Abstract
The dilemma of whether to treat elderly patients with diffuse large B-cell lymphoma (DLBCL) with a full or reduced dose intensity (DI) of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone+rituximab) is often faced by clinicians. We conducted a systematic review assessing the impact of R-CHOP DI on DLBCL survival outcomes, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines. We searched MEDLINE, EMBASE, and Cochrane CENTRAL for studies with ≥100 patients treated with R-CHOP/R-CHOP–like therapies published from January 2002 through November 2020. Studies were included if they reported the impact of R-CHOP DI on survival outcomes. We screened records, extracted data, and reviewed all the studies for quality and statistical appraisal. Of 380 screened records, 13 studies including 5188 patients were reviewed. DI was often calculated as the ratio of the cumulative delivered dose of prespecified drug(s) to the cumulative planned dose multiplied by a time-correction factor. Lower DI (intended or relative) was associated with inferior survival in 7 of 9 studies reporting crude survival analyses. Multivariable analysis using DI as a covariate was performed in 10 studies. Six showed an association (P < .05) with adjustment for other covariates, and 4 did not. Most studies and those larger studies of higher quality showed poorer outcomes associated with reduced DI. In subgroups aged ≥80 years, survival was not consistently affected by reduced DI. DI-specific randomized trials are warranted, but these data support full-dose R-CHOP in elderly and fit patients aged <80 years with DLBCL, but not in those aged ≥80 years, where dose-reduced R-CHOP does not appear to compromise survival.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) in the Western world. With a median age at diagnosis in the seventh decade of life, DLBCL is predominantly a disease of the elderly. The optimal strategy for elderly DLBCL patients requires a careful assessment of the risk of treatment-related mortality/morbidity vs residual life expectancy in a group for whom quality of life may outweigh the quantity of life. Treatment decisions are often highly individualized, considering the patient’s overall health, comorbidities, personal preferences, and willingness to accept the risk associated with conventional intensity therapy.
R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab) has remained the standard frontline treatment for almost 2 decades. The pivotal trial comparing R-CHOP with CHOP enrolled patients 60 to 80 years of age with an Eastern Cooperative Oncology Group performance score of 0 to 2 and a general health status that permitted 8 cycles of full-dose R-CHOP.1 Similar criteria have been used in other prospective trials.2 Patients treated outside of trials are generally older and frailer with more comorbidities resulting in more R-CHOP toxicity, dose reductions, or delays. Older fit patients are increasingly being considered for full-dose R-CHOP, despite the comorbidities caused by patient preferences and better supportive care (eg, granulocyte colony-stimulating factor [G-CSF] and prephase therapy). However, among very elderly (aged ≥80 years) patients, dose-reduced R-CHOP was supported by a phase 2 study (150 patients), in which the 2-year overall survival (OS) was 59%.3 The decision to opt for dose-reduced R-CHOP can be challenging because of a lack of randomized trials documenting equivalent efficacy to standard R-CHOP and a perception that dose reduction may compromise outcomes. Without universally accepted selection criteria for full-dose R-CHOP, a significant minority may start at full dose but then face early discontinuation and/or dose reduction of doxorubicin and/or cyclophosphamide related to poor tolerability. In a recent population-based study of 1011 elderly patients, 44% of patients aged >75 years and commencing full-dose R-CHOP experienced dose reduction or early discontinuation.4 The decision to reduce dose represents a trade-off in risk between toxicity and efficacy. The potential loss of efficacy is critical in elderly patients for whom high-dose therapy and autologous stem-cell transplant are not feasible and for whom universal access to chimeric antigen receptor CAR T-cell therapy remains limited.5
To date, the impact of reducing R-CHOP dose intensity (DI) on outcomes remains unclear, both for older individuals and for a broader newly diagnosed DLBCL population. Although a systematic review addressed the relative dose intensity (RDI) of chemotherapy in patients with breast cancer and aggressive lymphoma in 2011, it was not specific to any lymphoma histology and included multiple regimens, most of which predated rituximab.6 The purpose of this systematic review was to determine the effect of R-CHOP DI reduction on DLBCL outcomes and to understand how this effect varies across different age groups.
Materials and methods
Search strategy
The review was conducted systematically in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) guidelines7,8 and was registered on the PROSPERO database (CRD42020222073). A comprehensive search was conducted according to a systematic search strategy of the e-databases Ovid MEDLINE, Ovid EMBASE, and Cochrane Central Register of Controlled Trials. The Boolean operators “AND” and “OR” were employed.
Searches included the title and abstract, where possible, and were restricted to the English language. Searches were limited to the period from January 2002 to November 2020, as the pivotal rituximab trial was published in January 2002.1 The strategy comprised 2 main components, using relevant Medical Subject Headings (MeSH) terms where possible. Disease component(s) were searched for with the terms “diffuse large B cell lymphoma,” “DLBCL,” or “diffuse large B-cell lymphoma.” The therapeutic approach component(s) were searched with the terms “dose intensity,” “dose reduction,” “reduced dose,” or “miniCHOP” (supplementary Table 1). Searches were expanded by using the snowballing technique from reference lists, to ensure a fully comprehensive search.
Screening results
Search results were independently double screened in a blinded fashion by the research team (E.J.B., C.Y.C., T.A.E., and T.C.E.-G.) at the abstract and full-text screening stages, using predefined eligibility criteria (Table 1). Disagreements between any 2 researchers were referred to a third researcher to reach a consensus in a blinded fashion.
Key eligibility criteria
| Inclusion criteria . | Exclusion criteria . |
|---|---|
| Studies of DLBCL that included an assessment of the impact of DI of ≥1 drug component of R-CHOP on survival outcomes | Studies with <100 patients DLBCL receiving R-CHOP-21 |
| Most patients newly diagnosed with untreated DLBCL | Previously treated DLBCL |
| Inclusion of alternative histologies permitted, provided these represent a small minority of patients (e.g., transformed follicular lymphoma, PMBL) | Primary CNS lymphoma |
| Included ≥100 patients treated with R-CHOP-21 protocol* | No DDI |
| Full-text articles in the English language | No assessment of the impact of DI on survival outcome† |
| Retrospective studies | Case studies |
| Cohort studies | Review articles |
| Observational studies | Pharmacokinetic studies |
| Controlled trials that include an R-CHOP–treated arm† | Opinion papers |
| Commentaries | |
| Editorials | |
| Case reports | |
| Conference abstracts |
| Inclusion criteria . | Exclusion criteria . |
|---|---|
| Studies of DLBCL that included an assessment of the impact of DI of ≥1 drug component of R-CHOP on survival outcomes | Studies with <100 patients DLBCL receiving R-CHOP-21 |
| Most patients newly diagnosed with untreated DLBCL | Previously treated DLBCL |
| Inclusion of alternative histologies permitted, provided these represent a small minority of patients (e.g., transformed follicular lymphoma, PMBL) | Primary CNS lymphoma |
| Included ≥100 patients treated with R-CHOP-21 protocol* | No DDI |
| Full-text articles in the English language | No assessment of the impact of DI on survival outcome† |
| Retrospective studies | Case studies |
| Cohort studies | Review articles |
| Observational studies | Pharmacokinetic studies |
| Controlled trials that include an R-CHOP–treated arm† | Opinion papers |
| Commentaries | |
| Editorials | |
| Case reports | |
| Conference abstracts |
Variant regimens where doxorubicin is replaced with a derivative anthracycline were accepted.
