Key Points
Age, bone marrow reserve (CD34+ cells/µL, white blood cells, platelet count), and SCD-specific factors impact HSC mobilization.
Specific to SCD, hospitalization frequency, chronic pain, and duration hydroxyurea was held premobilization were predictive of HSC yield.
Abstract
Recent studies suggest that plerixafor mobilization and apheresis in patients with sickle cell disease (SCD) is safe and can allow collection of sufficient CD34+ hematopoietic stem cell (HSC) collection for clinical gene therapy applications. However, the quantities of plerixafor-mobilized CD34+ cells vary between different SCD patients for unknown reasons. Twenty-three participants with SCD underwent plerixafor mobilization followed by apheresis, processing, and HSC enrichment under a phase 1 safety and efficacy study conducted at 2 institutions. Linear regression or Spearman's correlation test was used to assess the relationships between various hematologic and clinical parameters with total CD34+ cells/kg collected. Median CD34+ cells/kg after 2 or fewer mobilization and apheresis cycles was 4.0 × 106 (range, 1.5-12.0). Similar to what is observed generally, CD34+ yield correlated negatively with age (P < .001) and positively with baseline (P = .003) and preapheresis blood CD34+ cells/µL (P < .001), and baseline white blood cell (P = .01) and platelet counts (P = .03). Uniquely for SCD, CD34+ cell yields correlated positively with the number of days hydroxyurea was held (for up to 5 weeks, P = .01) and negatively with markers of disease severity, including hospitalization frequency within the preceding year (P = .01) and the number of medications taken for chronic pain (P = .002). Unique SCD-specific technical challenges in apheresis were also associated with reduced CD34+ cell collection efficiency and purification. Here, we describe factors that impact plerixafor mobilization success in patients with SCD, confirming known factors as described in other populations in addition to reporting previously unknown disease specific factors in patients with SCD. This trial was registered at www.clinicaltrials.gov as #NCT03226691.
Introduction
Sickle cell disease (SCD) is an inherited monogenic disorder that can be corrected by allogeneic hematopoietic stem cell (HSC) transplantation or autologous transplantation of genetically modified HSCs.1-3 Genetic modification followed by subsequent engraftment of a sufficient number of autologous HSCs capable of lifelong hematopoietic potential is required for the success of genetic strategies. For most autologous HSC therapies outside of SCD, granulocyte colony-stimulating factor mobilization followed by apheresis collection results in high CD34+ cell yields, with faster hematologic recovery compared with bone marrow (BM)-derived HSCs.4 However, granulocyte colony-stimulating factor is contraindicated in SCD because of a high risk of life-threatening complications resulting from hyperleukocytosis and neutrophil activation.5-7
Until recently, HSCs for autologous therapy of SCD were collected by BM aspiration, a painful procedure that requires general anesthesia. However, we and others showed that plerixafor has an acceptable safety profile for mobilizing HSCs in individuals with SCD.8-11 Plerixafor is a bicyclam compound that reversibly inhibits the binding of stromal cell–derived factor-1α to its cognate receptor C-X-C chemokine receptor type 4 (CXCR4) on HSCs,12-15 resulting in the rapid mobilization of CD34+ HSCs into the circulation within a few hours of administration without inducing hyperleukocytosis or neutrophil activation.16-18 Plerixafor-mobilized HSCs demonstrate a superior quality compared with BM-derived HSCs in individuals with SCD as plerixafor-mobilized HSCs in SCD are enriched for long-term engrafting HSCs, which is not true of HSCs from SCD BM.9,19
The minimally acceptable dose of CD34+ cells needed for successful engraftment and long-term multilineage reconstitution in autologous HSC recipients is ∼2 × 106 CD34+ cells/kg; however, higher doses (∼10-15.0 × 106 cells/kg) are likely required for gene therapy applications to account for incomplete CD34+ recovery after selection, transduction inefficiencies, and reduced viability after genetic manipulation.20 We reported our initial experience with peripheral blood (PB) HSC mobilization and collection with single-agent plerixafor in 15 adult participants with SCD at the National Institutes of Health (NIH) and St. Jude Children’s Research Hospital (St. Jude).9 Median cell yield was 4.2 × 106 CD34+ cells/kg, 97% of which demonstrated a CD34high phenotype suggesting that they are long-term engrafting HSCs. Seven (46%) participants achieved a CD34+ cell yield ≥5.0 × 106 cells/kg after 1 to 2 plerixafor/apheresis cycles, suggesting that this approach allows high HSC yields sufficient for clinical gene therapy applications in approximately one-half of all subjects. Historically, approximately one-third of healthy donors fail to mobilize ≥2 × 106 CD34+ cells/mL after a single 240 µg/kg dose of plerixafor, necessitating a second cycle. Several factors are associated with higher CD34+ cell mobilization in healthy adults (ie, younger age, higher baseline platelet and circulating CD34+ counts, and male sex).21 The variables that impact HSC mobilization in patients with SCD are not known.
