Key Points
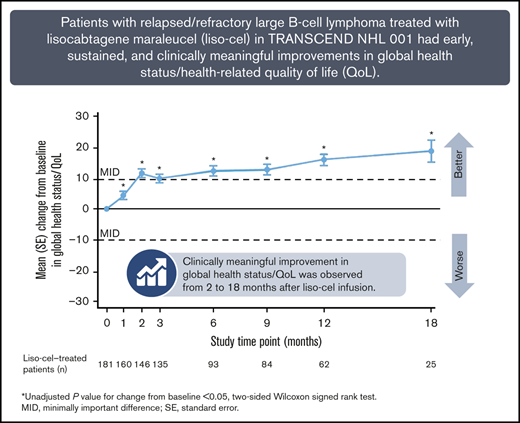
Patients with R/R LBCL in TRANSCEND NHL 001 showed HRQoL and symptom/functioning improvements from 1 to 18 months after liso-cel infusion.
HRQoL and symptom improvements across prespecified EORTC QLQ-C30 scales were clinically meaningful in a notable proportion of patients.
Abstract
CD19-directed chimeric antigen receptor (CAR) T-cell therapy has shown efficacy as a third-line or later treatment in patients with relapsed/refractory large B-cell lymphoma (LBCL). Using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and the EuroQol 5-Dimension 5-Level (EQ-5D-5L) questionnaire, we evaluated the impact of CAR T-cell treatment with lisocabtagene maraleucel (liso-cel) on health-related quality of life (HRQoL) and symptoms in patients with relapsed/refractory LBCL in the ongoing, open-label, nonrandomized TRANSCEND NHL 001 trial. Clinically meaningful improvement was observed in EORTC QLQ-C30 scores for global health status/QoL, based on a minimally important difference of 10 points at 2 to 18 months after liso-cel infusion. There were no clinically meaningful changes in physical functioning and pain, whereas clinically meaningful improvements were observed in fatigue at 2, 12, and 18 months. The proportion of patients with clinically meaningful improvement in global health status/QoL was generally higher for treatment responders than for nonresponders. A trend toward decreased mean EQ-5D-5L index scores was observed at 1 month after liso-cel infusion, followed by subsequent increases through 18 months. Mean EQ-5D-5L visual analog scale scores increased from 2 through 18 months. In summary, patients with relapsed/refractory LBCL treated with liso-cel had early, sustained, and clinically meaningful improvements in HRQoL and symptoms that correlated with antitumor activity. This study was registered at www.clinicaltrials.gov as #NCT02631044.
Introduction
Large B-cell lymphoma (LBCL) comprises a heterogeneous subset of non-Hodgkin lymphoma (NHL) subtypes that can be classified according to cell of origin and molecular/genetic characteristics1,2 and includes diffuse LBCL (DLBCL; de novo or transformed indolent lymphoma), primary mediastinal B-cell lymphoma, high-grade B-cell lymphoma, and follicular lymphoma grade 3B. DLBCL is the most common subtype and accounts for more than one-quarter of NHL cases.3
CD19-directed chimeric antigen receptor (CAR) T-cell therapy has shown efficacy as a third-line or later treatment in patients with relapsed/refractory (R/R) LBCL.4,5 Lisocabtagene maraleucel (liso-cel; JCAR017) is a CD19-directed, defined composition, 4-1BB CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells. Liso-cel has shown promising results in initial clinical testing. In the pivotal phase 1 TRANSCEND NHL 001 trial of liso-cel in patients with R/R LBCL, the overall response rate in the 256 efficacy-evaluable patients was 73%, and 53% of patients had a complete response.6
Patient-reported outcomes (PROs) are important for monitoring patients’ health-related quality of life (HRQoL), including their experience of both disease and treatment, and can help patients and health care providers make treatment decisions. Study results have shown that patients with DLBCL have a decreased HRQoL7,8 that is worse in survivors than in an age- and sex-matched normative population.9
The impact of treatment on HRQoL, which varies by treatment toxicity, the burden of procedures, and other factors, has been highlighted as an important factor to consider in treatment decision-making in LBCL.10,11 In the third-line treatment setting, patients with LBCL may have cumulative toxicity from prior treatments, which may include chemoimmunotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in the first-line setting12 and high-dose chemotherapy followed by hematopoietic stem cell transplantation or salvage therapies in the second-line setting.
Unlike standard chemotherapy and other treatments, CAR T-cell therapies are administered as a single dose, and the adverse event (AE) profiles differ. The AEs of most clinical interest for CAR T-cell therapies are cytokine release syndrome (CRS) and neurological events. CRS is usually experienced within 2 to 5 days of infusion, and neurological events occur within 4 to 9 days of infusion, with differences among the individual CAR T-cell products.6,13,14 In TRANSCEND, patients treated with liso-cel had low rates of grade ≥3 CRS (2%) and neurological events (10%).6 Because of the possibility of CRS and neurological events, postinfusion hospitalization for toxicity management is typically necessary after CAR T-cell therapy.5,15 Conversely, frequent hospital visits for multiple treatment administrations are avoided. The distinct impact of CAR T-cell therapies on HRQoL is not yet fully understood.
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) is a previously validated 30-item PRO instrument that has been used to assess the impact of different treatments on HRQoL in patients with cancer, including those with LBCL.7,8,16 It is increasingly being used in CAR T-cell therapy trials.15
In an earlier analysis of the TRANSCEND trial, liso-cel had a positive impact on patient HRQoL and symptom burden.17 The primary aim of the present analysis was to further assess the HRQoL impact of liso-cel, administered as a third-line or later treatment in patients with R/R LBCL in the TRANSCEND trial. We examined 4 prespecified EORTC QLQ-C30 scales, selected due to their clinical relevance to LBCL: global health status/QoL, physical functioning, fatigue, and pain. We also analyzed the health state index score and EuroQol visual analog scale (VAS) from the EuroQol 5-dimension 5-level (EQ-5D-5L) questionnaire. The EORTC QLQ-C30 role-, cognitive-, emotional-, and social-functioning scales were examined in exploratory analyses.