Nonrandomized, single-arm trials with dose-attenuated R-CHOP, in which no attempt was made to examine the effect of RDI on survival outcome.
Quality appraisal
Standardized Critical Appraisal Skills Program (CASP) tools (https://casp-uk.net/casp-tools-checklists) were used to appraise the quality of individual study designs, methodology, and reporting. CASP tools are specifically designed to appraise each study type reviewed (eg, clinical trial or cohort study). Scores were as follows: 5 (high), 4 (moderate-high), 3 (moderate), 2 (moderate-low), or 1 (low). Key limitations of individual studies were reported transparently.
Data extraction and analysis
Extracted data were reviewed by all the research team and tabulated to summarize key findings. Key data extracted were author and publication year, design, sample characteristics (including age, sample size, R-CHOP DI, and other key inclusion criteria), and reported survival outcomes (eg, progression-free survival [PFS] and OS). To be included, studies were required to have analyzed ≥100 R-CHOP–treated patients with DLBCL. Studies including alternative histologies or regimens other than R-CHOP were accepted, providing that they amounted to a small minority of those analyzed. A detailed description of the DI assessments is outlined in Table 2. Patients are presented according to (1) which agents were analyzed for R-CHOP DI, (2) how DI was calculated, and (3) which thresholds were used for correlative survival analysis. The design(s) and study characteristics, clarity of reporting, and statistical significance of reported data were assessed to determine the strengths and limitations of the evidence. All included studies underwent full statistical review by a statistician with expertise in clinical lymphoma research (M.J.M.).
Studies reporting the impact of DI on survival outcomes in DLBCL
| Reference . | N . | Study design and period . | Inclusion criteria . | Exclusion criteria . | Age, median (range) . | Drugs . | Derivation of dose intensity . | Cutoff RDI used for analysis, % . | Impact of reduced RDI on outcomes . | Assessment of confounders and competing risk . |
|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 100 | Retrospective, multicenter, 2003-2008 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: not specified Minimum no. cycles: 4 | Prior radiotherapy before CHOP; T-cell NHL | 60 y (range not specified) | C, H | RDI: DDI[drug]/SDI[drug].* ARDI: mean of RDIs for C, H The term “RDI” was used in lieu of ARDI in the study. | 87.9 | UVA OS: RDI (per 10% increase): HR, 0.7; 95% CI, 0.6-0.9); P = .02 MVA OS: RDI (per 10% increase): HR, 0.8; 95% CI, 0.6-1.0; P = .08 | MVA: adjusted for IPI ≥3 |
| 11 | 152 (R-CHOP, n = 101;R-THP-COP, n = 51†) | Retrospective, single-center, 1996-2009 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP or R-THP-COP (THP-ADM as an alternative to doxorubicin for >70 y) Age cutoff: not specified Minimum no. cycles: 3 | Initial dose reduction/discontinuation due to hepatic/renal/ cardiac dysfunction or poor PS, HIV-associated, history of advanced cancers | Not reported | C, H†, O, P | SDI, DDI, and RDI ARDI: individual drugs and combined based on regimens.* | 70 | MVA: OS ARDI <70%: HR, 9.0; 95% CI, 2.2-36.7); P = .002 PFS ARDI <70%: HR, 2.6; 95% CI, 1.3-5.2; P = .007 | MVA: adjusted for IPI ≥3, albumin <3.5 g/dL, febrile neutropenia, prophylactic G-CSF |
| 9 | 198 (DI analysis, n = 183;R-CHOP/CHOP, n = 190; R-EPOCH, n = 1) | Retrospective, multicenter, 1998-2008 | Lymphoma: untreated DLBCL Chemotherapy: anthracycline-based Age cutoff: ≥80 y Minimum no. cycles: 1 | Primary cutaneous DLBCL; CNS involvement at diagnosis; lymphoma diagnosis other than DLBCL | 83.1 y (mean) | H | SDI: 50 mg/m per 21 d (H) DDI RDI: DDI [H]/SDI[H] (Hryniuk et al23 )* | 85 | KM analysis: 1-y OS rate: RDI ≥85%: 59% RDI <85%: 70%; (log-rank P = .029) | Not addressed |
| 14 | 433 (≥70 y, n = 83; 19.2%) | Retrospective, single center, 2003-2011 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: not specified, stratified by age ≥70 vs <70 y Minimum no. cycles: not mentioned | Other treatments such as radiotherapy, surgery, chemo other than R-CHOP | 58 y (16-91) | H | SDI: 16.7 mg/m2 per week (H) DDI RDI: not defined, but a cutoff of 10 mg/m2 per week was 60%.* | 60 | Age <70 y, RDI ≤60% 2-y OS: HR, 3.46; 95% CI, 1.39-8.58; P = .007 2-y PFS: HR, 4.04; 95% CI, 1.76-9.31; P = .001 Age ≥70 y, RDI ≤60% 2-y OS: HR, 2.24; 95% CI, 1.04-4.85; P = .040 2-y PFS: HR, 2.52; 95% CI, 1.02-6.22; P = .045 Age ≥70 y only UVA: RDI <60% OS: HR, 0.45; 95% CI, 0.21-0.97; P = .04 MVA: RDI <60% OS: HR, 1.60; 95% CI, 0.61-4.20; P = .343 | MVA: adjusted for B symptoms, stage ≥III, ECOG PS ≥2, LDH>ULN, EN sites ≥2, IPI ≥3, BM involvement, Bulky tumor |
| 17 | 140 | Retrospective, single-center, 2004-2015 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: ≥70 y Minimum no. cycles: 1 | Transformed iNHL, CLL, CNS involvement; any regimen other than R-CHOP | 78 y (70 - 90) | C, H | (1) Absolute dose [H]cycle 1/intended dose [H]cycle 1; (2) Absolute dose [H]cycle 1+2/intended dose [H]cycle 1+2; (3) Absolute dose [C]cycle 1/intended dose [C]cycle 1; (4) Absolute dose [C]cycle 1+2/intended dose [C]cycle 1+2. Mentions dose only, not dose intensity (dose/unit time). | 90 | UVA: dose[H]cycle 1 (per 10% increase): HR, 0.80; 95% CI, 0.72-0.88; P < .0001 MVA (ECOG 0-1 subgroup): dose[C]cycle 1 (per 10% increase): HR, 0.77; 95% CI, 0.64-0.92; P = .005; dose[C]cycle 1+2 (per 10% increase): HR, 0.76; 95% CI, 0.60-0.96; P = .019; dose[H]cycle 1 (per 10% increase): HR, 0.81; 95% CI, 0.70-0.94; P = .005; dose[H]cycle 1+2 (per 10% increase): HR, 0.82; 95% CI, 0.69-0.97; P = .019 Subgroup analysis by age, dose for H >90%: age <80 y: OS, HR, 0.81; 95% CI, 0.69-0.94; P = .005; age ≥80 y: OS, HR, 0.88; 95% CI, 0.74-1.06; P = .16 | MVA: adjusted for age, sex, IPI, Hb, albumin |