Here, we present an expanded cohort of participants with SCD who underwent plerixafor mobilization, apheresis, processing, and HSC enrichment at the NIH and St. Jude, with attention to the hematologic and clinical factors associated with total CD34+ cell yield. Increased CD34+ cell yields were associated with clinical factors that are known to enhance HSC mobilization in healthy adults and non-SCD disorders, such as young age and BM reserve as measured by CD34+ cells/µL, white blood cell (WBC), and platelet counts. Conversely, unique SCD variables including hydroxyurea (HU) exposure, disease severity as measured by hospitalization frequency in the preceding year, and number of medications required to treat chronic pain, were associated with a reduced CD34+ yield. The severity of SCD may impair HSC collection by damaging those cells directly or by altering their hematopoietic niche.
Methods
Participant selection
This was an open-label phase 1 study to determine the safety of plerixafor mobilization and apheresis in patients with SCD. It was sponsored by the National Heart, Lung, and Blood Institutes at the NIH, and was conducted at the NIH Clinical Center and St. Jude. All patients provided written informed consent on a protocol approved by each local institutional review board. HU was stopped at least 2 weeks before mobilization, and all participants received red blood cell (RBC) exchange the day before mobilization and collection to target <30% sickle hemoglobin (HbS).
Study design
Study design, safety, and product assessment were performed as previously described.9 Participants received a single-dose subcutaneous (SC) administration of 240 µg/kg plerixafor (Mozobil, Sanofi, Bridgewater, NJ) followed approximately 4 hours later by leukapheresis using the Spectra Optia apheresis system (Terumo BCT Inc., Lakewood, CO). Kinetics data suggested that mobilization of CD34+ cells start within 2 hours after SC plerixafor administration,22 peaking at 3 to 6 hours in subjects with SCD compared with 6 to 12 hours in healthy donors.8,23 Acid citrate dextrose formula A was used for intraprocedural anticoagulation, with an initial whole blood to anticoagulant ratio of 12:1. Prophylactic intravenous calcium infusions were used in all cases. During apheresis, operators went slightly deeper into the buffy coat, targeting a hematocrit of 5% to 6%. If the minimum target CD34+ cell dose of 1.5 × 106 cells/kg (goal target, 2.0 × 106 cells/kg) was not obtained, a second SC dose (240 µg/kg) of plerixafor was administered the next day followed by repeat collection. PB CD34+ cells/mL were measured by flow cytometry (BD FACSCanto II, BD Biosciences, San Jose, CA). Blood collection intervals, product assessment, CD34+ selection, and participant follow-up were as previously described.9 Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Assessment parameters
Several clinical markers were assessed in relationship to total CD34+ cell/kg collection: demographics (age, sex); CD34+ cells/µL at baseline (before plerixafor) and preapheresis (4 hours after plerixafor, just before apheresis); baseline blood counts (WBC, hemoglobin, platelet count, absolute reticulocyte count [ARC]) as defined by the complete blood count before plerixafor; hemoglobin content (pre- and posttransfusion HbS%, fetal hemoglobin percent [HbF%]); markers of hemolysis (lactate dehydrogenase [LDH], aspartate aminotransferase [AST], total bilirubin, and alkaline phosphatase [ALK]); C-reactive protein as a marker of overall inflammation; duration HU was held before mobilization and collection; and indicators of disease severity as measured by hospitalization frequency in the preceding year and number of medications required to treat chronic pain. Unless otherwise noted, data are presented as total CD34+ cells/kg collected after a single mobilization and apheresis cycle.