Methods
Study design
The present analysis is based on the ongoing TRANSCEND study, an open-label, nonrandomized, multicenter, multicohort, seamless phase 1 study of the safety, antitumor activity, and pharmacokinetics of liso-cel in adults with R/R B-cell NHL.6 The study protocol and protocol amendments were approved by the institutional review boards at participating sites. All patients provided written informed consent. All authors were given access to the primary clinical trial data outputs and contributed to the review of the data.
Patients
TRANSCEND enrolled an LBCL cohort comprising patients with 1 of the following LBCL histologic subtypes as R/R disease after ≥2 prior lines of therapy (third-line or later LBCL): DLBCL not otherwise specified, DLBCL transformed from indolent lymphoma, high-grade B-cell lymphoma with MYC and BCL2, and/or BCL6 rearrangements and DLBCL histology, primary mediastinal B-cell lymphoma, or follicular lymphoma grade 3B.
After lymphodepleting chemotherapy, patients received liso-cel as 2 sequential infusions of CD8+ and CD4+ CAR+ T cells at 1 of 3 target doses (50 × 106, 100 × 106, or 150 × 106 CAR+ T cells), administered by intravenous infusion. Patients with progressive disease received other anticancer therapies after liso-cel infusion at the discretion of the treating investigator. Data collected after the other anticancer therapies were included in the present analysis.
PRO assessments
HRQoL, symptoms, and health utility were assessed with the EORTC QLQ-C30 and EQ-5D-5L. These PRO assessments were implemented through Protocol Amendment 4 (dated 5 January 2017). Only patients enrolled after institutional review board approval of this amendment were eligible for the PRO assessments. Assessments were completed via paper questionnaires or electronically on tablets, by the patients themselves or by a proxy who asked the patient the questions.
PRO assessments were conducted before treatment (before the lymphodepleting chemotherapy visit), at baseline (the day of liso-cel infusion), and at 1 (day 29), 2, 3, 6, 9, 12, 18, and 24 months after liso-cel treatment, as well as at disease progression/relapse and the end of the study. The EORTC QLQ-C30 and EQ-5D-5L were administered in English, Spanish, Mandarin, and Armenian. No Armenian translation was available for the EORTC QLQ-C30, and so it was administered with the help of a translator, when necessary.
EORTC QLQ-C30.
The EORTC QLQ-C30 comprises 5 functional scales (physical, role, cognitive, emotional, and social functioning), 3 symptom scales (fatigue, pain, and nausea/vomiting), and a global health status/QoL scale.18 The single-item components of these scales were not included in the present analysis.
The primary analysis examined 4 prespecified scales: global health status/QoL, physical functioning, fatigue, and pain. The role-, cognitive-, emotional-, and social-functioning scales were analyzed as exploratory outcomes. The scales were scored as described in the EORTC QLQ-C30 Scoring Manual.18 The clinically meaningful treatment effect for each scale was defined as a ≥10-point change from baseline, based on a review of the relevant literature.19 In addition, the global health status/QoL, physical functioning, fatigue, and pain scales were analyzed in patients who responded to treatment (best overall response of complete response or partial response) vs patients who did not respond to treatment (best overall response of stable disease, nonprogressive disease, progressive disease, or not evaluable).
EQ-5D-5L.
The EQ-5D-5L is a self-administered instrument comprising 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and a VAS ranging from 0 (worst health imaginable) to 100 (best health imaginable).20 Responses for the 5 dimensions and a United States (US) value set were used to calculate a summary index score, with a score of 1 indicating full health, 0 indicating death, and negative scores reflecting states perceived to be worse than death.20 The EQ-5D-5L index score was analyzed as a primary outcome and the VAS as an exploratory outcome.
Statistical analyses
The analyses used data obtained through 12 August 2019. Data for the assessment at 24 months were not interpretable, because only 3 patients had completed the assessment by the time of data cutoff. Therefore, those data are not presented.
Analyses were based on PRO-evaluable subpopulations of the liso-cel–treated LBCL set, which comprised all patients in the LBCL cohort treated with liso-cel. The EORTC QLQ-C30–evaluable population comprised patients for whom all scales were analyzable at baseline (ie, answers were provided for ≥50% of the items for each scale), and at least 1 scale was analyzable at postbaseline assessments. The EQ-5D-5L–evaluable population comprised patients with baseline data for both the EQ-5D-5L index score and the VAS and at least 1 postbaseline assessment for the EQ-5D-5L index score or VAS.
For each assessment, summary statistics were calculated for observed scores and change from baseline. The 2-sided Wilcoxon signed-rank test was performed without multiplicity adjustment to evaluate whether the change from baseline was different from 0 at each assessment time point after liso-cel infusion. Missing data were assumed to be missing at random and were not imputed.
EORTC QLQ-C30.
For each assessment, adherence was assessed as the proportion of patients who remained on study and provided answers for at least 15 of the 30 EORTC QLQ-C30 items. The denominator was the total number of patients in the EORTC QLQ-C30–evaluable subpopulation who were still on study at each assessment visit.
An individual-level analysis was performed to examine clinically meaningful changes, which were based on the minimally important difference (MID), the smallest score change that reflects a clinically meaningful treatment effect. The MID was defined a priori as a ±10-point change from baseline, according to the findings of an anchor-based analysis of MID for EORTC QLQ-C30,19 which were incorporated into the EORTC QLQ-C30 Scoring Manual.18 Change from baseline was categorized as “improvement” or “deterioration,” defined as a ≥10-point increase or decrease from baseline, respectively, or “no change,” defined as a <10-point increase or decrease from baseline. For each scale, proportions of patients categorized as having improvement, no change, or deterioration were calculated using the total number of patients who provided answers for ≥50% of the items on the scale. Time to first clinically meaningful improvement and time to first clinically meaningful deterioration were analyzed by Kaplan-Meier methodology.
EQ-5D-5L.
For each EQ-5D-5L assessment time point, adherence was assessed as the proportion of patients who remained on study and provided answers to at least 1 EQ-5D-5L dimension or who completed the VAS. The EQ-5D-5L index score was considered analyzable if answers were provided for all 5 dimensions; otherwise, it was marked as missing.