| 4 | 615 (total = 1011; R-CHOP/ CHOEP, n = 557; CHOP, n = 94; remainder, n = (R)CVP, palliative treatment) | Retrospective, nationwide multicenter, 2003-2012 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: ≥75 y Minimum no. cycles: not specified | CNS involvement; FL grade IIIb; transformed lymphoma; indolent lymphoma | 81 y (75-101) | C, H | SD: standard full dose [drug] per cycle Planned dose: delivered dose [drug] in cycle 1 Full dose: if the planned dose of [C] and [H] ≥80% in cycle 1 | 80 | KM analysis for OS (full dose ≥80%) 75-79 y, P = .068; 80-84 y, P = 0.414; ≥85 y, P = .962 | Stratification by age groups: 75-79, 80-84, ≥85 y MVA: none; RDI not included in MVA |
| 10 | 541 (R-CHOP/miniCHOP, n = 457; R-CHOEP, n = 84) | Retrospective, multicenter, 2000-2013 | Lymphoma: untreated DLBCL, PMBCL, testicular DLBCL, transformed FL (only radiotherapy for prior FL) Chemotherapy: R-CHOP, R-CHOEP, or R-mini-CHOP Age cutoff: >18 y Minimum no. cycles: 1 | PCNSL; Burkitt lymphoma; patients too frail to receive included chemotherapy regimens | 66 y (18-91) | H | SDI, DDI RDI: DDI[H]/SDI[H] (per Yamaguchi et al 2011)24 * | 70 | Bivariate analyses: data not published MVA: RDI ≤ 70%: OS: HR, 2.04; 95% CI, 1.15-3.61; P = .014; DFS: HR, 1.88; 95% CI, 0.97-3.67; P = .063 | MVA: adjusted for PS ≥2, stage ≥III, IPI ≥3, LDH >ULN, bulky disease, EN involvement, kidney/adrenal involvement; age, BMI ≥25, chemotherapy use (CHOP vs CHOEP; vincristine omission in any course |
| 15‡ | 690 (70-79 y, n = 452; ≥80 y, n = 238) | Retrospective, multicenter, 2009-2018 | Lymphoma: untreated DLBCL, transformed iNHL Chemotherapy: R-CHOP Age cutoff: ≥70 y Minimum no. cycles: 1 | Leg-type DLBCL, PTLD, and HIV-associated; CNS involvement; previously treated transformed iNHL | 77.1 y (70-96) | C, H | Intended DI (IDI): average delivered dose [C+H] in cycle 1, expressed as a % (i.e., relative to “standard dose”) DDI (but in this study, labeled “Received” DI (RDI): total delivered dose [C+H] × correction factor (to account for time delays) RDI/IDI: ratio of RDI to IDI.* (equivalent to ARDI in other studies), defined as the relative intensity of delivered vs planned (in this case, cycle 1) dose | 80 | UVA: IDI ≥80% OS: SHR, 0.35; 95% CI, 0.35-0.58; P < .001 PFS: SHR, 0.50; 95% CI, 0.39-0.64; P < .001 Relapse (competing risks regression; IDI ≥80% SHR, 0.70; 95% CI, 0.51-0.96; P = .026 IDI/RDI ratio was not predictive of relapse, PFS, or OS SHR, for relapse risk: 0.53; 95% CI, 0.22-1.27; P = .156 MVA relapse risk: IDI<80% Age 70-79 y: SHR, 1.61; 95% CI, 1.02-2.53; P = .04. Age ≥80 y: SHR: 1.48 95% CI, 0.96-2.29; P = .078 | MVA: adjusted for age, stage, ECOG PS ≥2, LDH > ULN, albumin, Hb, male, B-symptoms, EN >1. Competing-risks survival regression: cumulative risk of relapse with nonrelapse mortality as a competing risk |
| 16 | 223 | Retrospective, single center, 2005-2013 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: not specified Minimum no. cycles: 4 | Not reported | Not reported | R, C, H, O | SDI, DDI, RDI ARDI: mean RDIs for R, C, H, O* | 90 | UVA ARDI >90%: OS HR, 0.30; 95% CI, 0.20-0.46; P < .000001. PFS HR, 0.28; 95% CI, 0.18-0.44; P < .000001 MVA ARDI >90%: OS HR, 0.32; 95% CI, 0.21-0.48; P < .000001 PFS: HR, 0.31; 95% CI, 0.20-0.47; P < .000001 | MVA: adjusted for IPI ≥2 |
| 20 | 476 | Retrospective, single-center, 2004-2017 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP or R-THP-COP (THP-ADM as an alternative to doxorubicin) Age cutoff: not specified Minimum no. cycles: 3 | HIV-associated; PCNSL | 68.5 y (27-97) | C, H†, O | Standard Dose (SD): planned full dose[drug] Delivered dose (DD): total delivered dose [drug] RDI: DD[drug]/SD[drug] ARDI (termed “RDI” in the article): mean of all RDIs for C, H/THP-ADM, and O (per Hryniuk and Bush25 ) | 80 | UVA: 5-y OS: RDI ≥80%: 83.7%; 95% CI, 76.2-89.0 vs RDI <80%: 70.7%; 95% CI, 64.3-76.2; P = .01 5-y PFS, RDI ≥80%: 78.2%; 95% CI 70.2-84.4 vs RDI <80%: 63.1%; 95% CI, 56.6-68.9; P = .01 High CONUT score: RDI >80% vs ≤ 80%§ 5-y OS: 59.8% vs 50.9%; P = .73 | MVA: none; RDI not included in MVA |
| 20 | 608 (R-CHOP, n = 605; R-CVP, n =3) | Retrospective, single center, 2002-2012 | Lymphoma: DLBCL, transformed iNHL, and DLBCL variants (EBV+ DLBCL, primary cutaneous DLBCL, PMBL, LYG) Chemotherapy: R containing regimens Age cutoff: not specified Minimum no. of cycles: not specified | NR | 53.3 ± 14.1 y (mean) | C, H, O | SDI, DDI, RDI* Average RDI (ARDI): mean [RDIs] for C, H, O ARDI (per Kwak et al 199026 ) | 85 | UVA KM: ARDI<85% OS: P = .02 PFS: P = 0.01 MVA (according to NCCN-IPI): ARDI <85% OS: HR, 1.07; 95% CI, 0.72-1.61; P = .73 PFS: HR, 1.06; 95% CI, 0.75-1.50; P = .73 | MVAs: adjusted for B symptoms, Age, and IPI (either aaIPI, IPI, NCCN-IPI, or GELTAMO-IPI) |
| 13 | 127 (R-CH/THPCOP, n = 120; no R, n = 7) | Retrospective, multicenter, 2007-2017 | Lymphoma: untreated de novo DLBCL Chemotherapy: R-CHOP or R-THP-COP Age cutoff: ≥80 y Minimum no. cycles: 1 | Transformed iNHL; PTLD; HIV-associated; CNS involvement; any regimen other than CHOP or THP-COP; radiotherapy before/after chemotherapy | 83.7 y (80-96) | C, H†, O | SDI, DDI, RDI ARDI: mean [RDIs] for C, H/THP-ADM, and O per course.* tARDI: averages over each of the 6 cycles | 50 | KM analysis: tARDI >50% vs ≤50% 2-y OS = 61.8% vs 50.8%; P = .03 Cox hazards model with restricted cubic spline: effect of tARDI: P = 0.049 MVA OS: tARDI (/10%): HR, 0.89; 95% CI, 0.809-0.975; P = .013 | MVAs: adjusted for serum albumin, CCI score, IPI score (continuous) |