Statistical analysis
Linear regression or a Spearman's correlation test was used to assess the relationship between each variable and total CD34+ cells/kg collected. Spearman’s correlation was chosen when the normal normal/Gaussian distribution assumption was not met. To account for the interplay of known predictors of CD34+ collection in healthy donors and SCD-related specific variables, regression analyses using partial correlation were performed to control for the differences in baseline status of known confounders. A P value ≤.05 was considered statistically significant.
Results
Participant characteristics
Twenty-three participants with SCD (HbSS, n = 20; HbSC, n = 1; HbSβ+, n = 2) who met inclusion criteria were enrolled at St. Jude (n = 3) or NIH (n = 20) between July 2017 and October 2020. One participant described in the initial cohort9 was excluded. This individual had relapsed SCD after haploidentical transplantation, which is likely to produce a dominant effect on HSC mobilization kinetics and is atypical for most SCD individuals seeking autologous HSC therapy. Median age was 29 years (range, 20-50) and 61% were male (n = 14). Mean hemoglobin was 9.4 g/dL (range, 7.3-13.6) with an average %HbS pre- and postexchange transfusion of 55.9% (range, 18.1-87.1) and 26.4% (range, 15.1-37.7), respectively. Most participants were on HU before study entry (n = 20, 87%). Six subjects (26%) were on chronic transfusions (CT). The most common baseline SCD-related complications before study entry were vaso-occlusive crisis (VOC) (n = 18), iron overload (n = 15), acute chest syndrome (n = 13), and stroke (n = 9). The hematologic and clinical characteristics, including all SCD-related complications are summarized in supplemental Table 1.
The average number of hospitalizations in the preceding year before plerixafor administration was 5.0 (range, 0-13). The average number of medications taken for chronic pain was 2 (range, 0-6). Medications were included if they were self-reported to be taken on a regular basis. These included nonsteroidal anti-inflammatories, oxycodone and its derivatives, and acetaminophen plus codeine, methadone, morphine, hydromorphone, or gabapentin.
Plerixafor mobilization of HSCs is effective for most subjects with SCD
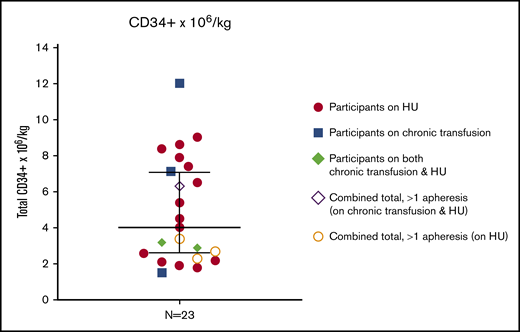
Eighty-seven percent of participants (n = 20) successfully met the target CD34+ cells/kg yield (2.0 × 106 CD34+ cells/kg) within 1 or 2 mobilization and apheresis procedures. The 3 remaining participants met the minimum goal (1.5 × 106 CD34+ cells/kg) required for day 1 apheresis (range, 1.5-1.9), but did not reach the target goal. Median CD34+ collection was 4.0 × 106 CD34+ cells/kg (range, 1.5-12.0) (Figure 1).
Total CD34+collection. Total CD34+ cells/kg were assessed in the final product after 1 (n = 19) or 2 (n = 4) mobilization and apheresis procedures. Median values with 95% confidence interval shown.
Total CD34+collection. Total CD34+ cells/kg were assessed in the final product after 1 (n = 19) or 2 (n = 4) mobilization and apheresis procedures. Median values with 95% confidence interval shown.
Three participants did not achieve the minimum CD34+ target after day 1 and underwent a second mobilization and apheresis procedure on the following day to achieve a sum total of 2.3 × 106, 2.7 × 106, and 3.4 × 106 CD34+ cell/kg, respectively. A fourth participant underwent repeat collection on day 2 despite meeting the initial target after one apheresis (total day 1 = 2.9 × 106 CD34+ cells/kg) to store additional backup HSCs before an allogeneic transplant protocol. Fewer than one-half of the participants (n = 10) achieved a total yield of ≥5.0 × 106 CD34+ cells/kg in 1 or 2 mobilization/collection cycles.
Nine grade 3 AEs (2 nonpain- and 7 pain-related) and 1 grade 4 AE (nonpain: delayed hemolytic transfusion reaction related to exchange transfusion) occurred, and each resolved with symptomatic treatment. In addition to the AEs previously described,9 2 patients in the expanded cohort had exacerbation of chronic pain requiring treatment with IV narcotics (grade 3).