For the EQ-5D-5L index score, an individual-level analysis of clinically meaningful change was performed using response categories defined a priori based on the previously defined MID of 0.07 for US value set–based scores.21 “Improvement” or “deterioration” was defined as an increase or decrease from baseline of ≥0.07, and “no change” was defined as an increase or decrease from baseline of <0.07. Proportions of patients categorized as having improvement, no change, or deterioration were calculated using the total number of patients with nonmissing EQ-5D-5L index scores. Time to first clinically meaningful improvement and time to first clinically meaningful deterioration were analyzed by Kaplan-Meier methodology.
This study was registered at www.clinicaltrials.gov as #NCT02631044.
Results
Patients
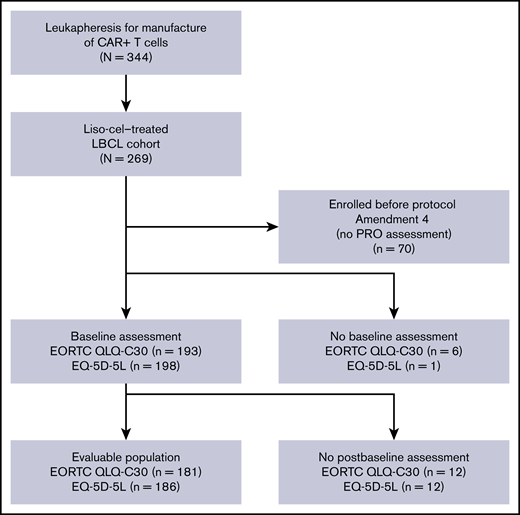
At data cutoff (12 August 2019), 344 patients had undergone leukapheresis for manufacture of CAR+ T cells (liso-cel), of whom 269 had received at least 1 dose of liso-cel and were included in the liso-cel–treated LBCL set.6 The EORTC QLQ-C30–evaluable population comprised 181 (91%) of the 199 patients in the liso-cel–treated LBCL set who were enrolled after the study was amended to include PRO assessments (Figure 1). Five patients were treated with target doses (50 × 106) of CAR+ T cells, 137 with 100 × 106 CAR+ T cells, and 39 with 150 × 106 CAR+ T cells. The median (range) on-study follow-up time for patients at data cutoff was 11.4 (1.2-27.8) months for the EORTC QLQ-C30–evaluable population.
Patient disposition for the EORTC QLQ-C30– and EQ-5D-5L–evaluable populations. Reasons for patients having no baseline assessment were that the patient declined to complete the assessment and the site failed to administer it. Reasons for patients having no postbaseline assessment were the patient declined to complete a postbaseline assessment, the site failed to administer a postbaseline assessment, and no postbaseline visit was conducted.
Patient disposition for the EORTC QLQ-C30– and EQ-5D-5L–evaluable populations. Reasons for patients having no baseline assessment were that the patient declined to complete the assessment and the site failed to administer it. Reasons for patients having no postbaseline assessment were the patient declined to complete a postbaseline assessment, the site failed to administer a postbaseline assessment, and no postbaseline visit was conducted.
For the EORTC QLQ-C30–evaluable population, mean (standard deviation [SD]) age was 60.2 (14.0) years (Table 1). Most patients were male (65%), white (86%), and not Hispanic or Latino (85%). One-hundred forty-one patients (78%) had refractory disease and 40 patients (22%) had relapsed. The most frequent type of B-cell NHL was DLBCL not otherwise specified (54%), followed by DLBCL transformed from follicular lymphoma (23%). Mean (standard deviation [SD]) time from diagnosis to the first liso-cel infusion was 32.3 (35.7) months.
Demographics and baseline characteristics of the EORTC QLQ-C30– and EQ-5D-5L–evaluable populations
| Parameter/category . | EORTC QLQ-C30–evaluable population (n = 181)* . | EQ-5D-5L–evaluable population (n = 186)† . |
|---|---|---|
| Age, y | ||
| Mean (SD) | 60.2 (14.0) | 60.1 (13.9) |
| Median | 63.0 | 63.0 |
| Age group, n (%), y | ||
| <65 | 105 (58) | 109 (59) |
| ≥65 | 76 (42) | 77 (41) |
| <75 | 162 (90) | 166 (89) |
| ≥75 | 19 (10) | 20 (11) |
| Sex, n (%) | ||
| Male | 117 (65) | 121 (65) |
| Female | 64 (35) | 65 (35) |
| Race, n (%) | ||
| White | 155 (86) | 158 (85) |
| Other | 17 (9) | 18 (10) |
| Unknown/missing | 9 (5) | 10 (5) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 20 (11) | 21 (11) |
| Not Hispanic/Latino | 153 (85) | 157 (84) |
| Unknown | 8 (4) | 8 (4) |
| ECOG PS at screening, n (%) | ||
| 0 | 73 (40) | 73 (39) |
| 1 | 107 (59) | 112 (60) |
| 2 | 1 (1) | 1 (1) |
| Pre–liso-cel ECOG PS, n (%)‡ | ||
| 0 | 44 (24) | 44 (24) |
| 1 | 127 (70) | 132 (71) |
| 2 | 8 (4) | 8 (4) |
| 3 | 1 (1) | 1 (1) |
| Type of B-cell non-Hodgkin lymphoma, n (%) | ||
| DLBCL NOS | 98 (54) | 99 (53) |
| HGBCL | 19 (10) | 21 (11) |
| DLBCL transformed from indolent lymphoma | 50 (28) | 51 (27) |
| Follicular lymphoma | 41 (23) | 42 (23) |
| CLL/SLL | 2 (1) | 2 (1) |
| Marginal zone lymphoma | 5 (3) | 5 (3) |
| Other | 2 (1) | 2 (1) |
| PMBCL | 13 (7) | 14 (8) |
| FL3B | 1 (1) | 1 (1) |
| Refractory or relapsed, n (%)§ | ||
| Refractory | 141 (78) | 145 (78) |
| Relapsed | 40 (22) | 41 (22) |
| Chemotherapy refractory or chemotherapy sensitive, n (%)|| | ||
| Chemotherapy refractory | 122 (67) | 125 (67) |
| Chemotherapy sensitive | 59 (33) | 61 (33) |
| Active CNS disease at first liso-cel infusion, n (%) | ||
| Yes | 3 (2) | 3 (2) |
| No | 178 (98) | 183 (98) |
| Number of prior systemic treatment regimens, n (%)¶ | ||
