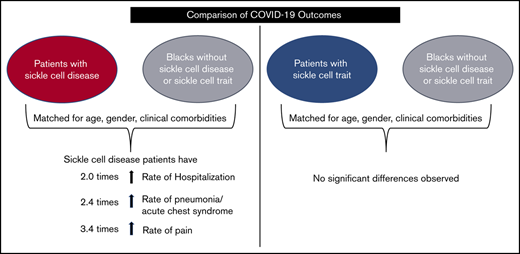
Key Points
Individuals with SCD are more likely to be hospitalized, develop pneumonia and pain due to COVID-19 than Black individuals without SCD/SCT.
There are no significant differences in COVID-19 outcomes between individuals with SCT and Black individuals without SCD/SCT.
Abstract
In the United States, COVID-19 has disproportionately affected Black persons. Sickle cell disease (SCD) and sickle cell trait (SCT) are genetic conditions that occur predominantly among Black individuals. It is unknown if individuals with SCD/SCT are at higher risk of severe COVID-19 illness compared with Black individuals who do not have SCD/SCT. The objective of our study was to compare COVID-19 outcomes, including the disease manifestations, hospitalization, and death, among individuals with SCD/SCT vs Black individuals who do not have SCD/SCT. We leveraged electronic health record data from a multisite research network to identify Black patients with COVID-19 who have SCD/SCT and those who do not have SCD/SCT. During the study period of 20 January 2020 to 20 September 2020, there were 312 patients with COVID-19 and SCD and 449 patients with COVID-19 and SCT. There were 45 517 Black persons who were diagnosed with COVID-19 but who did not have SCD/SCT. After 1:1 propensity score matching (based on age, sex, and other preexisting comorbidities), patients with COVID-19 and SCD remained at a higher risk of hospitalization (relative risk [RR], 2.0; 95% CI, 1.5-2.7) and development of pneumonia (RR, 2.4; 95% CI, 1.6-3.4) and pain (RR, 3.4; 95% CI, 2.5-4.8) compared with Black persons without SCD/SCT. The case fatality rates for those with SCD compared with Black persons without SCD/SCT were not significantly different. There also were no significant differences in COVID-19 outcomes between individuals with SCT and Black persons without SCD/SCT within the matched cohorts.
Introduction
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 was first detected/reported in the United States in January 2020. Since then, this pandemic has been a major public health crisis in the country. By August 2020, there were >4 million COVID-19 cases and >147 000 deaths reported in the United States due to this disease.1 Although previously published studies report mixed results regarding the independent effect of race on COVID-19–related outcomes,2-4 it is undebatable that Black persons in the United States have been disproportionally affected by this evolving pandemic. The Centers for Disease Control and Prevention reported that as of 5 August 2020, Black persons in the United States have experienced a disproportionately high percentage of COVID-19 deaths (22.2%) relative to their percentage in the US population (13%).5
Sickle cell disease and sickle cell trait are prevalent predominately among Black individuals in the United States. Sickle cell disease is caused by the homozygous inheritance of a mutated β-globin chain gene and occurs in almost 1 of every 400 Black individuals in the United States. Sickle cell trait is the result of heterozygous inheritance of a mutated β-globin chain gene and is thus more common, occurring in ∼1 of every 13 Black individuals in the United States. Growing evidence suggests that individuals with preexisting conditions such as hypertension, cardiovascular disease, diabetes, acute organ dysfunction, obesity, or cancer are at a higher risk of severe illness and death due to COVID-19.4,6-9 However, there is limited knowledge regarding the risk of severe COVID-19 disease among individuals with sickle cell disease and sickle cell trait compared with those who do not have sickle cell disease or trait.
In the current study, electronic health record data from a network of health care organizations were used to compare COVID-19 outcomes, including hospitalization, manifestations, and death, among individuals with sickle cell disease and sickle cell trait vs Black individuals who do not have sickle cell disease/trait. We hypothesized that individuals with sickle cell disease are at a higher risk of worse COVID-19 outcomes compared with Black patients without sickle cell disease/trait. We also hypothesized that the rate of COVID-19 outcomes in those with sickle cell trait is not significantly different from that of Black patients without sickle cell disease/trait.