Total CD34+ yield in participants with SCD is associated with factors that impact mobilization in healthy adults
There was a positive correlation between baseline CD34+ cells/µL and preapheresis CD34+ cells/µL (rs = 0.7606, P ≤ .001). Total CD34+ cells/kg therefore correlated positively with both baseline (rs = 0.5735, P = .003) and preapheresis CD34+ cells/µL (rs = 0.8159, P ≤ .001) (preapheresis correlation shown in Figure 2A). Participants with the lowest preapheresis CD34+ cells/µL demonstrated the lowest total CD34+ cells/kg yield, regardless of the number of blood volumes processed. The absolute fold change in baseline to preapheresis CD34+ cells/µL did not reach statistical significance.
Correlation of total CD34+collection with participant CD34+cells/µL, age, and sex. (A) Association of preapheresis CD34+ cells/µL to total CD34+ cells/kg. (B) Association of age with total CD34+ cells/kg. (C) Median CD34+ cell/kg for males vs females. Median values with 95% confidence interval shown. □, Participants on chronic transfusion and hydroxyurea (HU); ○, participants on chronic transfusions; ●, participants on HU.
Correlation of total CD34+collection with participant CD34+cells/µL, age, and sex. (A) Association of preapheresis CD34+ cells/µL to total CD34+ cells/kg. (B) Association of age with total CD34+ cells/kg. (C) Median CD34+ cell/kg for males vs females. Median values with 95% confidence interval shown. □, Participants on chronic transfusion and hydroxyurea (HU); ○, participants on chronic transfusions; ●, participants on HU.
Younger participants achieved higher total CD34+ cells/kg yield (rs = −0.6496, P ≤ .001) (Figure 2B). As demonstrated in Figure 2B, participants ≤30 years of age had a higher median total CD34+ collection (6.4 × 106 CD34+ cells/kg; range, 1.9-12.0) compared with participants ≥31 years of age (2.3 × 106 CD34+ cells/kg; range, 1.5-4.0). Three participants aged 21 to 23 years achieved lower than average collection compared with other similarly aged participants. These participants all had relatively low baseline (range, 5.9-6.8) and preapheresis CD34+ cells/µL (range, 14.9-41.6).
Median single collection was lower for females (2.6 × 106 CD34+ cells/kg; range, 1.1-7.1) vs males (3.6 × 106 CD34+ cells/kg; range, 0.9-12.0); however, the difference was not significant (Figure 2C). The average age of females vs males was not different; however, males were noted to have higher preapheresis CD34+ cells/µL (97.7 cells/µL ± 60.0 vs 45.7 cells/µL ± 44.6, P = .03). The 3 participants who met the minimum goal (1.5 × 106 CD34+ cells/kg) required for day 1 apheresis but did not achieve the target collection (>2 × 106 CD34+ cells/kg) were all female.
In general, participants with higher baseline WBC (rs = 0.4916, P = .01) and platelet counts (rs = 0.4333, P = .03) achieved higher total CD34+ cells/kg (Figure 3A-B). There was no correlation with RBC indices, including total hemoglobin (rs = −0.0172, ns) or ARC (rs = 0.2452, NS) (Figure 3C-D).
Correlation of total CD34+collection with hematologic parameters. (A-D) Association of hematologic parameters (WBC [A], platelets [B], hemoglobin [C], and ARC [D]) with total CD34+ cells/kg. □, Participants on chronic transfusion and hydroxyurea (HU), ○, participants on chronic transfusions; ●, participants on HU.
Correlation of total CD34+collection with hematologic parameters. (A-D) Association of hematologic parameters (WBC [A], platelets [B], hemoglobin [C], and ARC [D]) with total CD34+ cells/kg. □, Participants on chronic transfusion and hydroxyurea (HU), ○, participants on chronic transfusions; ●, participants on HU.