| 18 | 211 | Retrospective, multicenter, 2010-2018 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: ≥18 y Minimum no. cycles: 1 | Recurrent DLBCL | 72 y (19-92) | C, H, O | SDI, DDI, RDI ARDI (termed “RDI” in manuscript): mean RDIs for C, H, O (per Eyre et al 201915 )* | 70 | UVA: RDI <70% OS: HR, 4.38; 95% CI, 1.60-12.7; P = .003 MVA: RDI <70% OS: HR, 3.70; 95% CI, 1.12-12.2; P = .031 | MVA: adjusted for age ≥65 y or ≥80 y, raised LDH, PS ≥2, CCI ≤1 or ≥2, stage ≥3, EN ≥2 |
| Reference . | N . | Study design and period . | Inclusion criteria . | Exclusion criteria . | Age, median (range) . | Drugs . | Derivation of dose intensity . | Cutoff RDI used for analysis, % . | Impact of reduced RDI on outcomes . | Assessment of confounders and competing risk . |
|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 100 | Retrospective, multicenter, 2003-2008 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: not specified Minimum no. cycles: 4 | Prior radiotherapy before CHOP; T-cell NHL | 60 y (range not specified) | C, H | RDI: DDI[drug]/SDI[drug].* ARDI: mean of RDIs for C, H The term “RDI” was used in lieu of ARDI in the study. | 87.9 | UVA OS: RDI (per 10% increase): HR, 0.7; 95% CI, 0.6-0.9); P = .02 MVA OS: RDI (per 10% increase): HR, 0.8; 95% CI, 0.6-1.0; P = .08 | MVA: adjusted for IPI ≥3 |
| 11 | 152 (R-CHOP, n = 101;R-THP-COP, n = 51†) | Retrospective, single-center, 1996-2009 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP or R-THP-COP (THP-ADM as an alternative to doxorubicin for >70 y) Age cutoff: not specified Minimum no. cycles: 3 | Initial dose reduction/discontinuation due to hepatic/renal/ cardiac dysfunction or poor PS, HIV-associated, history of advanced cancers | Not reported | C, H†, O, P | SDI, DDI, and RDI ARDI: individual drugs and combined based on regimens.* | 70 | MVA: OS ARDI <70%: HR, 9.0; 95% CI, 2.2-36.7); P = .002 PFS ARDI <70%: HR, 2.6; 95% CI, 1.3-5.2; P = .007 | MVA: adjusted for IPI ≥3, albumin <3.5 g/dL, febrile neutropenia, prophylactic G-CSF |
| 9 | 198 (DI analysis, n = 183;R-CHOP/CHOP, n = 190; R-EPOCH, n = 1) | Retrospective, multicenter, 1998-2008 | Lymphoma: untreated DLBCL Chemotherapy: anthracycline-based Age cutoff: ≥80 y Minimum no. cycles: 1 | Primary cutaneous DLBCL; CNS involvement at diagnosis; lymphoma diagnosis other than DLBCL | 83.1 y (mean) | H | SDI: 50 mg/m per 21 d (H) DDI RDI: DDI [H]/SDI[H] (Hryniuk et al23 )* | 85 | KM analysis: 1-y OS rate: RDI ≥85%: 59% RDI <85%: 70%; (log-rank P = .029) | Not addressed |
| 14 | 433 (≥70 y, n = 83; 19.2%) | Retrospective, single center, 2003-2011 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: not specified, stratified by age ≥70 vs <70 y Minimum no. cycles: not mentioned | Other treatments such as radiotherapy, surgery, chemo other than R-CHOP | 58 y (16-91) | H | SDI: 16.7 mg/m2 per week (H) DDI RDI: not defined, but a cutoff of 10 mg/m2 per week was 60%.* | 60 | Age <70 y, RDI ≤60% 2-y OS: HR, 3.46; 95% CI, 1.39-8.58; P = .007 2-y PFS: HR, 4.04; 95% CI, 1.76-9.31; P = .001 Age ≥70 y, RDI ≤60% 2-y OS: HR, 2.24; 95% CI, 1.04-4.85; P = .040 2-y PFS: HR, 2.52; 95% CI, 1.02-6.22; P = .045 Age ≥70 y only UVA: RDI <60% OS: HR, 0.45; 95% CI, 0.21-0.97; P = .04 MVA: RDI <60% OS: HR, 1.60; 95% CI, 0.61-4.20; P = .343 | MVA: adjusted for B symptoms, stage ≥III, ECOG PS ≥2, LDH>ULN, EN sites ≥2, IPI ≥3, BM involvement, Bulky tumor |
| 17 | 140 | Retrospective, single-center, 2004-2015 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: ≥70 y Minimum no. cycles: 1 | Transformed iNHL, CLL, CNS involvement; any regimen other than R-CHOP | 78 y (70 - 90) | C, H | (1) Absolute dose [H]cycle 1/intended dose [H]cycle 1; (2) Absolute dose [H]cycle 1+2/intended dose [H]cycle 1+2; (3) Absolute dose [C]cycle 1/intended dose [C]cycle 1; (4) Absolute dose [C]cycle 1+2/intended dose [C]cycle 1+2. Mentions dose only, not dose intensity (dose/unit time). | 90 | UVA: dose[H]cycle 1 (per 10% increase): HR, 0.80; 95% CI, 0.72-0.88; P < .0001 MVA (ECOG 0-1 subgroup): dose[C]cycle 1 (per 10% increase): HR, 0.77; 95% CI, 0.64-0.92; P = .005; dose[C]cycle 1+2 (per 10% increase): HR, 0.76; 95% CI, 0.60-0.96; P = .019; dose[H]cycle 1 (per 10% increase): HR, 0.81; 95% CI, 0.70-0.94; P = .005; dose[H]cycle 1+2 (per 10% increase): HR, 0.82; 95% CI, 0.69-0.97; P = .019 Subgroup analysis by age, dose for H >90%: age <80 y: OS, HR, 0.81; 95% CI, 0.69-0.94; P = .005; age ≥80 y: OS, HR, 0.88; 95% CI, 0.74-1.06; P = .16 | MVA: adjusted for age, sex, IPI, Hb, albumin |
| 4 | 615 (total = 1011; R-CHOP/ CHOEP, n = 557; CHOP, n = 94; remainder, n = (R)CVP, palliative treatment) | Retrospective, nationwide multicenter, 2003-2012 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: ≥75 y Minimum no. cycles: not specified | CNS involvement; FL grade IIIb; transformed lymphoma; indolent lymphoma | 81 y (75-101) | C, H | SD: standard full dose [drug] per cycle Planned dose: delivered dose [drug] in cycle 1 Full dose: if the planned dose of [C] and [H] ≥80% in cycle 1 | 80 | KM analysis for OS (full dose ≥80%) 75-79 y, P = .068; 80-84 y, P = 0.414; ≥85 y, P = .962 | Stratification by age groups: 75-79, 80-84, ≥85 y MVA: none; RDI not included in MVA |
| 10 | 541 (R-CHOP/miniCHOP, n = 457; R-CHOEP, n = 84) | Retrospective, multicenter, 2000-2013 | Lymphoma: untreated DLBCL, PMBCL, testicular DLBCL, transformed FL (only radiotherapy for prior FL) Chemotherapy: R-CHOP, R-CHOEP, or R-mini-CHOP Age cutoff: >18 y Minimum no. cycles: 1 | PCNSL; Burkitt lymphoma; patients too frail to receive included chemotherapy regimens | 66 y (18-91) | H | SDI, DDI RDI: DDI[H]/SDI[H] (per Yamaguchi et al 2011)24 * | 70 | Bivariate analyses: data not published MVA: RDI ≤ 70%: OS: HR, 2.04; 95% CI, 1.15-3.61; P = .014; DFS: HR, 1.88; 95% CI, 0.97-3.67; P = .063 | MVA: adjusted for PS ≥2, stage ≥III, IPI ≥3, LDH >ULN, bulky disease, EN involvement, kidney/adrenal involvement; age, BMI ≥25, chemotherapy use (CHOP vs CHOEP; vincristine omission in any course |