Methods
Data source: TriNetX
TriNetX is a research network that optimizes clinical research and enables discoveries through the generation of real-world evidence. It achieves this by providing real-time access to longitudinal clinical data from the electronic health record of millions of individuals from >30 health care organizations across the United States. This research network has invested in data infrastructure and maintains high-quality, deidentified patient-level data from the electronic health records, which is refreshed on a regular basis (weekly or monthly, depending on the site). The network includes 29 958 individuals who have a diagnosis of sickle cell disease (and no trait), 49 919 individuals who have sickle cell trait (and no diagnosis codes for sickle cell disease), and 7 945 172 individuals who do not have sickle cell disease or trait. The network has a platform compliant with the Health Insurance Portability and Accountability Act of 1996 with built-for-purpose user interface and analytics capabilities.
Study design and patient population
We designed a retrospective cohort study to compare COVID-19 outcomes between individuals with sickle cell disease/trait and Black patients without sickle cell disease/trait. The TriNetX platform was used to identify patients with COVID-19 during the time period of 20 January 2020 to 20 September 2020, within 3 distinct patient cohorts: (1) COVID-19 in patients with sickle cell disease; (2) COVID-19 in patients with sickle cell trait; and (3) COVID-19 in Black patients without sickle cell disease/trait. The third cohort of COVID-19 Black patients without sickle cell disease/trait was used as a comparison group.
To identify COVID-19 cases, we used the International Classification of Diseases (ICD) diagnosis codes of U07.1, U07.2, B34.2, B97.29, and J12.81, or a positive COVID-19 indication on a nucleic acid amplification with probe-based detection test, present any time after 20 January 2020 (this is when the first COVID-19 case was detected in the United States), in patients’ electronic health records data. To be specific for COVID-19, any patient with a nonspecific code 079.29, which indicates other specified viral infection, was excluded. The cohort of COVID-19 in sickle cell disease included patients who had a diagnosis of COVID-19 using the aforementioned method and the presence of sickle cell disease ICD diagnosis codes (D57.0*, D57.1*, D57.2*, and D57.4*) but no sickle cell trait ICD diagnosis code (D57.3) any time before the COVID-19 diagnosis. The cohort of COVID-19 in sickle cell trait included patients who had: (1) a diagnosis of COVID-19 infection; (2) the presence of the sickle cell trait ICD diagnosis code (D57.3); and (3) no sickle cell disease ICD diagnosis codes any time before the COVID-19 diagnosis.
White patients were excluded from the sickle cell disease and sickle cell trait cohorts to be specific to the population of interest. The comparator cohort included Black patients with COVID-19 excluding patients who had a diagnosis of sickle cell disease or trait any time before their COVID-19 diagnosis.
Outcomes
Hospitalization.
A patient was considered to be hospitalized due to COVID-19 if his or her medical record indicated an inpatient visit or had an encounter with a linked procedure code for inpatient services, consultations, or critical care within 2 weeks and 30 days of the COVID-19 diagnosis.
COVID-19 manifestations.
ICD-10 codes were used to identify symptoms of cough/fever (ie, R05.*, R50.*), pneumonia or acute chest syndrome (ie, J12.*, J16.*, J18.*, D57.01, D57.811, D57.411), pain (ie, R07.*, R10.*, R52.*, R27, M25.5, M54.*, M79.6, M79.1, D57.00, D57.219, D57.419, D57.819), shortness of breath or hypoxia (ie, R06.00. R06.02, R09.02), and venous thromboembolism and pulmonary embolism (ie, I26.*, I81.*, I82.*). Need for mechanical ventilation (Current Procedural Code 94002 -94005) or an ICD-10 diagnosis code for acute respiratory distress (ie, J80.*, J96.*, R06.03) or respiratory failure were considered to be acute respiratory distress. We limited the data used to identify these manifestations to within 2 weeks from diagnosis of COVID-19 to ensure that they were specific to the infection.