CD34+ cell yields are associated with some indicators of disease severity and hydroxyurea exposure
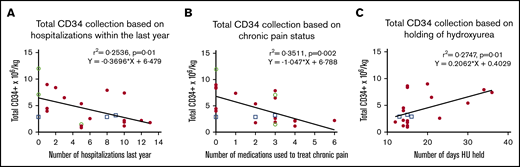
The number of hospitalizations in the year preceding enrollment was inversely correlated with total CD34+ cells/kg (r2 = 0.2536, P = .01) (Figure 4A). Participants who required multiple medications to control chronic pain demonstrated a lower total yield (r2 = 0.3511, P = .002) (Figure 4B). Although HU was held at least 2 weeks before mobilization in all participants, the CD34+ cell yield was greater in those who discontinued HU for a longer time (r2 = 0.2747, P = .01) (Figure 4C). This relationship did not plateau after the maximal interval of 35 days. There was no difference in CD34+ cell yield among participants on HU (n = 17) vs CT (n = 3) vs HU+CT (n = 3) (NS).
Correlation of total CD34+collection with disease severity and duration of HU washout. (A) Association of self-reported number of hospitalizations in the year before enrollment and total CD34+ cells/kg. (B) Association of self-reported number of medications taken on a daily basis to control chronic pain and total CD34+ cells/kg. (C) Association of number of days hydroxyurea (HU) was held before mobilization and apheresis total CD34+ cells/kg. □, Participants on chronic transfusion and hydroxyurea (HU), ○, participants on chronic transfusions; ●, participants on HU.
Correlation of total CD34+collection with disease severity and duration of HU washout. (A) Association of self-reported number of hospitalizations in the year before enrollment and total CD34+ cells/kg. (B) Association of self-reported number of medications taken on a daily basis to control chronic pain and total CD34+ cells/kg. (C) Association of number of days hydroxyurea (HU) was held before mobilization and apheresis total CD34+ cells/kg. □, Participants on chronic transfusion and hydroxyurea (HU), ○, participants on chronic transfusions; ●, participants on HU.
On secondary analysis controlling for known factors associated with total CD34+ yield (age, CD34 cells/µL [baseline and preapheresis], and platelet count), correlation to the number of hospitalizations (P = .01) and number of chronic pain meds (P = .03) remained. After additionally controlling for known factors and the length of time HU was held, the negative correlation to the number of pain medications remained (P = .03), whereas the number of hospitalizations did not (NS). Assessment parameters with corresponding P values are listed in Table 1.
Assessment parameters
| Correlation to total CD34 cells/kg yield . | Assessment variable . | P . |
|---|---|---|
| Positive correlation | Baseline CD34/µL | .003 |
| Preapheresis CD34/µL | <.001 | |
| WBC (109/L) | .01 | |
| Platelet count (109/L) | .03 | |
| Number of days HU held | .01 | |
| Negative correlation | Age | <.001 |
| Hospitalizations previous year | .01 | |
| Number of chronic pain medications | .002 | |
| No correlation | Sex | .11 |
| Hemoglobin (g/dL) | .94 | |
| ARC (103/µL) | .26 | |
| Pretransfusion HbS% | .82 | |
| Posttransfusion HbS% | .55 | |
| HbF% | .14 | |
| Lactate dehydrogenase (U/L) | .28 | |
| Aspartate aminotransferase (U/L) | .37 | |
| Total bilirubin (mg/dL) | .89 | |
| Alkaline phosphatase (U/L) | .63 | |
| C-reactive protein (mg/mL) | .92 |
| Correlation to total CD34 cells/kg yield . | Assessment variable . | P . |
|---|---|---|
| Positive correlation | Baseline CD34/µL | .003 |
| Preapheresis CD34/µL | <.001 | |
| WBC (109/L) | .01 | |
| Platelet count (109/L) | .03 | |
| Number of days HU held | .01 | |
| Negative correlation | Age | <.001 |
| Hospitalizations previous year | .01 | |
| Number of chronic pain medications | .002 | |
| No correlation | Sex | .11 |
| Hemoglobin (g/dL) | .94 | |
| ARC (103/µL) | .26 | |
| Pretransfusion HbS% | .82 | |
| Posttransfusion HbS% | .55 | |
| HbF% | .14 | |
| Lactate dehydrogenase (U/L) | .28 | |
| Aspartate aminotransferase (U/L) | .37 | |
| Total bilirubin (mg/dL) | .89 | |
| Alkaline phosphatase (U/L) | .63 | |
| C-reactive protein (mg/mL) | .92 |
Bold indicates P ≤ .05.