| 1 | 5 (3) | 6 (3) |
| 2 | 92 (51) | 93 (50) |
| 3 | 45 (25) | 47 (25) |
| 4 | 23 (13) | 24 (13) |
| 5-8 | 16 (9) | 16 (9) |
| Best response to any prior therapy, n (%) | ||
| Complete response | 106 (59) | 110 (59) |
| Partial response | 52 (29) | 53 (28) |
| Stable disease | 11 (6) | 11 (6) |
| Progressive disease | 12 (7) | 12 (6) |
| Time from diagnosis to first liso-cel infusion, months, mean (SD) | 32.3 (35.7) | 32.3 (35.4) |
| Received bridging therapy, n (%)# | 99 (55) | 102 (55) |
| Parameter/category . | EORTC QLQ-C30–evaluable population (n = 181)* . | EQ-5D-5L–evaluable population (n = 186)† . |
|---|---|---|
| Age, y | ||
| Mean (SD) | 60.2 (14.0) | 60.1 (13.9) |
| Median | 63.0 | 63.0 |
| Age group, n (%), y | ||
| <65 | 105 (58) | 109 (59) |
| ≥65 | 76 (42) | 77 (41) |
| <75 | 162 (90) | 166 (89) |
| ≥75 | 19 (10) | 20 (11) |
| Sex, n (%) | ||
| Male | 117 (65) | 121 (65) |
| Female | 64 (35) | 65 (35) |
| Race, n (%) | ||
| White | 155 (86) | 158 (85) |
| Other | 17 (9) | 18 (10) |
| Unknown/missing | 9 (5) | 10 (5) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 20 (11) | 21 (11) |
| Not Hispanic/Latino | 153 (85) | 157 (84) |
| Unknown | 8 (4) | 8 (4) |
| ECOG PS at screening, n (%) | ||
| 0 | 73 (40) | 73 (39) |
| 1 | 107 (59) | 112 (60) |
| 2 | 1 (1) | 1 (1) |
| Pre–liso-cel ECOG PS, n (%)‡ | ||
| 0 | 44 (24) | 44 (24) |
| 1 | 127 (70) | 132 (71) |
| 2 | 8 (4) | 8 (4) |
| 3 | 1 (1) | 1 (1) |
| Type of B-cell non-Hodgkin lymphoma, n (%) | ||
| DLBCL NOS | 98 (54) | 99 (53) |
| HGBCL | 19 (10) | 21 (11) |
| DLBCL transformed from indolent lymphoma | 50 (28) | 51 (27) |
| Follicular lymphoma | 41 (23) | 42 (23) |
| CLL/SLL | 2 (1) | 2 (1) |
| Marginal zone lymphoma | 5 (3) | 5 (3) |
| Other | 2 (1) | 2 (1) |
| PMBCL | 13 (7) | 14 (8) |
| FL3B | 1 (1) | 1 (1) |
| Refractory or relapsed, n (%)§ | ||
| Refractory | 141 (78) | 145 (78) |
| Relapsed | 40 (22) | 41 (22) |
| Chemotherapy refractory or chemotherapy sensitive, n (%)|| | ||
| Chemotherapy refractory | 122 (67) | 125 (67) |
| Chemotherapy sensitive | 59 (33) | 61 (33) |
| Active CNS disease at first liso-cel infusion, n (%) | ||
| Yes | 3 (2) | 3 (2) |
| No | 178 (98) | 183 (98) |
| Number of prior systemic treatment regimens, n (%)¶ | ||
| 1 | 5 (3) | 6 (3) |
| 2 | 92 (51) | 93 (50) |
| 3 | 45 (25) | 47 (25) |
| 4 | 23 (13) | 24 (13) |
| 5-8 | 16 (9) | 16 (9) |
| Best response to any prior therapy, n (%) | ||
| Complete response | 106 (59) | 110 (59) |
| Partial response | 52 (29) | 53 (28) |
| Stable disease | 11 (6) | 11 (6) |
| Progressive disease | 12 (7) | 12 (6) |
| Time from diagnosis to first liso-cel infusion, months, mean (SD) | 32.3 (35.7) | 32.3 (35.4) |
| Received bridging therapy, n (%)# | 99 (55) | 102 (55) |
CLL, chronic lymphocytic leukemia; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; FL3B, follicular lymphoma grade 3B; HGBCL, high-grade B-cell lymphoma; NOS, not otherwise specified; PMBCL, primary mediastinal B-cell lymphoma; SLL, small lymphocytic lymphoma.
Liso-cel–treated patients for whom all scales were analyzable (ie, answers were provided for ≥50% of the items for each scale) at baseline, and at least 1 scale was amenable to postbaseline analysis.
Liso-cel–treated patients with baseline data for both the EQ-5D-5L index score and the VAS and at least 1 postbaseline assessment for the EQ-5D-5L index score or the VAS.
The most recent ECOG PS after lymphodepleting chemotherapy and before liso-cel infusion.
Relapse was defined as best response to last systemic or transplant treatment with curative intent of complete response; refractory was defined as best response of partial response, stable disease, or progressive disease.
Chemotherapy refractory was defined as stable disease or progressive disease during the last chemotherapy-containing regimen or relapse <12 months after autologous stem cell transplantation. Patients who did not fulfill these criteria were categorized as chemotherapy sensitive.
The original study protocol enrolled patients with at least 2 previous lines of treatment, but there was no requirement for at least 2 previous lines of systemic treatment. As a result, 9 enrolled patients had received only 1 previous line of systemic treatment (plus consolidation with hematopoietic stem cell transplantation or radiation therapy). During enrollment, the protocol was amended to require at least 2 previous lines of systemic treatment.
Systemic treatment and/or radiotherapy provided after consent and before lymphodepleting chemotherapy.
EORTC QLQ-C30
Mean (SD) score at baseline was 62.3 (20.3) for global health status/QoL, 77.8 (19.2) for physical functioning, 38.2 (21.8) for fatigue, and 25.6 (25.8) for pain. EORTC QLQ-C30 adherence was 88% (160 of 181), 66% (93 of 141), 73% (62 of 85), and 69% (25 of 36) at 1, 6, 12, and 18 months, respectively.