Mortality.
Mortality was determined based on death documented within 2 weeks and 30 days of COVID-19 diagnosis. The extended time frame of 30 days was added to capture deaths occurring due to the lingering course of COVID-19 illness.
Analyses
We used descriptive statistics to report patient demographic characteristics, including mean ± standard deviation age, proportion of female patients, and prevalence of preexisting comorbidities for each of the 3 cohorts: (1) COVID-19 in sickle cell disease; (2) COVID-19 in sickle cell trait; and (3) COVID-19 in Black patients without sickle cell disease/trait. Preexisting clinical comorbidities were determined based on ICD-10 codes present in patients’ electronic health record any time before the COVID-19 diagnosis. We compared the demographic and preexisting comorbidities in COVID-19 patients with sickle cell disease/trait vs COVID-19 in Black patients who do not have sickle cell disease/trait, using a Student t test or z test as appropriate.
Rates of specific COVID-19–related outcomes were calculated as the percentage of patients in the cohort with the particular outcome. We compared COVID-19 outcomes between individuals with sickle cell disease or trait vs Black patients without sickle cell disease or trait after 1:1 propensity score matching. Propensity scores were calculated based on the characteristics listed in Table 1, and matching was based on the greedy nearest neighbor matching algorithm with a caliper of 0.1 pooled standard deviation. The matching procedure ensures that the comparison groups are similar in age, sex, and distribution of clinical comorbidities so that attributable risk of sickle cell disease and trait can be estimated. Risk ratios and risk differences are reported along with 95% confidence intervals for the respective comparisons. Given multiple outcomes of interest, we considered a more stringent two-sided α of <0.01, based on a z test, to determine statistical significance for differences in outcome rates.
Demographic and clinical characteristics of cohorts of COVID-19 patients in the study
| Characteristic . | ICD code* . | Sickle cell disease, n = 312 (1) . | Sickle cell trait, n = 449 (2) . | Black patients without sickle cell disease or trait, n = 45 517 (3) . | P, (1) vs (3) . | P, (2) vs (3) . |
|---|---|---|---|---|---|---|
| Age, mean ± SD, y | — | 31.4 ± 16.8 | 37.7 ± 18.5 | 45.0 ± 19.7 | <.001 | <.001 |
| Female sex, n (%) | — | 195 (63) | 366 (82) | 26 963 (59) | .24 | <.001 |
| Comorbidities, n (%) | ||||||
| Asthma | J45 | 76 (24) | 132 (29) | 4993 (11) | <.001 | <.001 |
| Hypertension | I10 | 84 (27) | 176 (39) | 14 340 (32) | .08 | <.001 |
| Overweight/obesity | E66 | 57 (18) | 189 (42) | 9692 (21) | .07 | <.001 |
| Diabetes mellitus | E08-E13 | 35 (11) | 95 (21) | 7747 (17) | .0065 | .02 |
| Heart failure | I50 | 28 (9) | 36 (8) | 2881 (6) | .06 | .14 |
| Chronic ischemic heart disease | I25 | 20 (6) | 25 (6) | 2822 (6) | .88 | .58 |
| History of acute failure/chronic kidney disease | N17-N19 | 56 (18) | 53 (12) | 4902 (11) | <.001 | .49 |
| Liver diseases | K70-K77 | 30 (10) | 32 (7) | 2090 (5) | <.001 | .01 |
| Chronic obstructive pulmonary disease | J44 | 16 (5) | 20 (4) | 1811 (4) | .30 | .60 |