| 15‡ | 690 (70-79 y, n = 452; ≥80 y, n = 238) | Retrospective, multicenter, 2009-2018 | Lymphoma: untreated DLBCL, transformed iNHL Chemotherapy: R-CHOP Age cutoff: ≥70 y Minimum no. cycles: 1 | Leg-type DLBCL, PTLD, and HIV-associated; CNS involvement; previously treated transformed iNHL | 77.1 y (70-96) | C, H | Intended DI (IDI): average delivered dose [C+H] in cycle 1, expressed as a % (i.e., relative to “standard dose”) DDI (but in this study, labeled “Received” DI (RDI): total delivered dose [C+H] × correction factor (to account for time delays) RDI/IDI: ratio of RDI to IDI.* (equivalent to ARDI in other studies), defined as the relative intensity of delivered vs planned (in this case, cycle 1) dose | 80 | UVA: IDI ≥80% OS: SHR, 0.35; 95% CI, 0.35-0.58; P < .001 PFS: SHR, 0.50; 95% CI, 0.39-0.64; P < .001 Relapse (competing risks regression; IDI ≥80% SHR, 0.70; 95% CI, 0.51-0.96; P = .026 IDI/RDI ratio was not predictive of relapse, PFS, or OS SHR, for relapse risk: 0.53; 95% CI, 0.22-1.27; P = .156 MVA relapse risk: IDI<80% Age 70-79 y: SHR, 1.61; 95% CI, 1.02-2.53; P = .04. Age ≥80 y: SHR: 1.48 95% CI, 0.96-2.29; P = .078 | MVA: adjusted for age, stage, ECOG PS ≥2, LDH > ULN, albumin, Hb, male, B-symptoms, EN >1. Competing-risks survival regression: cumulative risk of relapse with nonrelapse mortality as a competing risk |
| 16 | 223 | Retrospective, single center, 2005-2013 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: not specified Minimum no. cycles: 4 | Not reported | Not reported | R, C, H, O | SDI, DDI, RDI ARDI: mean RDIs for R, C, H, O* | 90 | UVA ARDI >90%: OS HR, 0.30; 95% CI, 0.20-0.46; P < .000001. PFS HR, 0.28; 95% CI, 0.18-0.44; P < .000001 MVA ARDI >90%: OS HR, 0.32; 95% CI, 0.21-0.48; P < .000001 PFS: HR, 0.31; 95% CI, 0.20-0.47; P < .000001 | MVA: adjusted for IPI ≥2 |
| 20 | 476 | Retrospective, single-center, 2004-2017 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP or R-THP-COP (THP-ADM as an alternative to doxorubicin) Age cutoff: not specified Minimum no. cycles: 3 | HIV-associated; PCNSL | 68.5 y (27-97) | C, H†, O | Standard Dose (SD): planned full dose[drug] Delivered dose (DD): total delivered dose [drug] RDI: DD[drug]/SD[drug] ARDI (termed “RDI” in the article): mean of all RDIs for C, H/THP-ADM, and O (per Hryniuk and Bush25 ) | 80 | UVA: 5-y OS: RDI ≥80%: 83.7%; 95% CI, 76.2-89.0 vs RDI <80%: 70.7%; 95% CI, 64.3-76.2; P = .01 5-y PFS, RDI ≥80%: 78.2%; 95% CI 70.2-84.4 vs RDI <80%: 63.1%; 95% CI, 56.6-68.9; P = .01 High CONUT score: RDI >80% vs ≤ 80%§ 5-y OS: 59.8% vs 50.9%; P = .73 | MVA: none; RDI not included in MVA |
| 20 | 608 (R-CHOP, n = 605; R-CVP, n =3) | Retrospective, single center, 2002-2012 | Lymphoma: DLBCL, transformed iNHL, and DLBCL variants (EBV+ DLBCL, primary cutaneous DLBCL, PMBL, LYG) Chemotherapy: R containing regimens Age cutoff: not specified Minimum no. of cycles: not specified | NR | 53.3 ± 14.1 y (mean) | C, H, O | SDI, DDI, RDI* Average RDI (ARDI): mean [RDIs] for C, H, O ARDI (per Kwak et al 199026 ) | 85 | UVA KM: ARDI<85% OS: P = .02 PFS: P = 0.01 MVA (according to NCCN-IPI): ARDI <85% OS: HR, 1.07; 95% CI, 0.72-1.61; P = .73 PFS: HR, 1.06; 95% CI, 0.75-1.50; P = .73 | MVAs: adjusted for B symptoms, Age, and IPI (either aaIPI, IPI, NCCN-IPI, or GELTAMO-IPI) |
| 13 | 127 (R-CH/THPCOP, n = 120; no R, n = 7) | Retrospective, multicenter, 2007-2017 | Lymphoma: untreated de novo DLBCL Chemotherapy: R-CHOP or R-THP-COP Age cutoff: ≥80 y Minimum no. cycles: 1 | Transformed iNHL; PTLD; HIV-associated; CNS involvement; any regimen other than CHOP or THP-COP; radiotherapy before/after chemotherapy | 83.7 y (80-96) | C, H†, O | SDI, DDI, RDI ARDI: mean [RDIs] for C, H/THP-ADM, and O per course.* tARDI: averages over each of the 6 cycles | 50 | KM analysis: tARDI >50% vs ≤50% 2-y OS = 61.8% vs 50.8%; P = .03 Cox hazards model with restricted cubic spline: effect of tARDI: P = 0.049 MVA OS: tARDI (/10%): HR, 0.89; 95% CI, 0.809-0.975; P = .013 | MVAs: adjusted for serum albumin, CCI score, IPI score (continuous) |
| 18 | 211 | Retrospective, multicenter, 2010-2018 | Lymphoma: untreated DLBCL Chemotherapy: R-CHOP Age cutoff: ≥18 y Minimum no. cycles: 1 | Recurrent DLBCL | 72 y (19-92) | C, H, O | SDI, DDI, RDI ARDI (termed “RDI” in manuscript): mean RDIs for C, H, O (per Eyre et al 201915 )* | 70 | UVA: RDI <70% OS: HR, 4.38; 95% CI, 1.60-12.7; P = .003 MVA: RDI <70% OS: HR, 3.70; 95% CI, 1.12-12.2; P = .031 | MVA: adjusted for age ≥65 y or ≥80 y, raised LDH, PS ≥2, CCI ≤1 or ≥2, stage ≥3, EN ≥2 |
aaIPI, age adjusted IPI; ARDI, average RDI; C, cyclophosphamide; CCI, Charlson Comorbidity Index; CLL, chronic lymphocytic leukemia; E, etoposide; EBV, Epstein-Barr virus; EN, extranodal disease; FL, follicular lymphoma; H, doxorubicin; Hb, hemoglobin; iNHL, indolent non-Hodgkin lymphoma; IPI, international prognostic index; LDH, lactate dehydrogenase; LYG, lymphomatoid granulomatosis; NCCN, National Comprehensive Cancer Network; NHL, non-Hodgkin lymphoma; O, vincristine; P, prednisolone; PCNSL, primary CNS lymphoma; PFS, progression-free survival; PMBL, primary mediastinal B-cell lymphoma; PS, performance status; PTLD, posttransplant lymphoproliferative disorder; R, rituximab; SHR, sub–hazard ratio; tARDI, total ARDI; ULN, upper limit of normal; UVA, univariable analysis.