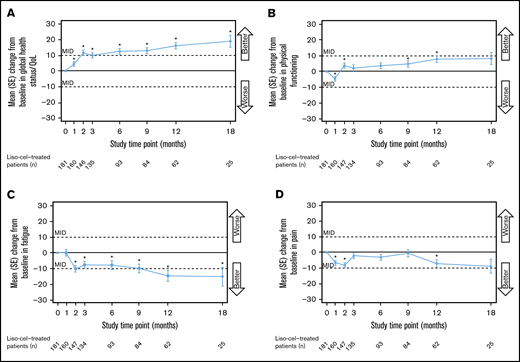
Global health status/QoL was significantly improved compared with baseline from 1 month (day 29) through 18 months after liso-cel infusion (Figure 2A). At 2 to 18 months, the improvements were clinically meaningful. The mean (SD) score change between baseline and 18 months was 19.7 (25.6) (supplemental Table 1). Physical functioning was also significantly improved after treatment (at 2, 9, and 12 months), although significant deterioration was initially observed at 1 month, and none of the subsequent improvements were clinically meaningful (Figure 2B). The symptom scale for fatigue showed significant improvement compared with baseline from 2 to 18 months (Figure 2C). The improvements at 2, 12, and 18 months were clinically meaningful. Finally, pain scores improved significantly at 2 months, before deteriorating to near-baseline values at 3 to 9 months, and then improving again at 12 and 18 months (Figure 2D). None of the improvements were clinically meaningful.
Changes in EORTC QLQ-C30 scores over time for the scales included in the primary analysis. Global health status/QoL (A), physical functioning (B), fatigue (C), and pain (D). Data are for the EORTC QLQ-C30–evaluable population. The MID was defined as a ≥10-point change in either direction. *Unadjusted P value for change from baseline <.05, 2-sided Wilcoxon signed-rank test. The test was performed only for assessments where the sample size was ≥10. SE, standard error.
Changes in EORTC QLQ-C30 scores over time for the scales included in the primary analysis. Global health status/QoL (A), physical functioning (B), fatigue (C), and pain (D). Data are for the EORTC QLQ-C30–evaluable population. The MID was defined as a ≥10-point change in either direction. *Unadjusted P value for change from baseline <.05, 2-sided Wilcoxon signed-rank test. The test was performed only for assessments where the sample size was ≥10. SE, standard error.
In the exploratory analysis, role functioning was significantly improved at 2, 3, 9, and 12 months after liso-cel infusion, with clinically meaningful improvement observed at 12 and 18 months (supplemental Figure 1A; supplemental Table 2). Emotional functioning was significantly improved at 2 months and at 6 through 18 months, with clinically meaningful improvement observed at 18 months (supplemental Figure 1B). Cognitive functioning improved significantly between baseline and 2 months but showed no significant improvements vs baseline at later time points and no clinically meaningful changes (supplemental Figure 1C). Finally, social functioning showed both significant improvement and clinically meaningful improvement from 2 through 18 months (supplemental Figure 1D).
Individual-level analysis.
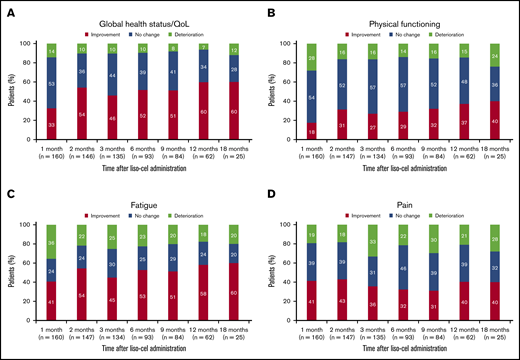
In an individual-level analysis of the EORTC QLQ-C30 scales included in the primary analysis, the proportion of patients with clinically meaningful improvement in global health status/QoL was 33% at 1 month after liso-cel infusion, 52% at 6 months, and 60% at both 12 and 18 months (Figure 3A). Conversely, the proportion of patients with clinically meaningful deterioration was 14% at 1 month, 10% at 6 months, 7% at 12 months, and 12% at 18 months.
Individual-level analysis for the EORTC QLQ-C30 scales included in the primary analysis. Global health status/QoL (A), physical functioning (B), fatigue (C), and pain (D). Data are for the EORTC QLQ-C30–evaluable population. Percentages were calculated using the total number of patients who had provided answers for ≥50% of the items on the given scale. Improvement was defined as a ≥10-point increase from baseline (global health status/QoL and physical functioning) or ≥10-point decrease from baseline (fatigue and pain). No change was defined as a <10-point increase or decrease from baseline. Deterioration was defined as a ≥10-point decrease from baseline (global health status/QoL and physical functioning) or ≥10-point increase from baseline (fatigue and pain).
Individual-level analysis for the EORTC QLQ-C30 scales included in the primary analysis. Global health status/QoL (A), physical functioning (B), fatigue (C), and pain (D). Data are for the EORTC QLQ-C30–evaluable population. Percentages were calculated using the total number of patients who had provided answers for ≥50% of the items on the given scale. Improvement was defined as a ≥10-point increase from baseline (global health status/QoL and physical functioning) or ≥10-point decrease from baseline (fatigue and pain). No change was defined as a <10-point increase or decrease from baseline. Deterioration was defined as a ≥10-point decrease from baseline (global health status/QoL and physical functioning) or ≥10-point increase from baseline (fatigue and pain).
For physical functioning, 18% of patients achieved clinically meaningful improvement at 1 month, 29% at 6 months, 37% at 12 months, and 40% at 18 months; the proportion of patients who experienced clinically meaningful deterioration was 28% at 1 month, 14% at 6 months, 15% at 12 months, and 24% at 18 months (Figure 3B). Forty-one percent of patients achieved clinically meaningful improvement in fatigue at 1 month, 53% at 6 months, 58% at 12 months, and 60% at 18 months, whereas the proportion of patients with clinically meaningful deterioration of symptoms was 36% at 1 month, 23% at 6 months, 18% at 12 months, and 20% at 18 months (Figure 3C). Finally, the proportion of patients with clinically meaningful improvement in pain was 41% at 1 month, 32% at 6 months, and 40% at both 12 and 18 months, whereas 19%, 22%, 21%, and 28% of patients experienced clinically meaningful deterioration of pain at the respective time points (Figure 3D).