| Cerebral infarction | I63 | 24 (8) | 13 (3) | 1322 (3) | <.001 | .97 |
| Pulmonary embolism | I26 | 16 (5) | 18 (4) | 673 (1) | <.001 | <.001 |
| Other venous embolism and thrombosis | I82 | 35 (11) | 17 (4) | 1149 (3) | <.001 | .09 |
| Characteristic . | ICD code* . | Sickle cell disease, n = 312 (1) . | Sickle cell trait, n = 449 (2) . | Black patients without sickle cell disease or trait, n = 45 517 (3) . | P, (1) vs (3) . | P, (2) vs (3) . |
|---|---|---|---|---|---|---|
| Age, mean ± SD, y | — | 31.4 ± 16.8 | 37.7 ± 18.5 | 45.0 ± 19.7 | <.001 | <.001 |
| Female sex, n (%) | — | 195 (63) | 366 (82) | 26 963 (59) | .24 | <.001 |
| Comorbidities, n (%) | ||||||
| Asthma | J45 | 76 (24) | 132 (29) | 4993 (11) | <.001 | <.001 |
| Hypertension | I10 | 84 (27) | 176 (39) | 14 340 (32) | .08 | <.001 |
| Overweight/obesity | E66 | 57 (18) | 189 (42) | 9692 (21) | .07 | <.001 |
| Diabetes mellitus | E08-E13 | 35 (11) | 95 (21) | 7747 (17) | .0065 | .02 |
| Heart failure | I50 | 28 (9) | 36 (8) | 2881 (6) | .06 | .14 |
| Chronic ischemic heart disease | I25 | 20 (6) | 25 (6) | 2822 (6) | .88 | .58 |
| History of acute failure/chronic kidney disease | N17-N19 | 56 (18) | 53 (12) | 4902 (11) | <.001 | .49 |
| Liver diseases | K70-K77 | 30 (10) | 32 (7) | 2090 (5) | <.001 | .01 |
| Chronic obstructive pulmonary disease | J44 | 16 (5) | 20 (4) | 1811 (4) | .30 | .60 |
| Cerebral infarction | I63 | 24 (8) | 13 (3) | 1322 (3) | <.001 | .97 |
| Pulmonary embolism | I26 | 16 (5) | 18 (4) | 673 (1) | <.001 | <.001 |
| Other venous embolism and thrombosis | I82 | 35 (11) | 17 (4) | 1149 (3) | <.001 | .09 |
SD, standard deviation.
Platform uses ICD-10 codes. These are mapped to ICD-9 codes in the backend of the program.
Sample size justification
With a sample of 200 per independent group, we have more than 80% power (α = 0.01) to detect a 15% difference in outcomes, assuming 35% of patients with sickle cell disease/trait experience a particular outcome.
Sensitivity analyses
Because sickle cell trait is known to be underrecognized in the United States, we conducted sensitivity analyses to determine the robustness of our findings by examining the extent to which our results are affected by the misclassification of individuals with sickle cell trait. We systematically varied the size of the cohort of COVID-19 patients with sickle cell trait and conducted the same analyses on the data to determine the impact on outcomes. To do so, we randomly selected a subgroup of the COVID-19 Black patients who were considered to not have sickle cell disease or trait such that the prevalence of venous thromboembolism and pulmonary embolism (combined) before COVID-19 is twice that in the group of Black patients without sickle cell disease and trait. This subgroup was then assumed to have sickle cell trait. This was done based on the premise that previous studies showed no significant differences in comorbidities between those with sickle cell trait and those who do not have sickle cell trait, except for venous thromboembolism/pulmonary embolism.10-12 We varied the size of the subgroup from 1000 to 3000. All outcomes were compared between the enhanced sickle cell trait group and Black patients without sickle cell disease or trait using the methodology described in "Analyses."