Definitions for DDI: standard dose intensity (SDI): planned full-dose[drug]/planned time to complete chemotherapy; delivered dose intensity (DDI): total delivered dose [drug]/total time to complete chemotherapy; relative dose intensity (RDI), DDI[drug]/SDI[drug]; average relative dose intensity (ARDI), mean of RDIs for drugs (varied by study).
R-THP-COP: used in Japan for >70 years, THP-ADM replaces doxorubicin, and doses of C, 500 mg/m2, and O, 1.0 mg/m2, are decreased.
Eyre et al 201627 was excluded, because all cases in that analysis were included in the larger cohort (Eyre et al15 ).
CONUT, controlling nutritional status, derived from serum albumin, cholesterol, and absolute lymphocyte count. High CONUT score, ≥4.
Results
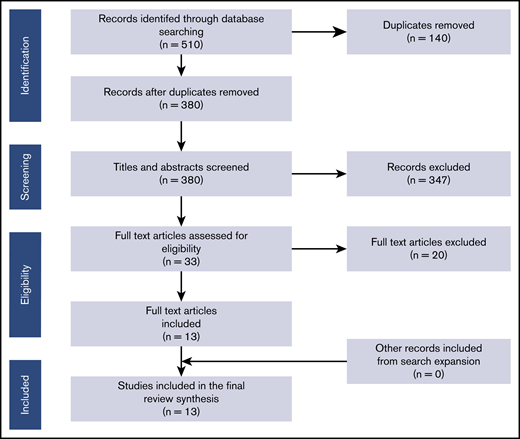
A total of 380 unique abstracts/titles were identified through database searches, 33 were selected for full-text review. Thirteen articles met the eligibility criteria, with no additional eligible records identified after snowballing. The PRISMA inclusion/exclusion process is presented in Figure 1. All 13 included studies were retrospective series. Six were single-center, and 7 were multicenter studies, including 3 analyses that were fully or partially based on nationwide cancer registries.4,9,10 Five studies were conducted in Japan, 2 in South Korea, and 1 each in the United States, the United Kingdom, Israel, Denmark, Sweden, and Poland (Table 2).
A cumulative total of 4083 patients received R-CHOP (or an R-CHOP-like regimen) across 12 studies, with an additional ∼160 patients from 1 study where there was a lack of granularity,9 There were 5188 patients overall, of whom 4499 were included in a DI analysis. In 7 studies, all patients received R-CHOP in 21-day cycles, intended at either full dose or reduced dose (R-miniCHOP). Three studies included a small minority of patients who received alternative regimens, including CHOP, (R)-CVP (cyclophosphamide, vincristine, prednisolone, ±rituximab), (R)-CNOP (cyclophosphamide, mitoxantrone, vincristine, prednisolone, with or without rituximab), (R)-CHOEP/EPOCH-R (etoposide with cyclophosphamide, doxorubicin, vincristine, prednisolone, with or without rituximab), or other regimens. In 3 Japanese studies,11-13 all patients received either R-CHOP or a variant R-THP-COP in which doxorubicin was substituted for pirarubicin (4′-O-tetrahydropyranyl doxorubicin [THP-ADM]), a derivative anthracycline purported to have a superior cardiotoxicity profile. The minimum number of cycles of chemotherapy received across all studies ranged from 1 to 4. Information on the maximum number of cycles (planned and/or administered) was not provided in 6 studies. Of the 13 studies, 5 reported a planned maximum of 6 or ≥6 cycles, and 3 studies reported the range of administered cycles (median, 6 in all 3 studies; maximum, 8 in 2 studies and 9 in 1 study).
Seven studies had specific inclusion criteria for age; 2 studies included patients ≥18 years, and 5 studies focused exclusively on elderly patients aged ≥70 (n = 2), ≥75 (n = 1), and ≥80 (n = 2) years. The other 6 studies did not have a defined age range. Ha et al14 did not specify an age range in their inclusion criteria, but Cox regression analysis was performed exclusively on data from a subgroup aged ≥70 years. All studies included most patients with treatment-naive, de novo DLBCL; however, the exclusion of alternative diagnoses was variable. The most frequently excluded entities were central nervous system (CNS) involvement at diagnosis (n = 5), HIV-associated DLBCL (n = 4); primary CNS lymphoma (n = 2); and posttransplant lymphoproliferative disorder (n = 2). Two studies13,15 excluded all of these entities.
Derivation of RDI
All 13 included studies assessed RDI; however, there was marked variation in how RDI was defined, the terminology used, and the derivation method. In 10 studies, RDI was calculated as the ratio of the cumulative delivered dose of the prespecified drug(s) in the individual study to the cumulative planned dose multiplied by a time factor: the ratio of the planned treatment time to the actual time taken to complete chemotherapy. The planned maximum DI was generally based on the standard dosing of rituximab (375 mg/m2), cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), vincristine (1.4 mg/m2), and prednisolone (100 mg daily for 5 days), administered every 21 days for 6 cycles. Most studies expressed RDI as an average of the individual RDIs of ≥2 components of R-CHOP. Components included in the RDI calculation varied significantly. Doxorubicin (or pirarubicin) was the common denominator in all studies. Three studies assessed doxorubicin (H) RDI only; 4 studies assessed both cyclophosphamide and doxorubicin (C, H); and 4 studies evaluated cyclophosphamide, doxorubicin, and vincristine (C, H, O). Długosz-Danecka et al16 included all components of R-CHOP excepting prednisolone, whereas Hirakawa et al11 assessed all components excepting rituximab.
Nagata et al12 derived RDI as the ratio of the cumulative delivered dose to the cumulative planned dose across 6 cycles, with no time-correction factor, to our knowledge. In 3 studies,4,15,17 DI was explicitly calculated for cycle 1, referred to as the intended dose intensity (IDI). IDI reflects dose reductions from the standard (maximum) R-CHOP dose planned in advance of initiating therapy, instead of secondary DI reductions caused by toxicity or physician or patient choice. Eyre et al15 was the only study to derive both IDI and RDI.
In the studies that evaluated time-dependent DI across all cycles, early discontinuation before completing the standard ≥6 cycles generally did not result in a reduced RDI. Four studies9,13,15,18 reported that, in case of premature discontinuation caused by progressive disease, toxicity, or death, RDI was calculated up to discontinuation or death. Although not specifically addressed in the remaining studies, RDI was calculated as the ratio of 2 time-dependent dose intensities, meaning that premature discontinuation would not result in RDI reduction.