All subjects received exchange transfusion therapy before HSC mobilization. However, the total CD34+ yield was independent of hemoglobin content including pre-exchange transfusion HbS% (rs = −0.0494, NS), postexchange transfusion HbS% (rs = −0.1384, ns), and HbF% (rs = −0.3094, NS) (supplemental Figure 1A-C). There was no correlation to markers of hemolysis including total bilirubin (rs = −0.0285, ns), LDH (rs = −0.2354, ns), ALK (rs = 0.1023, NS), and AST (rs = 0.1924, NS) (supplemental Figure 2A-D). Additionally, a high C-reactive protein did not correlate to overall yield (rs= 0.0235, ns) (supplemental Figure 2E).
Technical challenges in apheresis are associated with reduced CD34 yield and collection efficiency
The average total liters of blood processed was 18.7 L (range, 10.6-30.0). The overall mean ± standard deviation for CD34+ ×106 per blood volume processed was 97.5 ± 80.6 × 106/blood volume processed. The mean CD34+ cell yield/L of whole blood processed was significantly lower (19.4 ± 14.8 × 106/L processed) than seen in healthy allogeneic HSC apheresis donors of comparable age and race at the NIH (60.6 ± 39.0 × 106/L processed). There was a strong positive correlation between yield/L processed and preapheresis CD34+ cells/µL (R2 = 0.89, P < .001). The maximal extracorporeal blood volume was 285 to 297 mL, with a mean bag hematocrit at the completion of the procedure of 5.5 ± 2.2% (target, 5%-6%). Mean CD34 collection efficiency was lower (38.6 ± 22.8%) than demographically comparable allogeneic donors (66.3 ± 12.4%). Significant instability of the collection interface was noted in 32% procedures, with occasional visible clumps in the apheresis layer. In these cases, the whole blood:anticoagulant ratio was reduced (from 12:1 to 10:1 or 8:1) to increase the acid citrate dextrose formula A infusion rate in an attempt to resolve the interface instability. However, in some cases, instability persisted for the entire procedure. Being subjective and operator dependent, apheresis by experienced personnel for patients with SCD is recommended.
Discussion
We and others have demonstrated that plerixafor mobilization and apheresis in patients with SCD has an acceptable safety profile and allows sufficient collection of CD34+ HSCs in most but not all participants. To be eligible for gene therapy applications, collection of a sufficiently high number of autologous HSCs is required. To begin to address that not all participants with SCD will mobilize sufficient HSCs after 1 or 2 mobilization/apheresis cycles, we expanded our cohort and reanalyzed our data to specifically identify factors that impact mobilization success in these patients.
Plerixafor mobilization in this expanded cohort of participants with SCD from 2 clinical sites resulted in a median of 4.0 × 106 CD34+ cells/kg. Participants with the lowest total CD34+ collection were older, had lower baseline and preapheresis CD34+ cells/µL, had lower WBC and platelet counts, and held home HU for the minimally recommended period. Clinically, participants with lower collection had indicators of greater disease severity, including frequent hospitalizations and chronic pain requiring multiple medications.
Consistent with apheresis literature, participants with the lowest baseline and preapheresis CD34+ cells/µL counts had the lowest total CD34+ collection.24-26 There was a stronger correlation in preapheresis CD34+ cells/µL count to total collection as some participants with a low baseline CD34+ cells/µL count responded well to plerixafor mobilization. Four participants with a baseline CD34+ cells/µL <12 cells/µL (range, 7.5-12) had greater than 10-fold change in CD34+ cells/µL at preapheresis relative to their baseline, and achieved some of the highest total CD34+ collections of the cohort (5.4-12.0 × 106 CD34+/kg). A baseline CD34+ cells/µL is readily available on all patients who may present for mobilization and collection and may set some but not all expectations for the success of collection; those with baseline CD34+ cells/µL <5 cells/µL are more likely to have poor collection and/or require repeat mobilization, but other factors may impact plerixafor response. Of the 4 participants with a lower baseline CD34+ cells/µL but good mobilization and collection, all were male and younger (<30 years). Three participants in their early 20s with lower than average collection compared with other similarly aged participants could be identified by a baseline CD34 count <7 CD34/µL with smaller fold change in CD34+ cells/µL at preapheresis relative to their baseline.