Results
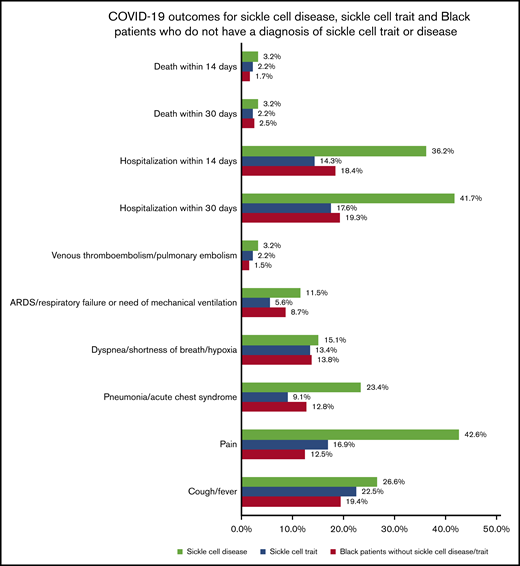
In the database, there were 312 cases of COVID-19 in individuals with sickle cell disease and 449 cases of COVID-19 in individuals with sickle cell trait during the time frame 20 January 2020 to 20 September 2020. Our comparator group included 45 517 Black patients who were diagnosed with COVID-19 in the same time frame but did not have sickle cell disease/trait. The demographic and clinical characteristics for each cohort are shown in Table 1. Compared with Black individuals without sickle cell disease/trait, individuals with sickle cell disease who had COVID-19 were significantly younger, a lower proportion of them had diabetes mellitus, and a higher proportion had asthma, liver conditions, cerebral infarction, history of acute kidney failure or chronic kidney disease, pulmonary embolism, and venous thrombosis and embolism (P < .01). Compared with Black individuals without sickle cell disease/trait, individuals with sickle cell trait who had COVID-19 were significantly younger, a higher proportion were female, overweight/obese, and a higher proportion had asthma, hypertension, diabetes, liver disease, and pulmonary embolism (P < .01). Figure 1 displays the rate of COVID-19 outcomes for the 3 distinct cohorts of patients with COVID-19.
COVID-19 outcomes in cohorts of patients who have sickle cell disease, sickle cell trait, and Black patients who do not have sickle cell disease/trait. ARD, acute respiratory distress.
COVID-19 outcomes in cohorts of patients who have sickle cell disease, sickle cell trait, and Black patients who do not have sickle cell disease/trait. ARD, acute respiratory distress.
COVID-19 outcomes among individuals with sickle cell disease compared with Black patients without sickle cell disease/trait
There were 312 patients with COVID-19 and sickle cell disease who were matched to 312 Black patients with COVID-19 but without sickle cell disease/trait; thus, there were no significant differences in demographic and clinical characteristics between the 2 groups. The matched cohort had a mean age of 32 years, 60% were female, 28% had hypertension, 25% had asthma, 20% had a history of acute kidney failure/chronic kidney failure, 20% were obese/overweight, 12% had diabetes mellitus, 10% had liver disease, 7% had a history of cerebral infarction, 5% had chronic obstructive pulmonary disease, 10% had heart failure, 7% had chronic ischemic heart disease, 5% had pulmonary embolism, and 11% had a history of venous thromboembolism and embolism before the COVID-19 diagnosis. After matching, individuals with sickle cell disease had a significantly higher rate of hospitalization, pneumonia, and pain due to COVID-19 compared with Black patients without sickle cell disease or trait; however, there was no significant difference in death rates within 14 days or 30 days between the 2 groups (Table 2).