Impact of DI on survival outcomes: all ages
The median RDI was reported in only 4 studies, ranging from 58.9% to 87.9%. The impact of RDI on OS was evaluated in all included studies, with 7 also assessing PFS. Additional end points analyzed included disease-free survival, event-free survival, treatment-related mortality (TRM), and cumulative risk of relapse. Survival end points were estimated using the Kaplan-Meier (KM) method and compared across different RDI groups using the log-rank test. Nine studies performed KM analysis across the entire cohort (regardless of age), of which 7 showed a significant reduction in OS in the reduced RDI group compared with the higher RDI group (P < .05). Terada et al19 found that the cohort receiving doses less than the median RDI had similar OS (P = .23). In contrast, Carson et al9 analyzed elderly US veterans and found an inverse association between RDI and survival. Median survival in the reduced RDI cohort (doxorubicin RDI, <85%) was 28.1 months, compared with 21.8 months at higher RDI (P = .029).
The impact of RDI on survival was also analyzed using the Cox proportional hazards model in most studies. RDI was treated as a categorical variable in 7 studies, whereas 3 studies treated it as a continuous variable. RDI was included in the univariable analysis (UVA) in 7 studies, with 6 of those showing an association between reduced RDI and inferior OS. Multivariable analysis (MVA) using RDI as a covariate was performed in 10 studies, with 6 of those showing an association when adjusted for other covariates and 3 showing no association. Eight studies were adjusted for International Prognostic Index (IPI), 5 for age (categorical, 4; continuous, 1), and 4 for stage and performance score. Only 2 studies were adjusted for comorbidities, as represented by the Charlson Comorbidity Index (CCI). In Eyre et al,15 IDI was significantly associated with OS, PFS, and relapse risk in both UVA and MVA; however, RDI was not predictive of any of these outcomes, most likely because IDI and RDI correlated strongly in the population studied.
Where RDI was treated as a dichotomous variable in either survival or Cox regression, there was considerable variation in the cutoff values used, ranging from 50% to 90%. Four studies that used higher RDI cutoffs (80%, 85%, or 90%) justified the higher percentage by approximating the higher dose group to full-dose intensity.4,9,15,17 Terada et al used the median RDI of 87.9% as a cutoff.19 In only 2 studies was RDI systematically analyzed as a semicontinuous variable in 5% or 10% intervals from 50% to 90%. Yamamoto et al18 selected a 70% cutoff for all subsequent analyses, as this cutoff corresponded to the most significant impact on event-free survival, whereas, in Hirakawa et al,11 although significant OS differences were observed using a cutoff of 80%, the researchers elected to use a 70% cutoff, as this approximated the most commonly observed RDI when R-CHOP was dose reduced due to toxicity or patient-related factors. The remaining 6 studies either did not give any justification for the chosen cutoff or referred to precedent.10,12-14,16,20
Differential impact of DI across age groups
The effect of reducing DI in older patients varied across studies (supplementary Table 3). Two studies that exclusively investigated patients ≥80 years of age showed conflicting results. Carson et al9 analyzed 530 patients with DLBCL (almost all men) from the US Veteran’s registry (of whom 193 received anthracyclines), and 183 were included in the final RDI analysis. Patients who received RDI ≥85% had significantly inferior OS vs those whose doses were reduced (P = .029). There was no accounting for possible confounding factors, as RDI was not included in multivariable Cox regression analysis. In contrast, Lee et al13 found that, in 120 patients aged ≥80 years, those receiving a higher average RDI had superior outcomes, albeit with a lower cutoff of 50%. An MVA that included RDI as a continuous variable confirmed this association, adjusting for possible confounders (sex, albumin, CCI, IPI, and Geriatric 8 health status score). Moreover, a Cox regression analysis using restricted cubic spline demonstrated a nearly linear association between average RDI and mortality. The reason for the discrepancy between these 2 studies is unclear, but notably, the median RDI in Lee et al was exceptionally low (58.9%). The TRM in this study was particularly low (3.1%) compared with that in previous studies of age-matched R-CHOP–treated patients.3 Lee et al13 speculated that the frequent use of primary G-CSF and antibiotic prophylaxis and near-universal hospitalization throughout treatment was partly responsible. By comparison, the TRM in the anthracycline-treated group in Carson et al was 15%, of which two-thirds occurred after the first cycle. In the latter study, survival became similar by 2 years after diagnosis (53% reduced RDI vs 48% full RDI),9 insinuating a higher frequency of relapse and disease-related mortality in patients receiving a reduced RDI.
Four studies performed subgroup analyses, allowing for comparison across different age categories. Juul et al4 performed separate survival analyses in patients aged 75 to 79, 80 to 84, and ≥85 years. A trend toward superior OS was observed in patients aged 75 to 79 years who received a higher IDI (P = .068); however, there was no significant difference in OS in those ≥80 years (P = .414 and P = .962, respectively). Similarly, Eyre et al15 found that IDI had a differential impact across a large population (n = 690) when patients were divided by age < vs ≥80 years. Patients aged 70 to 79 years who received an IDI ≥80% had significantly higher PFS and OS (both P < .001), whereas in patients aged ≥80 years, there was no significant difference in either PFS (P = .88) or OS (P = .75). In an MVA adjusting for other factors, the cumulative incidence of relapse was significantly higher in patients 70 to 79 years of age who received an IDI <80% (P = .04), but not in patients aged ≥80 years (P = .32). In a substantially smaller study (n = 140) by Vidal et al,17 analysis by age subgroups showed that cycle 1 dose reduction <90% of doxorubicin and cyclophosphamide was associated with worse OS in patients aged 70 to 79 years (P = .005), as well as in patients aged ≥80 years. However, after controlling for possible confounding factors (sex, IPI, and albumin) in MVA, doxorubicin dose reduction was still found to be significantly associated with worse OS in younger patients (HR, 0.81; 95% CI, 0.69-0.94; P = .005), but there was no association among the older patients (HR, 0.88; 95% CI, 0.74-1.06; P = .16). In Ha et al,14 subgroup analysis showed that a reduction of doxorubicin RDI <60% was associated with worse OS and PFS in younger (<70 years) and older (≥70 years) groups; however, the difference was more significant in younger patients.
Quality appraisal and statistical analysis
A CASP analysis was performed for all 13 studies (supplementary Table 2). The main factors contributing to the poor-quality performance were their retrospective design; small size, single-institution–derived population; and inadequate adjustment for potential confounding factors. Upon expert statistical review (M.J.M.), significant heterogeneity in study design and statistical analyses were identified. RDI was a covariate and not the primary statistical predictor in 3 studies.10,12,20 The heterogeneity in statistical analyses and reporting across studies were sufficient to preclude any formal meta-analysis or summary figures of outcome across studies. Most studies appeared sufficiently powered for the performed analyses; however, the number of deaths was not reported for 5 studies,9,10,12,14,20 and 2 studies11,19 were clearly underpowered for the analyses reported.
Discussion
We describe the first comprehensive systematic review in the rituximab era conducted to study the role of DI of frontline R-CHOP in all DLBCL patients, with a focus on the elderly. The study has several significant findings resulting from a systematic overview of this complex topic and can guide clinicians to better informed decision making when treating elderly patients or younger patients with comorbidities. We identified 13 retrospective studies with a total of 4499 patients included in an overall DI analysis. No study has prospectively evaluated the impact of R-CHOP DI on survival. A cutoff population size of ≥100 was chosen to represent a reasonable trade-off between inclusivity and statistical robustness. Many of the studies identified were small (n = 100-200) and/or single-center studies, with only 2 studies wholly derived from nationwide registries. Furthermore, the populations studied were heterogeneous, with considerable variation in the age ranges, chemotherapy regimens, and alternative diagnoses (other than de novo DLBCL) within eligibility criteria. Few studies reported the median RDI across the entire cohort; however, for those that did, the range was wide (58.9% to 87.9%), consistent with the wide variation between study populations, which suggests possible biases in prescribing practice.