Some studies suggest that HSC mobilization decreases with age, for example at ages >50 years in normal donors and individuals with hematopoietic malignancies.27-31 Our data indicate that in SCD, HSC yields begin to decline at an even earlier age. Strikingly, 8 of 9 individuals >30 years had CD34+ cell yields <2 × 106/kg after 2 cycles of mobilization/apheresis (Figure 2B). SCD is a chronic disease associated with cumulative ischemic and inflammatory multiorgan damage. It is likely that these effects in the hematopoietic compartment are likely to accelerate age-related declines in PB HSC mobilization noted in non-SCD individuals.28-31 Overtime, the recurrent use of hospital services to manage disease complications is associated with adverse clinical outcomes including early mortality.32-43 In this study, individuals with greater hospitalization frequency within the previous year in addition to individuals on multiple medications for chronic pain demonstrated the lowest CD34+ collection. Of note, the 3 participants with 8 to 10 hospitalizations in the previous year who achieved a final yield of >6 × 106 CD34/kg after 1 or more apheresis were age <30 years with an elevated baseline CD34+ cells/µL. Our findings suggest that HSC mobilization/collection before age 30 may therefore facilitate the success of autologous gene therapy in SCD. It may be prudent to counsel patients accordingly and consider HSC collection and banking for future consideration of autologous therapy as subjects approach age 30 years. In future studies, it will be informative to study the effects of aging in SCD on HSC quality19 and to determine whether HSC yield could be improved in aging patients by increased dose plerixafor or alternative mobilization regimens including combination regimens.
HU cessation is associated with an increase in the number of circulating CD34+ cells in patients with SCD,44 and is associated with successful mobilization in patients with thalassemia when there is a washout period of at least 2 weeks before mobilization and collection.45 Here, we demonstrate higher CD34+ collection in those who held HU longer suggesting a washout period of 20 to 30 days or longer may be required to negate the negative impact on HSC collection. Because patients were switched to transfusion (simple or exchange) therapy upon HU cessation, it is also possible that transfusions played a role in improving CD34+ cell mobilization. Compared with thalassemia, HU treatment is the major approved medication with decades of experience and proven benefits in the treatment of SCD. The beneficial effects of HU persist when pharmacokinetics are maintained, with effects that are reversible upon cessation. Given that many patients interested in autologous gene therapy are likely to have been on HU for a prolonged period, a longer washout may be necessary to negate the myelosuppressive effect of HU.4 We suggest cessation of HU 30 days before planned mobilization with a consideration of simple or exchange transfusion just before cessation to minimize any potential flare of SCD symptoms while off of HU. We did not detect a difference in CD34+ cell yield in participants on HU vs CT; however, the number of participants on CT were small with a wide range of total CD34+ cell collection. Though there was a reasonable number of participants with iron overload in this study, we did not detect an impact on CD34+ yield.
None of the participants in this study were on the more recently US Food Drug Administration–approved therapies such as crizanlizumab or voxelotor, and it is unknown how these agents may impact CD34+ collection. The use of crizanlizumab compared with placebo reduced the median VOC rate/year during a 1-year period; however, median rates of days hospitalized were not significantly different.46 In post hoc analysis, crizanlizumab treatment increased the likelihood of patients with a high number of prior VOCs being VOC event-free and delaying the time to first VOC,47 which could have an impact on CD34+ collection as described in this study. Voxelotor was reported to improve total hemoglobin48 ; however, we found no association to hemoglobin level and total CD34+ cell collection and thus it is unlikely to impact total yield.