Comparison of COVID-19 outcomes between individuals with sickle cell disease and Black individuals without sickle cell disease/trait
| Outcome . | Absolute risks in matched cohorts, % . | SCD vs Black patients without sickle cell disease/trait . | P . | ||||
|---|---|---|---|---|---|---|---|
| Sickle cell disease (N = 312) . | Black patients without sickle cell disease/trait (N = 312) . | Risk ratio . | 95% CI . | Risk difference, % . | 95% CI . | ||
| COVID-19 manifestations | |||||||
| Cough/fever | 26.9 | 22.7 | 1.2 | 0.9 to1.6 | 4.2 | –2.6 to 10.9 | .23 |
| Pain | 41.9 | 12.2 | 3.4 | 2.5 to 4.8 | 29.8 | 23.2 to 36.4 | <.001 |
| Pneumonia/acute chest syndrome | 23.1 | 9.6 | 2.4 | 1.6 to 3.4 | 13.4 | 7.8 to 19.2 | <.001 |
| Dyspnea/shortness of breath/hypoxia | 15.1 | 15.7 | 0.9 | 0.6 to 1.4 | −0.6 | –6.3 to 5.0 | .82 |
| ARD/respiratory failure or need of mechanical ventilation | 11.2 | 8.9 | 1.3 | 0.8 to 2.0 | 2.2 | –2.5 to 7.0 | .35 |
| Venous thromboembolism/pulmonary embolism | 3.2 | 3.2 | 1 | 0.4 to 2.4 | 0.0 | –2.8 to 2.8 | >.99 |
| Hospitalization | |||||||
| Within 14 d | 35.9 | 17.6 | 2.0 | 1.5 to 2.7 | 18.2 | 11.5 to 28.3 | <.001 |
| Within 30 d | 41.3 | 19.2 | 2.2 | 1.6 to 2.8 | 22.1 | 15.1 to 29.1 | <.001 |
| Mortality | |||||||
| Within 14 d | 3.2 | 3.2 | 1 | 0.4 to 2.4 | 0.0 | –2.8 to 2.8 | >.99 |
| Within 30 d | 3.2 | 3.2 | 1 | 0.4 to 2.4 | 0.0 | –2.8 to 2.8 | >.99 |
| Outcome . | Absolute risks in matched cohorts, % . | SCD vs Black patients without sickle cell disease/trait . | P . | ||||
|---|---|---|---|---|---|---|---|
| Sickle cell disease (N = 312) . | Black patients without sickle cell disease/trait (N = 312) . | Risk ratio . | 95% CI . | Risk difference, % . | 95% CI . | ||
| COVID-19 manifestations | |||||||
| Cough/fever | 26.9 | 22.7 | 1.2 | 0.9 to1.6 | 4.2 | –2.6 to 10.9 | .23 |
| Pain | 41.9 | 12.2 | 3.4 | 2.5 to 4.8 | 29.8 | 23.2 to 36.4 | <.001 |
| Pneumonia/acute chest syndrome | 23.1 | 9.6 | 2.4 | 1.6 to 3.4 | 13.4 | 7.8 to 19.2 | <.001 |
| Dyspnea/shortness of breath/hypoxia | 15.1 | 15.7 | 0.9 | 0.6 to 1.4 | −0.6 | –6.3 to 5.0 | .82 |
| ARD/respiratory failure or need of mechanical ventilation | 11.2 | 8.9 | 1.3 | 0.8 to 2.0 | 2.2 | –2.5 to 7.0 | .35 |
| Venous thromboembolism/pulmonary embolism | 3.2 | 3.2 | 1 | 0.4 to 2.4 | 0.0 | –2.8 to 2.8 | >.99 |
| Hospitalization | |||||||
| Within 14 d | 35.9 | 17.6 | 2.0 | 1.5 to 2.7 | 18.2 | 11.5 to 28.3 | <.001 |
| Within 30 d | 41.3 | 19.2 | 2.2 | 1.6 to 2.8 | 22.1 | 15.1 to 29.1 | <.001 |
| Mortality | |||||||
| Within 14 d | 3.2 | 3.2 | 1 | 0.4 to 2.4 | 0.0 | –2.8 to 2.8 | >.99 |
| Within 30 d | 3.2 | 3.2 | 1 | 0.4 to 2.4 | 0.0 | –2.8 to 2.8 | >.99 |
Bold P values indicate statistical significance.
ARD, acute respiratory distress, CI, confidence interval.