For global health status/QoL, median (95% confidence interval [CI]) time to first clinically meaningful improvement was 2.2 (2.0-2.9) months and median (95% CI) time to first clinically meaningful deterioration was not reached (12.3 months–not reached). Median (95% CI) time to first clinically meaningful improvement and deterioration in physical functioning was 17.8 (9.4–not reached) and 12.3 (6.6–not reached) months, respectively. For fatigue, median (95% CI) time to first clinically meaningful improvement was 2.0 (1.8-2.2) months, and median (95% CI) time to first clinically meaningful deterioration was 3.3 (2.8-9.2) months. Finally, median (95% CI) time to first clinically meaningful improvement and deterioration in pain was 3.8 (1.9-12.1) and 6.5 (3.2-9.0) months, respectively.
In the individual-level analysis of EORTC QLQ-C30 scales included in the exploratory analysis, the proportion of patients with clinically meaningful improvement at 12 months was 45% for role functioning, 31% for emotional functioning, 36% for cognitive functioning, and 60% for social functioning (supplemental Figure 2). The proportion of patients with clinically meaningful deterioration at 12 months was 19% for role functioning, 7% for emotional functioning, 21% for cognitive functioning, and 16% for social functioning.
Analyses of treatment responders and nonresponders.
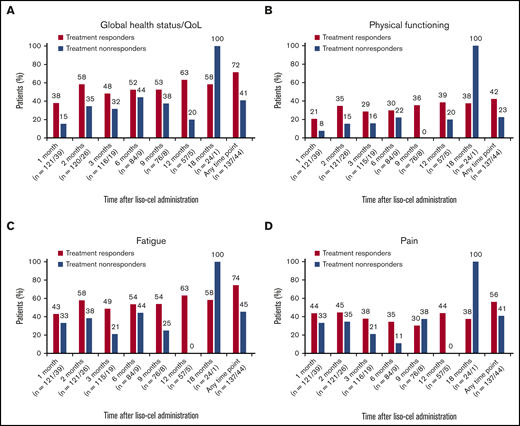
Clinically meaningful improvements were analyzed separately in treatment responders (those achieving a complete or partial response; n = 137) and nonresponders (those not achieving a complete or partial response or whose treatment response was not evaluable; n = 44). Higher proportions of treatment responders, compared with nonresponders, respectively, showed a clinically meaningful improvement at any time point after liso-cel infusion in global health status/QoL (72% vs 41%), physical functioning (42% vs 23%), fatigue (74% vs 45%), and pain (56% vs 41%) (Figure 4). At 1 month, a higher proportion of treatment responders vs nonresponders showed improvements in global health status/QoL (38% vs 15%), physical functioning (21% vs 8%), fatigue (43% vs 33%), and pain (44% vs 33%). A similar pattern was observed at later time points, although clinical interpretation was limited by the small number of treatment nonresponders.
Individual-level analysis of clinically meaningful improvements for the EORTC QLQ-C30 scales included in the primary analysis for treatment responders vs nonresponders. Global health status/QoL (A), physical functioning (B), fatigue (C), and pain (D). Data are for the EORTC QLQ-C30–evaluable population. Percentages of patients with clinically meaningful improvements were calculated from the total number of patients who provided answers for ≥50% of the items on the given scale. Improvement was defined as a ≥10-point increase from baseline (global health status/QoL and physical functioning) or ≥10-point decrease from baseline (fatigue and pain). Any time point means 1 or more time points from 1 month to 18 months.
Individual-level analysis of clinically meaningful improvements for the EORTC QLQ-C30 scales included in the primary analysis for treatment responders vs nonresponders. Global health status/QoL (A), physical functioning (B), fatigue (C), and pain (D). Data are for the EORTC QLQ-C30–evaluable population. Percentages of patients with clinically meaningful improvements were calculated from the total number of patients who provided answers for ≥50% of the items on the given scale. Improvement was defined as a ≥10-point increase from baseline (global health status/QoL and physical functioning) or ≥10-point decrease from baseline (fatigue and pain). Any time point means 1 or more time points from 1 month to 18 months.
Median (95% CI) time to first clinically meaningful improvement was shorter or similar in treatment responders vs nonresponders: 2.0 (1.9-2.2) months vs 3.0 (2.3-not reached) months for global health status/QoL, 18.2 (8.8-not reached) months vs 17.8 (3.3-17.8) months for physical functioning, 1.9 (1.7-2.1) months vs 6.0 (1.1-not reached) months for fatigue, and 3.0 (1.8-18.2) months vs 4.2 (1.0-not reached) months for pain.
EQ-5D-5L
The EQ-5D-5L–evaluable population comprised 186 (93%) of the 199 patients in the liso-cel–treated LBCL set who were enrolled after the study was amended to include PRO assessments (Figure 1). Demographics and baseline characteristics of the EQ-5D-5L–evaluable population are shown in Table 1.
At baseline, the mean (SD) US value set–based EQ-5D-5L index score was 0.82 (0.12) and the mean (SD) EQ-5D-5L VAS was 68.3 (19.5). EQ-5D-5L adherence was 89% (165 of 186), 67% (97 of 145), 70% (62 of 88), and 66% (25 of 38) at 1, 6, 12, and 18 months, respectively.
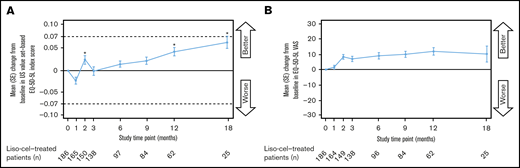
The mean EQ-5D-5L index score showed a trend toward decrease at 1 month after liso-cel infusion, but was significantly increased at 2, 12, and 18 months (Figure 5A). None of the EQ-5D-5L index score increases were clinically meaningful. Mean EQ-5D-5L VAS score showed a trend of being increased from 2 through 18 months after liso-cel infusion (Figure 5B). Higher proportions of treatment responders (48%) vs nonresponders (27%) showed a clinically meaningful improvement in EQ-5D-5L index scores at any time point after liso-cel infusion.
