; N/A, sample was not available for that patient; RR, reference range; URD, unrelated donor.
Fever sample occurred at the same time point as the day 7 sample and they are therefore the same.
CXCL9
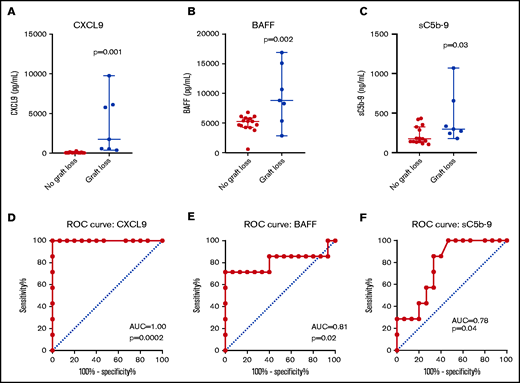
CXCL9 values (Figure 1) were significantly higher in patients with GR at the time of fever compared with febrile controls (median, 1744 vs 45 pg/mL; P = .001). ROC curve analysis of CXCL9 levels obtained at time of fever identified an area under the curve (AUC) of 1 (P = .0002). A cutoff value of 321.5 pg/mL was predictive of GR with sensitivity of 100% and specificity of 100%. The highest recorded CXCL9 value (9760 pg/mL) occurred in patient 20 during fever and GR. CXCL9 levels were higher at day +7 after stem cell infusion in patients who rejected their graft; however, this did not meet statistical significance (median, 94 vs 44 pg/mL; P = .12). There was no difference between baseline CXCL9 values in patients who rejected their graft compared with febrile HCT controls (median, 36 vs 56 pg/mL; P = .3).
CXCL9, BAFF, and sC5b-9 are higher in patients with graft rejection compared with febrile HCT controls. CXCL9, BAFF, and sC5b-9 levels at the time of fever are shown. Fever samples were obtained at the closest available time point to when fevers rose above 102.2°F (39°C) or at the closest time point to Tmax if less than 102.2°F. The median value with 95% confidence interval is marked (A-E). ROC curves are shown for each marker at time of fever (F).
CXCL9, BAFF, and sC5b-9 are higher in patients with graft rejection compared with febrile HCT controls. CXCL9, BAFF, and sC5b-9 levels at the time of fever are shown. Fever samples were obtained at the closest available time point to when fevers rose above 102.2°F (39°C) or at the closest time point to Tmax if less than 102.2°F. The median value with 95% confidence interval is marked (A-E). ROC curves are shown for each marker at time of fever (F).
BAFF
BAFF values (Figure 1) were also significantly higher in patients with GR at the time of fever compared with febrile controls (median, 8811 vs 5288 pg/mL; P = .002). ROC curve analysis identified an AUC of 0.81 (P = .02). A cutoff value of 7556 pg/mL was predictive of GR with a sensitivity of 71.4% and specificity of 100%. The highest recorded BAFF value (16 893 pg/mL) occurred in patient 19 during fever and GR. BAFF levels at day +7 after stem cell infusion were higher in patients who rejected their graft, but this did not meet statistical significance (median, 5240 vs 3420 pg/mL; P = .07). There was no difference between baseline BAFF values in patients who rejected their graft compared with febrile HCT controls (median, 1298 vs 1312 pg/mL; P = .79)
Complement
Soluble C5b-9 levels (a marker of terminal complement activation; Figure 1) were higher at the time of fever in patients with GR compared with febrile controls (median, 299 vs 176ng/mL; P = .03). ROC curve analysis identified an AUC of 0.78 (P = .04). A cutoff value of 213 ng/mL was predictive of GR with a sensitivity of 85.7% and specificity of 66.7%. The highest recorded sC5b-9 value (1070 ng/mL) occurred in patient 19 during fever and GR. Day +7 sC5b-9 levels were not different between the cohorts; however, baseline sC5b-9 levels were significantly lower in patients who rejected their graft compared with febrile HCT controls (median, 105 vs 143 ng/mL; P = .03). C3a (median, 124.4 vs 110 ng/mL; P = .54) and C5a (median, 21.1 vs 18.4 ng/mL; P = .39) levels at the time of fever were not statistically different between the GR cohort and those who kept their grafts. There were no significant differences between C3a and C5a levels at day +7 and baseline.