COVID-19 outcomes among individuals with sickle cell trait compared with Black patients without sickle cell disease/trait
There were 449 patients with COVID-19 and sickle cell trait who were matched to 449 Black patients with COVID-19 without sickle cell disease/trait such that there were no significant differences in demographic and clinical characteristics between the 2 groups. In the matched cohort, the mean age was 38 years, 81% were female, 39% had hypertension, 30% had asthma, 12% had a history of acute kidney failure/chronic kidney failure, 42% were obese/overweight, 22% had diabetes mellitus, 8% had liver disease, 3% had a history of cerebral infarction, 4% had chronic obstructive pulmonary disease, 7% had heart failure, 5% had chronic ischemic heart disease, 3% had pulmonary embolism, and 3% had a history of venous thromboembolism before the COVID-19 diagnosis. After matching, there were no significant differences in COVID-19 manifestations, hospitalizations, or mortality between individuals with sickle cell trait and Black patients who do not have sickle cell disease/trait. The sensitivity analyses also showed no significant differences in COVID-19 manifestations and hospitalizations between individuals with sickle cell trait and Black patients who did not have sickle cell disease/trait.
Discussion
Our study findings describe the increased risk of hospitalization, pain, and pneumonia due to COVID-19 among individuals with sickle cell disease compared with Black individuals who do not have sickle cell disease/trait, after adjusting for age, sex, and other preexisting comorbidities. We also found that COVID-19 outcomes for individuals with sickle cell trait, after adjusting for demographic and clinical characteristics, are not significantly different from Black individuals without sickle cell disease or trait.
Our results show that individuals with sickle cell disease have an increased risk of severe infection if they contract COVID-19. These findings are not surprising, as individuals with sickle cell disease are known to have an increased risk of having severe illness with viral infections that can escalate due to sickle cell disease–specific pathophysiological changes, specifically influenza.13,14 In addition, infections can trigger further sickle cell disease–related complications such as pain and acute chest syndrome. Moreover, after matching for relevant comorbidities, our findings support that the severity of COVID-19 can be attributed to sickle cell disease. The death rate observed in this study for those with sickle cell disease (3.2%) is similar to that reported by the largest international COVID-19 sickle cell registry (3.1%).15 After matching based on age, sex, and other comorbidities, there was no difference in death rates between the 2 groups. This implies that the death rates are not significantly different between those with sickle cell disease and the general Black population who have similar rates of comorbidities and end-organ damage. It further indicates that organ damage may increase COVID-19 mortality risk whether it is due to sickle cell disease or another cause. However, it is interesting to note that this fatality rate between the matched groups was not significantly different, despite individuals with sickle cell disease experiencing more severe COVID-19–related manifestations. This could potentially be because individuals with sickle cell disease may be more likely to seek care due to their prior known risk of sickle cell disease complications during times of infection than Black individuals who do not have sickle cell disease. This is also supported by the overrepresentation of sickle cell disease/COVID-19 cases in our study. Future research is needed to determine whether access to care or time to receive COVID-19 treatment influences outcomes across the population.
We found that individuals with sickle cell trait have COVID-19 outcomes similar to those in Black individuals who do not have sickle cell disease or trait. This is consistent with sickle cell trait being largely an asymptomatic disease.16-18 After matching on age, sex, and other known comorbidities, there were no significant differences between the 2 groups. It is noteworthy that in our sickle cell trait cohort, the majority were female, most likely because they are tested for trait at the time of their obstetrics/gynecology care. This finding reflects that knowledge of trait status is limited to a subset of patients and may be unknown to both the individuals who may have sickle cell trait and their health care team. However, if a patient has a diagnosis of sickle cell trait in their records, it is likely that they truly have the trait. This is evident by a recent study which evaluated the performance of ICD codes for identification of sickle cell trait and showed that ICD codes for sickle cell trait are highly specific but have a low sensitivity.19 Because newborn screening is universal in the United States, it is surprising that trait status is not documented for many individuals. Systematic reporting of trait status and its incorporation into medical records can further help understand the risks and outcomes for this population, irrespective of COVID-19.