Last, there was no association to final HSC yield and known biochemical markers of hemolysis or inflammation. RBC survival studies show LDH, AST, and bilirubin do not correlate to RBC survival, whereas the ARC is a useful surrogate marker of hemolysis and a reflection of the BM’s response to anemia.49,50 Several of the participants in this study with an elevated ARC but a low total CD34+ collection had baseline CD34+ cells/µL <5 cells/µL, potentially explaining the lack of correlation of ARC to total yield. There was no correlation to total hemoglobin and pre- and postexchange transfusion HbS%, whereas those with a higher WBC and platelet count at baseline had a higher yield. Higher baseline WBC and platelet counts in patients with SCD are associated with increased clinical severity43 ; however, the association in this study to a higher total CD34+ yield is more likely a reflection of bone marrow activation and enhanced hematopoiesis.29,51
Despite a relatively small sample size, we have identified multiple potential predictors of mobilization success in patients with SCD. The failure to collect enough HSCs is an important and potentially frequent and underreported problem in this patient population; therefore, mobilization data should be reported as part of all gene therapy studies and can confirm disease-specific findings reported here. Real-time CD34+ measurements throughout the apheresis process will further elucidate plerixafor mobilization kinetics in this patient population, enabling providers an ability to individualize and maximize collection. Furthermore, better qualitative and quantitative measurements of HSCs are needed to qualify mobilized HSCs, measure capacity for long-term survival after genetic manipulation, and allow greater understanding of hematopoietic changes after long-term HU use and with age. For poor mobilizers, higher dose plerixafor, combination therapy with newer mobilization agents such as GroBeta,52 or HSC expansion agents such as aryl hydrocarbon receptor may be required to make autologous gene therapy a realistic option, particularly to those with severe disease who may benefit most.
Conclusion
A sufficient quantity of long-term engrafting HSCs is required for successful gene therapy applications. Based on the results of this study we recommend the following considerations: (1) cessation of HU at least 30 days before HSC collection; (2) informed discussion of HSC collection and banking for future consideration of autologous therapy before age 30 or sooner in those with a more severe phenotype; and (3) robust reporting of mobilization data in patients with SCD to validate this study and others. Curative strategies for patients with SCD are reserved for those with severe disease; therefore, accurate prediction and expectation for mobilization success is imperative to allow informed discussion for patients. Simultaneously, investigation into expanded options for HSC collection in patients with SCD is urgently needed.
For deidentified original data, please contact alexis.leonard@nih.gov or matthewhs@nhlbi.nih.gov.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute and National Institute of Diabetes and Digestive and Kidney Diseases. This study was also partially funded by the Doris Duke Charitable Foundation Sickle Cell Disease/Advancing Cures (grant 2017093) (S.Q.T., A.S., and M.J.W.) and National Institutes of Health, National Heart, Lung, and Blood Institute (P01 HL053749) (M.J.W. and S.Q.T.), The Assisi Foundation of Memphis (M.J.W.), and St. Jude/ALSAC. Plerixafor (Mozobil) was provided by Sanofi, Bridgewater, NJ.
Authorship
Contribution: A.L. collected data and analyzed results, made the figures, and wrote the paper; D.S. designed the research; S.R.P., T.E.H., S.H., T.T., M.W., E.M., and S.Q.T. performed experiments; K.W., J.S.H., and A.S. performed experiments and edited the paper; C.F. and M.J.W. designed the research and edited the paper; and N.U., M.H., and J.F.T. designed the research, wrote the clinical protocol, performed experiments, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew Hsieh, Cellular and Molecular Therapeutics Branch, NHLBI/NIDDK, NIH, 9000 Rockville Pike, Building 10, 9N112, Bethesda, MD 20892; e-mail: matthewhs@nhlbi.nih.gov.
References
Author notes
The full-text version of this article contains a data supplement.



![Correlation of total CD34+collection with hematologic parameters. (A-D) Association of hematologic parameters (WBC [A], platelets [B], hemoglobin [C], and ARC [D]) with total CD34+ cells/kg. □, Participants on chronic transfusion and hydroxyurea (HU), ○, participants on chronic transfusions; ●, participants on HU.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/9/10.1182_bloodadvances.2021004232/4/m_advancesadv2021004232f3.png?Expires=1765929910&Signature=ULcbYanLG7M3DXsB4PEWlcpvSj8ukSIyX-FhG8u3wpZf1HOuqqI9EitKY~fQsX3q8IGeKJMFVssT441twOqu~p53Bh689g6beGtceDnvIb897nHpMZWinW7LYNKW9ktCu8b9pqaqBbqwy4OkB9B3Y1pnZ5aoJqk6TuP4PC8YYV-hpRwJzR1Gv40DP4W3H6JsrJ9iSlGwfRpJE4OriMQCP44bV0B1RFHSVND6ESVoYFL~BW55VSOw8fVDVjhcyU3bYmsP9l~XYiFtmYT7voJZJNOcgKFxFRrb~kntAOBCVhifOKa~m8dbAED17-EWszZAc4~KXNhae7S-XnUvAbG43Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)