Fever kinetics after HCT
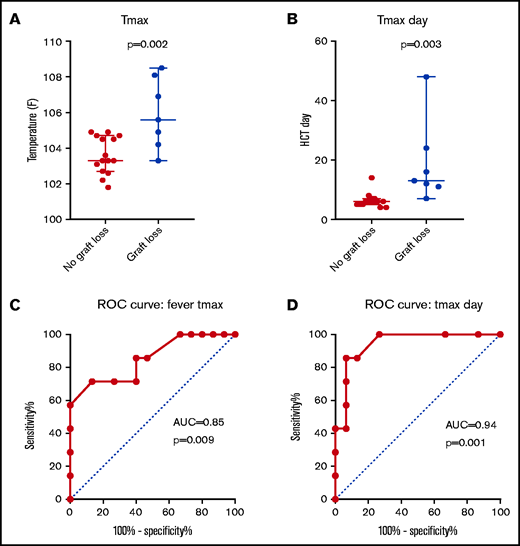
Patients with GR had significantly higher Tmax (Figure 2) than febrile controls (median, 105.6°F vs 103.3°F; P = .002). ROC curve analysis identified an AUC of 0.85 (P = .009). A cutoff value of 104.8°F was predictive of GR with a sensitivity of 71.5% and specificity of 88.7%. The 2 highest recorded Tmax (108.5°F and 108.1°F) occurred in patients 19 and 21 at 11 and 24 days after HCT, respectively. Both these patients rejected their graft at the time of those fevers. Tmax also occurred significantly later in patients with GR compared with febrile controls (median, day 13 vs day 6; P = .003). ROC curve analysis of Tmax timing after HCT identified an AUC of 0.94 (P = .001). A cutoff value of 9 days was predictive of GR with sensitivity of 85.7% and specificity of 93.3%. Although Tmax classification was done retrospectively, these findings show that patients with GR have higher and later fever spikes compared with febrile HCT controls.
Tmax is higher and occurs later in patients with graft rejection compared with febrile patients who underwent HCT without graft rejection. Tmax magnitude (A) and timing relative to HCT (B) are shown with corresponding ROC curves (C-D).
Tmax is higher and occurs later in patients with graft rejection compared with febrile patients who underwent HCT without graft rejection. Tmax magnitude (A) and timing relative to HCT (B) are shown with corresponding ROC curves (C-D).
Interferon-γ and C5 inhibition in patients with BMF with a prior history of GR
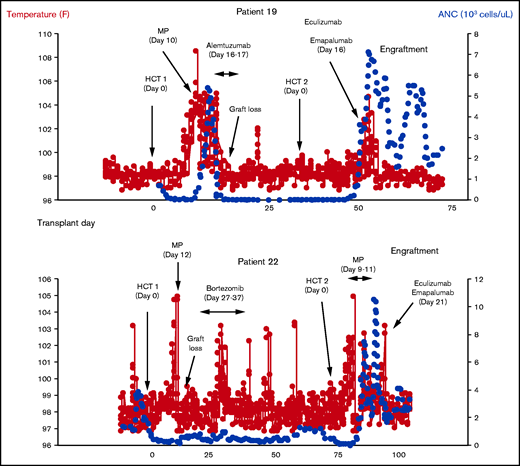
Two patients with GR with high spiking fevers and graft loss during their first HCT underwent a second HCT during our study and experienced high fevers again after HCT (Figure 3). Clinical and laboratory data for these patients are detailed in Table 3. Patient 19 had a diagnosis of SAA and received a PBSC graft from an 8/10 unrelated donor after fludarabine conditioning for his second HCT. Patient 19 first became acutely febrile 14 days after his second HCT. Engraftment studies sent on day +14 showed 98% donor chimerism. There were no other attributable causes of fever identified. He continued to spike fevers and reached a Tmax of 104.7°F on day +15. CXCL9 was elevated at 149 pg/mL (normal, <122 pg/mL). sC5b-9 was normal (92 ng/mL; normal, <244 ng/L) on day +15 but nearly double his pre-HCT baseline for his second HCT (57 ng/mL). Given the prior history of graft rejection with high-spiking fevers in this patient, he received emapalumab (500 mg) and eculizumab (900 mg) on day +16. For dosing reference, the patient was 12 years old and weighed 44.3 kg at the time of medication administration. Despite receiving a terminal complement inhibitor, his sC5b-9 level increased to 156 ng/mL the day after eculizumab, indicative of upregulated terminal complement activation. Engraftment studies sent on day +17 showed 97% donor chimerism, and fevers resolved by day +18. He has achieved transfusion independence, immune reconstitution, and has continued to have stable mixed chimerism predominantly ≥95% donor at a follow-up time of 9 months.