Many of the noted comorbidities, such as history of stroke and impaired renal function, may be related to the natural history of sickle cell disease. This highlights the need for harmonized electronic health record data and data sharing between institutions in these emergent times. Our findings emphasize that knowledge of a patient’s medical history, in addition to sickle cell disease status, is paramount in understanding the risk of outcomes and in guiding COVID-19 management. In addition, risks associated with underlying comorbidities might vary in different population groups or settings.20 Future research investigating the risks associated with underlying comorbidities in specific groups of patients can further inform clinical care. Irrespective of sickle cell disease or trait status, there is a need to examine health equity across races not only specific to management of COVID-19 illness but throughout one’s lifetime. Health inequities can affect the prevalence and management of comorbidities and increase the risk of poor outcomes not only from COVID-19 but also from other comorbidities. Structural and systemic racism21 within our society and health care systems can also affect the management of sickle cell disease and other comorbidities, as well as COVID-19, leading to poor health outcomes.
The existing literature on COVID-19 in sickle cell disease and trait is mostly limited to case reports/series or does not have a comparative group.22-29 Only one other study in France has compared COVID-19 outcomes among individuals with sickle cell disease vs the general population. However, the study lacked statistical power to detect differences and was limited to the inpatient setting.30 Our study is the first, to our knowledge, to compare COVID-19 outcomes, including hospitalization, symptoms, and death, among individuals with sickle cell disease/trait vs Black individuals without sickle cell disease/trait. Our analyses used a large sample and account for many of the comorbidities known to be associated with severe COVID-19 illness to understand risks attributable to sickle cell disease and trait specifically.
The current study used data from multiple health care organizations and hence is generalizable to a large extent; however, it remains restricted to institutions participating in the TriNetX research network and thus reflects the population of patients receiving care at the contributing institutions. Also, the ICD-10 codes to identify sickle cell disease and trait are not always accurate. However, we used strict criteria to ensure we were specific in capturing the respective cohort. We excluded individuals who have sickle cell trait and White individuals from the sickle cell disease cohort. In addition, for the sickle cell trait cohort, White patients or those who have any instance of sickle cell disease in their medical records were excluded. It is possible that there are individuals with sickle cell trait for whom trait status is not recorded in medical records in the cohort of Black individuals without sickle cell disease/trait, which can bias results toward the null. Given the present practice of not systematically documenting trait status in medical records, it is difficult to overcome this challenge. However, our analyses used propensity score matching, which balanced cohorts for sex, age, and other comorbidities. Thus, it would have captured patients of similar age and sex in the comparison group who are likely aware that they do not have sickle cell trait. In addition, our sensitivity analyses varying the number of individuals in the sickle cell trait cohort yielded similar findings. Finally, our analyses did not account for secular trends in the pandemic and treatments received, which might influence outcomes. Comparative effectiveness of various treatment strategies for COVID-19 is much needed as this pandemic evolves.
In summary, these data provide evidence that sickle cell disease imposes the additional risk of severe COVID-19 illness and hospitalization, after balancing for age, sex, and other preexisting conditions. The death rate between sickle cell disease and Black individuals without sickle cell disease/trait who have a similar distribution of age, sex, and preexisting comorbidities was not significantly different. We found no significant differences in COVID-19 outcomes between sickle cell trait and Black individuals without sickle cell trait/disease, after balancing for age, sex, and other preexisting conditions.
Presented in abstract form at the 62nd annual meeting (virtual) of the American Society of Hematology, 5-8 December 2020.
This study used deidentified data housed within a research network. Hence, individual-level data will not be shared.
Authorship
Contribution: A.S. designed and performed research, and wrote the paper; A.M.B. provided critical inputs to research design, clinical relevance, and edited the manuscript draft; and J.A.P. designed research, provided clinical insight, and edited the manuscript draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashima Singh, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Suite 3050, Milwaukee, WI 53226; e-mail: ashimasingh@mcw.edu.