There was a noteworthy lack of standardization of the RDI definition and calculation across studies. Although many accounted for the total cumulative administered drug dose and the time to complete treatment relative to a set standard, 3 studies did not include a time factor in the DI derivation.4,12,17 Moreover, the choice of drug(s) included in the RDI calculation varied greatly between studies, with few attempting to rationalize or justify this choice. Doxorubicin was the common denominator across all 13 studies, presumably because it is the least well-tolerated and most frequently dose-reduced or omitted component of R-CHOP. Furthermore, anthracyclines are among the most important chemotherapeutics in lymphoma treatment, and dose reductions or omission could risk inferior disease control. However, cyclophosphamide, vincristine, and even rituximab and prednisolone were also variably included in RDI calculations, making cross-study comparisons challenging.
The issue of DI derivation is complicated further by many studies that do not distinguish between instances in which DI intensity is reduced from the outset (ie, planned dose reduction) and DI reduction as a result of poor tolerability or toxicity. This distinction is significant both clinically and statistically, as planned dose reductions in vulnerable patients may be associated with less toxicity and potentially lower TRM than is obtained with full-dose therapy. Three studies4,15,17 addressed this question by deriving IDI, defined as the administered dose in the first cycle of chemotherapy relative to the full dose per cycle. In only 1 study (Eyre et al15 ) were both IDI and RDI examined independently. In general, providing data on starting dose (ie, IDI) is critical when assessing the clinical importance of DI on survival outcomes, because the association between dose reduction and outcome is, to some degree, confounded by the toxicities that led to the dose reduction and can also result in death without genuine loss of disease control. Detailed analyses of causes of death allow for assessment of the overall contribution of lymphoma and nonlymphoma (including toxicity) deaths to OS, but this was only provided in 7 studies. Also, recording the outcomes of patients starting lower than full dose and completing without further reduction as a subgroup would be important, as it would remove the confounding by life-threatening toxicities that lead to lower-than-full doses, which would be enriched among patients who experience dose reductions. The outcomes by dose group were not provided in any of the studies. Finally, including the planned number of cycles in RDI and IDI, calculations should be considered, as patients who received full DI before early discontinuation would be exposed to significantly less drug but would still be included in analyses as having received the full treatment, which may bias analyses toward an underestimation of the true impact of RDI on survival outcomes.
Despite the heterogeneous populations and inconsistency in DI derivation, there is cumulative evidence that maintaining a higher DI is broadly important across whole cohorts, spanning a wide age range. In most studies, those patients who received a lower RDI/IDI had worse OS. However, the cutoff RDI used for survival and MVA varied considerably (50% to 90%). Moreover, there was little attempt to correct for the confounding association between survival events and RDI in the MVAs. On the other hand, simple analyses of RDI may also underestimate the true impact on survival, as dose reductions in many patients happen after some treatment cycles, thereby introducing an immortal bias and enrichment of patients who respond to treatment and are considered for additional courses of a lower dose.
Another key finding is that the impact of R-CHOP DI on survival diminishes with increasing age, particularly in those aged ≥80 years. Maintaining a higher RDI had either less strong or no association with improved OS and, in 1 study, was found to be detrimental.9 This result may be explained by the fact that among the very elderly, the risk of dying of lymphoma-unrelated causes is much more significant and dilutes the potential deaths caused by suboptimal lymphoma treatment. Consistent with this finding, the use of R-miniCHOP is supported by prospective phase II data and the recently reported SENIOR trial (NCT02128061) where the standard R-miniCHOP arm and the experimental arm of R-miniCHOP plus lenalidomide showed a 2-year OS of 66% in patients ≥80 years.21 To this end, R-miniCHOP now represents a standard of care for elderly (≥80 years of age) or frail patients in many countries and is also accepted as a control arm when testing novel therapies in these populations (eg, R-miniCHOP vs R-mini-CHP and polatuzumab vedotin). However, these studies do not address whether full-dose R-CHOP is superior overall in those aged ≥80 years. Finally, population-based studies have shown that commencing a standard therapy such as R-CHOP at any dose is associated with better survival, even in very elderly, appropriately selected patients.22
Conclusions
We found evidence of improved survival with higher RDIs (up to R-CHOP-21) in those aged <80 years, but the literature to date does not support full-dose intensity (ie, typically IDI >80%) in those ≥80 years. There is significant inconsistency in RDI calculation, thresholds used for analysis, and variable use of RDI and IDI parameters. Collaborative, carefully predefined, and detailed data sets using consistent parameters for these measurements are essential, moving forward, to enable reasonable interpretation of data within this field. RDI is commonly used but, as described, is confounded directly by survival outcome, and IDI has been studied in only a limited number of series. There is a need for prospective clinical trials that analyze the effect of IDI and RDI in the very elderly (≥80 years). In the absence of improved options beyond R-CHOP in DLBCL over the past 20 years, prospective studies of DI are warranted, despite the recognized challenges involved.
Original data are available by e-mail request to the corresponding author (e.bataillard@nhs.net).
Acknowledgments
T.A.E. acknowledges support by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme.
The views expressed are those of the authors and not necessarily those of the funding body.
Authorship
Contribution: E.J.B. was involved in conceptualization, database search, screening of abstracts and full texts, data extraction and analysis, quality appraisal, and writing the original draft; C.Y.C. was involved in conceptualization, screening of abstracts and full texts, adjudication, and editing of the manuscript; M.J.M. involved in statistical analysis and appraisal; A.K. was involved in data analysis and editing of the manuscript; and T.A.E. and T.C.E.-G. were involved in conceptualization, screening of abstracts and full texts, revising the original draft, editing the manuscript, and supervision of the review process.
Conflict-of-interest disclosure: E.J.B. and T.C.E.-G. were previously employed by Roche. C.Y.C. served as a consultant and advisor and received honoraria from Roche, Janssen, MSD, Gilead, Ascentage Pharma, Acerta, Loxo Oncology, and TG Therapeutics and received research funding from Celgene, Roche, and Abbvie and travel expenses from Roche. M.J.M. served as a consultant and advisor to MorphoSys, Kite Pharma, and Pfizer; and received research funding from Celgene/BMS, Nanostring, Genentech, and Morphosys. A.K. received research funding from the Lymphoma Research Foundation. T.A.E. received honoraria from Roche, Gilead, Janssen, Abbvie, and AstraZeneca; served on the advisory board for Roche, KITE, Loxo Oncology, Beigene, and Incyte; received travel expenses from Gilead, Takeda, and Abbvie; served on the trial steering committee for Loxo Oncology; and received research funding from AstraZeneca and Beigene.
Correspondence: Edward J. Bataillard, Department of Hematology, Imperial College Healthcare NHS Trust, The Catherine Lewis Centre, Hammersmith Hospital, Du Cane Rd, London W12 0HS, United Kingdom; e-mail: e.bataillard@nhs.net.
References
Author notes
T.A.E. and T.C.E.-G. contributed equally to this study.
The full-text version of this article contains a data supplement.