Clinical outcomes in 2 patients treated with eculizumab and emapalumab for graft rejection. Two patients in our cohort (19 and 22) experienced high fevers again after their second HCT. Based on our data showing elevated interferon and terminal compliment activation in graft rejection with high fevers, each of these patients received 1-time doses of eculizumab and emapalumab at the time of fever and both maintained engraftment. ANC, absolute neutrophil count; MP, methylprednisolone.
Clinical outcomes in 2 patients treated with eculizumab and emapalumab for graft rejection. Two patients in our cohort (19 and 22) experienced high fevers again after their second HCT. Based on our data showing elevated interferon and terminal compliment activation in graft rejection with high fevers, each of these patients received 1-time doses of eculizumab and emapalumab at the time of fever and both maintained engraftment. ANC, absolute neutrophil count; MP, methylprednisolone.
Clinical and laboratory data for patients treated with emapalumab and eculizumab for graft rejection
| . | Patient 19 . | Patient 22 . |
|---|---|---|
| Age, y | 12 | 10 |
| Sex | Male | Female |
| Diagnosis | Severe aplastic anemia | Genetically undefined chromosomal fragility syndrome |
| HCT 2 | ||
| Conditioning | Fludarabine | Alemtuzumab/fludarabine/melphalan |
| Graft | PBSC | PBSC |
| Donor | 8/10, URD | 8/10, URD |
| GVHD PPx | Ex vivo T-cell depletion | Ex vivo T-cell depletion |
| Cell dose (CD34+ cells × 106/kg) | 13.6 | 37.6 |
| Days from first HCT | 38 | 72 |
| Fevers | ||
| Tmax (°F) | 104.7 | 104.9, 103.1* |
| Tmax day | Day +15 | Day +9, day +21 Patient 22 was afebrile for 3 d in between fever episodes. Early fevers were diagnosed as engraftment syndrome and the later fevers attributed to rejection. |
| Interferon γ (CXCL9, pg/mL) | ||
| Day emapalumab given | Day +16 | Day +21 |
| CXCL9 baseline | <31 | 36 |
| CXCL9 pre-emapalumab | 149 (day +15) | 271 (day +21) |
| CXCL9 post-emapalumab | 49 (day +18) | 226 (day +22), 76 (day +24) |
| Subsequent CXCL9 studies | All additional CXCL9 levels were normal with a maximum of value 40. The last recorded levels was <31 on day +91. | CXCL9 was remeasured on day +35 and was <31. No additional CXCL9 levels were measured for graft rejection surveillance. |
| Terminal complement (sC5b9, ng/mL) | ||
| Day eculizumab given | Day +16 | Day +21 |
| sC5b-9 baseline | 57 | 59 |
| sC5b-9 pre-eculizumab | 92 (day +15) | 234 (day +21) |
| sC5b-9 post eculizumab | 156 (day +17) | 223 (day +35), 152 (day +38) |
| Subsequent sC5b-9 studies | Repeat levels on day +20, day +24, and day +26 were 122, 150, and 139, respectively. No additional increases were observed, and the last recorded level was 104 on day +98. | sC5b-9 levels were measured for graft rejection surveillance through day +48 and the last recorded value was 187. |
| Donor chimerism | ||
| Before eculizumab/emapalumab | 98% (day +14) | 93% (day +19) |
| After eculizumab/emapalumab | 97% (day +17) | 96% (day +23), 97% (day +25) |
| Long term | Donor chimerism dropped to 91% on day +24 but recovered and largely remained ≥95%. The most recent donor chimerism was 95% on day +371. | Donor chimerism remained ≥98% and the majority of measurements were 100% including on day +95, shortly before death. |
| . | Patient 19 . | Patient 22 . |
|---|---|---|
| Age, y | 12 | 10 |
| Sex | Male | Female |
| Diagnosis | Severe aplastic anemia | Genetically undefined chromosomal fragility syndrome |
| HCT 2 | ||
| Conditioning | Fludarabine | Alemtuzumab/fludarabine/melphalan |
| Graft | PBSC | PBSC |
| Donor | 8/10, URD | 8/10, URD |
| GVHD PPx | Ex vivo T-cell depletion | Ex vivo T-cell depletion |
| Cell dose (CD34+ cells × 106/kg) | 13.6 | 37.6 |
| Days from first HCT | 38 | 72 |
| Fevers | ||
| Tmax (°F) | 104.7 | 104.9, 103.1* |
| Tmax day | Day +15 | Day +9, day +21 Patient 22 was afebrile for 3 d in between fever episodes. Early fevers were diagnosed as engraftment syndrome and the later fevers attributed to rejection. |
| Interferon γ (CXCL9, pg/mL) | ||
| Day emapalumab given | Day +16 | Day +21 |
| CXCL9 baseline | <31 | 36 |
| CXCL9 pre-emapalumab | 149 (day +15) | 271 (day +21) |
| CXCL9 post-emapalumab | 49 (day +18) | 226 (day +22), 76 (day +24) |