Change in EQ-5D-5L index score and EQ-5D-5L VAS over time. US value set–based EQ-5D-5L index score (A) and EQ-5D-5L VAS (B). Data are for the EQ-5D-5L–evaluable population. *Unadjusted P-value for change from baseline <.05, 2-sided Wilcoxon signed-rank test. The test was performed only for assessments where the sample size was ≥10.
Change in EQ-5D-5L index score and EQ-5D-5L VAS over time. US value set–based EQ-5D-5L index score (A) and EQ-5D-5L VAS (B). Data are for the EQ-5D-5L–evaluable population. *Unadjusted P-value for change from baseline <.05, 2-sided Wilcoxon signed-rank test. The test was performed only for assessments where the sample size was ≥10.
Individual-level analysis.
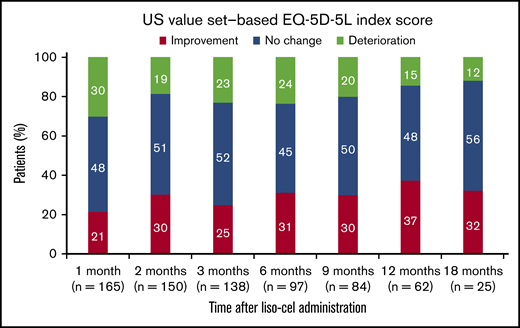
The proportion of patients with clinically meaningful improvement in EQ-5D-5L index scores after liso-cel infusion was 21% at 1 month, 31% at 6 months, 37% at 12 months, and 32% at 18 months (Figure 6). The proportion of patients with clinically meaningful deterioration was 30% at 1 month, 24% at 6 months, 15% at 12 months, and 12% at 18 months. Median (95% CI) time to first clinically meaningful improvement in EQ-5D-5L index score was 11.7 (6.3–not reached) months and median (95% CI) time to first clinically meaningful deterioration was 8.8 (5.6-17.4) months.
Individual-level analysis for US value set–based EQ-5D-5L index score. Data are for the EQ-5D-5L–evaluable population. Percentages were calculated using the total number of patients with nonmissing EQ-5D-5L index scores. Improvement was defined as an increase of ≥0.07 from baseline. No change was defined as an increase or decrease of <0.07 from baseline. Deterioration was defined as a decrease of ≥0.07 from baseline.
Individual-level analysis for US value set–based EQ-5D-5L index score. Data are for the EQ-5D-5L–evaluable population. Percentages were calculated using the total number of patients with nonmissing EQ-5D-5L index scores. Improvement was defined as an increase of ≥0.07 from baseline. No change was defined as an increase or decrease of <0.07 from baseline. Deterioration was defined as a decrease of ≥0.07 from baseline.
Discussion
In this analysis based on data from the pivotal TRANSCEND trial, liso-cel treatment substantially improved HRQoL and symptom burden in patients with LBCL. Although there was nonclinically meaningful deterioration at 1 month in EORTC QLQ-C30 physical functioning, improvements in global health status/QoL and fatigue were detected as early as 2 months after liso-cel infusion and were maintained through 18 months after infusion. Despite previous studies showing the negative impact of LBCL on HRQoL,7,8 our patients had reasonable baseline functioning based on EQ-5D-5L index score and had nonclinically meaningful improvements in EQ-5D-5L index scores from 2 months after liso-cel infusion. EQ-5D-5L VAS score tended to be increased from 2 through 18 months after liso-cel infusion. Importantly, more patients reported clinically meaningful improvements in HRQoL and symptom burden than clinically meaningful deterioration. In addition, with the exception of physical functioning, median time to first clinically meaningful improvement for the EORTC QLQ-C30 scales included in the primary analysis was shorter than median time to first clinically meaningful deterioration. Furthermore, patients who responded to liso-cel experienced better improvements in HRQoL and symptom burden than did nonresponders.
Previous trials of CAR T-cell therapies have investigated their impact on HRQoL. In a follow-up study of 40 patients with R/R chronic lymphocytic leukemia, NHL, and acute lymphoblastic leukemia 1 to 5 years after treatment with autologous CD19-directed CAR+ T cells, 19 patients (47.5%) reported cognitive difficulties, depression, or anxiety, and 7 patients (17.5%) scored ≤40 (≥1 SD lower than the reference population) on the Patient-Reported Outcomes Measurement Information System Global Mental Health scale.22 In the JULIET study of adults with chemotherapy-refractory LBCL,23 the autologous CD19-directed CAR T-cell therapy tisagenlecleucel resulted in meaningful improvements at 3 months in patient-reported scores for Functional Assessment of Cancer Therapy (FACT)–Lymphoma, FACT Trial Outcome Index, and FACT General (FACT-G) and in the general health and vitality Short Form 36 subscales.24 Long-term follow-up of treatment responders showed that HRQoL improvements were maintained at 18 months.25
The improvements in HRQoL at 3 months in JULIET occurred despite half of the patients reporting serious AEs in the first 8 weeks after treatment.24 This result illustrates the need to look beyond acute toxicities when evaluating HRQoL after CAR T-cell treatment along with examining earlier time points to understand the impact of acute toxicities on HRQoL. In the present study, the observed transient deterioration in physical functioning (EORTC QLQ-C30) at the group level most likely reflects the short-term negative impact that CAR T-cell therapy can have on HRQoL,26 with CRS and neurological events generally occurring within the first 2 weeks after infusion. This decrement has been reported anecdotally by patients in clinical care. In addition, lymphodepleting chemotherapy before CAR T-cell infusion can lead to prolonged cytopenia. Although it would have been interesting to explore the impact of acute toxicities on HRQoL more deeply, the relatively low rates of grade ≥3 CRS (2%) and neurological events (10%) in TRANSCEND6 precluded a meaningful subgroup analysis. However, it should be noted that 18% of patients had a clinically meaningful improvement in physical functioning at 1 month. Moreover, the longer-term observed effects on HRQoL and symptoms must also be understood, as they give a clearer indication of how a patient is feeling after recovering from toxicities and potentially achieving a therapeutic response.