| Subsequent CXCL9 studies | All additional CXCL9 levels were normal with a maximum of value 40. The last recorded levels was <31 on day +91. | CXCL9 was remeasured on day +35 and was <31. No additional CXCL9 levels were measured for graft rejection surveillance. |
| Terminal complement (sC5b9, ng/mL) | ||
| Day eculizumab given | Day +16 | Day +21 |
| sC5b-9 baseline | 57 | 59 |
| sC5b-9 pre-eculizumab | 92 (day +15) | 234 (day +21) |
| sC5b-9 post eculizumab | 156 (day +17) | 223 (day +35), 152 (day +38) |
| Subsequent sC5b-9 studies | Repeat levels on day +20, day +24, and day +26 were 122, 150, and 139, respectively. No additional increases were observed, and the last recorded level was 104 on day +98. | sC5b-9 levels were measured for graft rejection surveillance through day +48 and the last recorded value was 187. |
| Donor chimerism | ||
| Before eculizumab/emapalumab | 98% (day +14) | 93% (day +19) |
| After eculizumab/emapalumab | 97% (day +17) | 96% (day +23), 97% (day +25) |
| Long term | Donor chimerism dropped to 91% on day +24 but recovered and largely remained ≥95%. The most recent donor chimerism was 95% on day +371. | Donor chimerism remained ≥98% and the majority of measurements were 100% including on day +95, shortly before death. |
GVHD, graft-versus-host disease; PPx, prophylaxis; URD, unrelated donor after second HCT.
Patient 22 had a genetically undefined chromosomal fragility syndrome and a history of GR (primary graft loss) during her first transplant. This patient received a PBSC graft from an 8/10 unrelated donor after alemtuzumab, fludarabine, and melphalan conditioning for her second HCT. Fevers initially began 6 days after her second HCT, and the patient received 3 days of methylprednisolone for engraftment syndrome treatment. Engraftment studies were performed on day +12 and showed 100% donor chimerism. Fever frequency and height decreased and completely resolved by day +16. Repeat chimerism studies were sent on day +19 and showed 93% donor chimerism. The patient became febrile again on day +20 and reached a temperature of 103.1°F on day +21. There were no other attribute causes of fever identified. CXCL9 and sC5b-9 levels were obtained clinically and were 271 and 234 ng/mL on day +21, respectively. Although the sC5b-9 level was technically within normal range (<244 ng/mL), it was nearly double the pre-HCT baseline level for this patient’s second HCT (128 ng/mL) and more than 4 times higher than her baseline before her first HCT (59 ng/mL). This suggested an increase in terminal complement activation in addition to an elevated CXCL9. Given the prior history of graft rejection with high fevers, the patient received emapalumab (150 mg) and eculizumab (600 mg) on day +21. For dosing reference, the patient was 10 years old and weighed 17.2 kg at the time of medication administration. Repeat engraftment studies on day +25 showed 97% donor chimerism. Fevers resolved on day +22. Donor chimerism remained ≥98% and was mostly 100%. The patient unfortunately died on day +127 from respiratory failure caused by sepsis (viral and bacterial) and human herpesvirus 6 encephalitis.
No adverse events were directly attributed to emapalumab or eculizumab. We do acknowledge that patient 22 died of infection on day +127 after receiving immunosuppressive therapy with eculizumab and emapalumab on day +21. All patients who receive eculizumab at our center receive antibiotic prophylaxis, and prior work by Jodele et al15 has shown that the incidence of bloodstream infections is not different between patients who received eculizumab and those who did not. We therefore feel this infection was unlikely from eculizumab therapy; however, it is important to consider the potential infection risk of combination interferon and terminal complement blockade in these patients.
Discussion
This study aimed to investigate markers and mechanisms of GR in patients with BMF undergoing HCT by studying potentially targetable inflammatory markers that might mediate GR. Our study has demonstrated potential clinical and biological markers of GR that might in the future be used to avert GR, and we report 2 cases in whom likely incipient GR was averted by a simple strategy of infusion of medications targeting potential mediators of GR.