Because there are no validated PRO instruments for CAR T-cell therapy, clinical trials have used previously validated cancer-specific instruments, such as the EORTC QLQ-C30 and FACT-G, to assess general health status.15 Of these 2 instruments, the EORTC QLQ-C30 covers a broader range of HRQoL facets.27 Moreover, the EORTC QLQ-C30 has been widely used in recent trials of CAR T-cell therapies,15 which should facilitate comparison of the findings with those of other studies. These are the reasons that the EORTC QLQ-C30 was selected for the present study.
The present study includes the same limitations that are inherent in assessing PROs of any clinical trial, including managing missing data and shifts in response15 ; nevertheless, PRO assessments that use previously validated instruments provide vital information that cannot be captured through other means. In this study, EORTC QLQ-C30 adherence declined between 1 and 6 months after liso-cel infusion, before stabilizing. The adherence rate at 6 months (66%) is comparable to the rate of ≥70% at 24 weeks reported in the KEYNOTE-087 trial of pembrolizumab in patients with R/R classic Hodgkin lymphoma,28 but lower than adherence at 6 months in the JULIET study of tisagenlecleucel in adults with LBCL (81%).25 The adherence rate at 18 months (69%) is comparable to that in JULIET (65%).25 In another recent trial, adherence decreased over time in patients with newly diagnosed multiple myeloma treated with thalidomide or lenalidomide, from 100% at baseline to 69% at ∼15 months.29
Some data were assumed to be missing at random, but this was not confirmed. If data were not missing at random, the missing data may be a source of bias. Loss of patients as a result of death or study discontinuation may have exacerbated this bias. Overall survival in TRANSCEND was 21 months overall, but only 5 months for patients with stable disease or progressive disease as their best response.6 In addition, some patients had not been involved in the study long enough to reach some of the time points. Treatment responders with improved HRQoL may choose to remain on study, whereas nonresponders and patients who relapse are liable to have poor HRQoL on account of progressive disease and may choose to discontinue. Only 5 treatment nonresponders included in the EORTC QLQ-C30–evaluable population remained on study at 12 months and only 1 treatment nonresponder remained at 18 months. The PRO analysis based on response to treatment was limited by the small number of nonresponders who completed assessments at later time points. Because of these biases, PRO analyses by treatment response status, particularly those at later time points, should be interpreted with caution. P values should also be interpreted with caution because they were calculated without multiplicity adjustment.
Patients who progressed and subsequently received another anticancer therapy were asked to continue to complete PRO assessments. Of the 96 patients who received another anticancer therapy following liso-cel infusion, 42 answered the EORTC QLQ-C30 survey after initiating the anticancer therapy. Including these patients in the analysis may have confounded our findings by making it impossible to distinguish between the effects of anticancer therapies and those of liso-cel. A further potential limitation was the use of a fixed threshold of 10 to define all clinically meaningful treatment effects for the EORTC QLQ-30. EORTC QLQ-30 guidelines suggest that a fixed threshold may be too simplistic for failing to differentiate between different scales.30,31 Moreover, it may not be appropriate to use the same threshold for group- and individual-level analyses.32 As the analysis was focused on long-term trends in HRQoL and it would be difficult to administer questionnaires to patients experiencing acute CRS or neurological events, no assessments were scheduled for the first 4 weeks after liso-cel infusion. By not performing PRO assessments during the first 2 weeks after liso-cel infusion, we were unable to capture useful information on the immediate impact of liso-cel on HRQoL. In addition, we were unable to determine whether HRQoL at screening was associated with eventual receipt of liso-cel, because the HRQoL assessments were not performed at screening. This analysis could be explored in future studies. Finally, patients enrolled before Protocol Amendment 4 was approved did not have PRO assessments, which resulted in <70% of the liso-cel–treated LBCL set being included in the present analysis.
In summary, although physical functioning deteriorated overall during the first month after liso-cel infusion, patients with R/R LBCL experienced short- and long-term improvements, extending to 12 months, in HRQoL and symptom severity across several EORTC QLQ-C30 scales. Overall, a notable proportion of patients demonstrated clinically meaningful improvements in HRQoL and symptoms at various time points across prespecified scales.
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Acknowledgments
Medical writing and editorial support were provided by Stephen Gilliver of Evidera (Bethesda, MD) and Meredith Rogers of The Lockwood Group (Stamford, CT), funded by Bristol Myers Squibb.
This study was funded by Juno Therapeutics, a Bristol Myers Squibb Company.
Authorship
Contribution: A.P., D.G.M., and J.G. conceived and planned the framework and analysis; C.D. and D.G.M. assisted with acquisition of the data; D.L.P. analyzed the quality of life data; all authors assisted with data analysis and data interpretation and participated in drafting the manuscript, provided critical feedback, and read and approved the final version; and all authors had full access to the data in the study and take responsibility for the integrity of the data, the relevance of the methodology, and the accuracy of the data analysis.
Conflict-of-interest disclosure: D.L.P. has received honoraria from Juno Therapeutics, Celgene, Allakos, and Bristol Myers Squibb, and research funding through the University of Washington and Fred Hutchinson Cancer Center. C.D., Y.K., M.P.J., and A.P. are employees of Bristol Myers Squibb and hold stock in the company. J.G. was an employee of Bristol Myers Squibb when the work reported in this article was done and holds stock in the company; his current affiliation is Umoja Biopharma, Seattle, WA. D.G.M. has received honoraria for consulting from A2 Biotherapeutics, Amgen, BioLineRx, Bristol Myers Squibb, Celgene, Genentech, Gilead, Juno Therapeutics, Kite Pharma, MorphoSys, Novartis, and Pharmacyclics and research funding (paid to his institution, Fred Hutchinson Cancer Research Center) from Bristol Myers Squibb, Celgene, Juno Therapeutics, and Kite Pharma; holds stock options in A2 Biotherapeutics and has patents pending (not issued or licenses, no royalties or licenses) from Juno Therapeutics.
The current affiliation for J.G. is Umoja Biopharma, Seattle, WA.
Correspondence: Donald L. Patrick, School of Public Health, University of Washington, 3980 15th Ave NE, Fourth Floor, Box 351621, Seattle, WA 98195-1621; e-mail: donald@uw.edu.
References
Author notes
The full-text version of this article contains a data supplement.