T cells are traditionally blamed for GR after HCT. T-lymphocytes are potent interferon γ producers, and the clinical availability of interferon γ blockade has created a heightened interest in understanding the exact role of T cells and interferon γ in GR. Among the biomarkers and inflammatory pathways included in our study, interferon γ-mediated inflammation had the strongest published association with GR after HCT. Merli et al9 reported higher interferon γ and CXCL9 levels in patients with graft failure as early as 3 days after HCT and as far out as day +14.9 CXCL9, a downstream marker of interferon production, had the strongest association with graft failure in that same study.9,16 Our work similarly shows that CXCL9 is a strong predictor of GR when measured at the time of fever. Although we did not detect a statistical difference between CXCL9 levels at day +7 after stem cell infusion, CXCL9 was higher in patients with GR at day +7. The ROC curve generated from these data shows perhaps implausibly good sensitivity and specificity of elevated CXCL9 levels, and these data may lose some strength as additional cases are studies, but the current data are compelling for the need for larger studies. The possibility of CXCL9 levels indicating the start of GR is of particular interest, as such a finding if confirmed would be of great clinical value but requires additional study.
The exact role of humoral immunity in GR after HCT is ill defined, although donor-specific antibodies have a clear pathogenesis in HLA-mismatched and haploidentical HCT.17,18 Antibody-mediated rejection is more commonly studied in solid organ transplant, where multiple studies have described BAFF as a biomarker for antibody-mediated rejection.11,19 BAFF is responsible for regulating the proliferation and survival of B cells (and to some extent plasma cells).20 In a study of 68 patients who received kidney transplant by Pongpirul et al,11 half of patients with high BAFF levels (>573 pg/mL) at day 7 developed antibody-mediated rejection compared with just 3.7% of patients with low BAFF levels. Our study is the first to investigate BAFF in patients with GR after HCT. Patients with GR had significantly higher BAFF levels at the time of fever compared with febrile HCT controls, which suggests BAFF may be a useful biomarker for GR in patients who underwent HCT as well. Mechanistically, this suggests that GR promotes a significant shift toward a pro–B-cell state that is even higher than what occurs in a normal post-HCT environment or in a febrile state. At this point we cannot definitively determine whether the observed B-cell stimulation is merely the downstream result of surging interferon γ or an important instigator of GR itself. However, given the strength of the observed association, further investigation to B cell–mediated mechanisms of GR and the role of BAFF inhibition are needed.
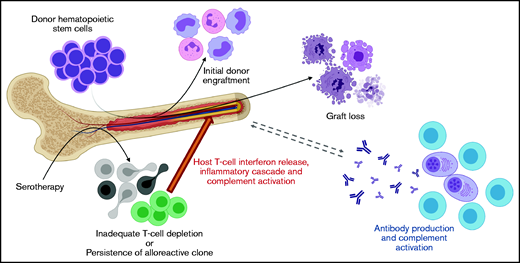
The contribution of complement activation to GR after HCT is also understudied but may be an important connection between cellular and humoral pathways of GR. Our data show that terminal complement activation (sC5b-9) is higher in patients with GR compared with febrile controls. Alternative pathway complement activation is well described in the pathogenesis of transplant-associated thrombotic microangiopathy, and previous work by our group has shown that complement and interferon work in a stimulatory loop in this disease.21 Complement activation is also well studied in solid organ transplantation, and C4d deposition in solid organ allograft biopsies is an established biomarker of antibody-mediated rejection.10 Notably, multiple prior studies have demonstrated that antibody-mediated complement activation increases T-cell alloreactivity, interferon production, and HLA expression.10,22-24 This established connection between B cell/plasma cell, interferon, and complement-mediated inflammation in solid organ rejection is very consistent with the findings in the current study. We therefore hypothesize that the persistence of alloreactive host T cells generates a heightened interferon-driven inflammatory response and ignites a feedback loop of donor-specific antibodies and membrane attack complex (sC5b-9) formation (Figure 4).
Proposed mechanism of graft rejection in patients with BMF syndromes.
All patients with GR in our study experienced fevers during GR, consistent with previously published observations.9 However, our study is the first to evaluate detailed fever kinetics in these patients. We found that maximum temperatures were higher and occurred later in patients with GR compared with those who kept their graft. These findings have immediate clinical applicability because temperature measurements are easy, inexpensive, and noninvasive studies. The differential diagnosis for fevers in the immediate posttransplant period includes engraftment syndrome, infection, and GR. Although engraftment syndrome and infection are more common than GR, unexplained high-spiking fevers in the second week or later after HCT should prompt diligent monitoring for GR. This is particularly true for patients who received mismatched grafts, reduced intensity conditioning, T cell–depleted grafts, or have other known risk factors for GR. Our data suggest that temperature monitoring may serve as a sensitive and specific screening tool for identifying patients with potential GR who would benefit from monitoring of potential biomarkers and may also guide the decision to intervene with immunomodulating therapies.
We report successful intervention in 2 cases with potential incipient GR using agents targeting interferon and complement. These add to the current literature on the use of interferon blockade in a small number of patients with GR and HLH.9,25 It is important to also note the intricacies of using immune modulating therapies at the time of stem cell engraftment, particularly drugs that affect T-cell function. It is possible that interferon-blocking therapies adversely affect engraftment in some patients by inhibiting donor T-cell activity. Although the current published data are limited, the use of emapalumab for the prevention of GR has been described in 4 patients with HLH.9,25 Three of these patients successfully engrafted after emapalumab therapy. Interestingly, the patient who did not engraft did not achieve adequate interferon γ neutralization despite emapalumab therapy, which favors the need to thoroughly block interferon γ during GR.9 No large study has described the effect of eculizumab on T-cell function during engraftment. However, a prior study by Rubinstein et al26 showed that eculizumab therapy did not impair viral specific T-cell function in patients who underwent HCT. Furthermore, multiple studies have shown no adverse effect of pre-HCT eculizumab therapy on engraftment in patients with paroxysmal nocturnal hemoglobinuria.27,28 Mei et al29 published their experience with post-HCT eculizumab prophylaxis in 8 patients with paroxysmal nocturnal hemoglobinuria, and Cooper et al describe 2 additional patients.27 Nine of 10 patients engrafted without issue, whereas 1 patient experienced graft failure. The data presented in our current study must of course be regarded as preliminary but provide support for a systematic and likely multi-center study of optimal intervention strategies.
Our study has strengths and weaknesses. Our study benefited from availability of routine prospectively stored research blood samples for biomarker testing. Moreover, our patients with potential GR who received intervention benefited from availability of rapid turnaround testing in a CLIA-certified laboratory. It is important to recognize, however, that that any conclusions made from this study are limited by the small sample size of patients. However, we are also encouraged that, despite this small sample size, multiple statistically significant findings were identified. The applicability of these findings is also limited by the narrow range of diagnoses included in the study, and future studies must include patients with a broad array of diagnoses, including malignancies. It is conceivable that rejection mechanisms could vary in these distinct populations, which makes this an essential next step. Although our findings are consistent with previously published mechanisms of graft rejection in solid organ transplant, the extension of those findings to HCT rejection must be done with caution and additional studies are needed in HCT cohorts.
In conclusion, GR after HCT is characterized by late, high-spiking fevers and a heightened inflammatory response that supersedes what is seen in febrile patients who underwent HCT and preserve their graft. These data suggest fever monitoring is a simple yet highly effective screening tool for the identification of GR. Furthermore, our results suggest a potential role for terminal complement, BAFF, and interferon γ inhibition for the acute treatment and prevention of GR after HCT for BMF.
Acknowledgments
The authors thank Kristi Smiley, the Diagnostic Immunology Laboratory at Cincinnati Children’s Hospital Center, Mary Block, and the Hemostasis Thrombosis Laboratory for valuable contributions to this work. All animated figures in this manuscript were created with BioRender software.
Authorship
Contribution: A.S. designed the study, collected the data, analyzed the data, and wrote the manuscript; K.C.M. collected clinical data, edited the manuscript, and provided BMF syndrome expertise; J.J.B. designed the study, selected inflammatory markers and provided immunologic expertise; A.D. collected, organized, and provided clinical samples for laboratory studies; A.L. performed and reviewed statistical analyses; A.T.-C. collected clinical data and provided pharmacologic expertise; S.M.D. oversaw the study, wrote the manuscript, and provided BMF syndrome expertise; and S.J. designed the study, oversaw the study, analyzed the data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: S.J. holds US Patent US 10 815,296 B2, has received research support from Alexion Pharmaceuticals, and received travel support from Omeros. The remaining authors declare no competing financial interests.
Correspondence: Anthony Sabulski, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: anthony.sabulski@cchmc.org.
References
Author notes
Requests for data sharing may be submitted to Anthony Sabulski (anthony.sabulski@cchmc.org).