Key Points
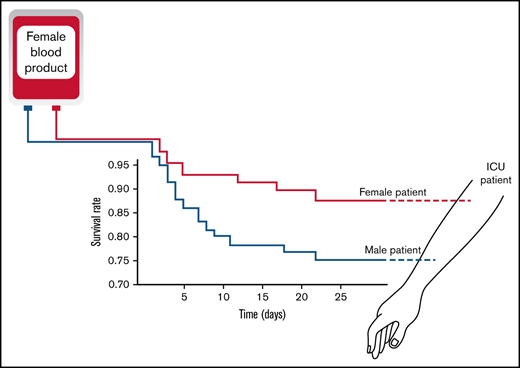
Transfusion of female RBCs to male recipients increases the risk of ICU mortality compared with female blood to female recipients.
Receiving RBCs from female donors is associated with a trend toward ARDS.
Abstract
Transfusion of red blood cells (RBCs) from female donors has been associated with increased risk of mortality. This study aims to investigate the associations between donor-recipient sex and posttransfusion mortality and morbidity in critically ill patients who received RBC transfusions from either male-only donors or from female-only donors (unisex-transfusion cases). Survival analysis was used to compare 4 groups: female-to-female, female-to-male, male-to-female, and male-to-male transfusion. Multivariate logistic model was used to evaluate the association between donor sex and intensive care unit (ICU) mortality. Associations between transfusion and acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), and nosocomial infections were assessed. Of the 6992 patients included in the original cohort study, 403 patients received unisex-transfusion. Survival analysis and the logistic model showed that transfusion of female RBCs to male patients was associated with an increased ICU mortality compared with transfusion of female RBCs to female patients (odds ratio, 2.43; 95% confidence interval, 1.02-5.77; P < .05). There was a trend toward increased ARDS in patients receiving RBC from female donors compared with those receiving blood from males (P = .06), whereas AKI was higher in donor-recipient sex-matched transfusion groups compared with sex-mismatched groups (P = .05). This was an exploratory study with potential uncontrolled confounders that limits broad generalization of the findings. Results warrant further studies investigating biological mechanisms underlying the association between donor sex with adverse outcomes as well as studies on the benefit of matching of blood between donor and recipient.
Introduction
Nearly 1 in 3 critically ill patients receive a red blood cell (RBC) transfusion during their stay in the intensive care unit (ICU).1,2 RBC transfusion has been widely used as a life-saving treatment in several situations to maintain adequate oxygen delivery to tissues. However, a growing body of evidence suggests that RBC transfusion might be associated with an increased risk of mortality.3,4 Mechanisms underlying this association are not known but may include donor-related factors, such as donor sex.5-8 Specifically, observational studies suggest that transfusing blood from female donors increases the risk of mortality.5,8 In addition to receipt of female blood, donor-recipient sex-mismatched transfusion was also reported to be associated with mortality.6,7 However, other studies have reported no association between donor sex and increased mortality.9,10 Discrepancies in study results may originate from differences in study designs, with a majority of studies including patients who received RBC transfusions from both female and male donors, which hampers relating effects to a specific donor sex. In addition, most of the previous studies have focused mainly on mortality as the primary outcome of RBC transfusions, without exploring other outcomes.11 Possibly, donor-related factors are associated with morbidity outcomes but do not necessarily lead to mortality.9
This multicenter study aimed to investigate the association between donor sex and posttransfusion mortality and ICU-acquired complications in critically ill patients who received unisex transfusions (receiving blood only from male donors or only from female donors). The hypothesis states that transfusing female blood to male patients is associated with worse outcomes. In this study, 2 statistical approaches were applied to investigate the association between donor sex and ICU mortality, which were survival analysis and multivariate logistic analysis.
Methods
Study design
This study is part of the Molecular Diagnosis and Risk Stratification of Sepsis (MARS), an observational cohort study conducted in critically ill adult patients that were acutely admitted to 2 tertiary hospitals in the Netherlands (clinicaltrials.gov #NCT01905033) between January 2011 and December 2013. The Medical Ethical Committees of both centers approved an opt-out consent method (IRB no. 10-056C). Participants were informed about the study by a brochure provided at ICU admission attached with an opt-out card that could be completed by the patient or legal representative in case of unwillingness to participate. Readmissions and patients who were transferred from another ICU were excluded. Only patients who received RBC transfusion from 1 sex group (unisex-transfusion cases) were considered in this study. Per protocol at both institutions, a single unit of RBC transfusion was given at a hemoglobin level of 7 g/dL. Patients were then divided into 4 groups: female patients who received RBC transfusions exclusively from female donors (female-to-female group), male patients who received RBC transfusions exclusively from female donors (female-to-male group), female patients who received RBC transfusions exclusively from male donors (male-to-female group), and male patients who received RBC transfusions exclusively from male donors (male-to-male group). Plasma products were from male donors only. Platelets were not sex matched.
Patient data
Dedicated ICU research physicians prospectively collected patient information including sex, age, and chronic comorbidity. Severity scores of Acute Physiology and Chronic Health Evaluation IV12 and Sequential Organ Failure Assessment13 were conducted at ICU admission. Sepsis was defined according to the sepsis-3 definition.14 The presence of acute kidney injury (AKI) and acute respiratory distress syndrome (ARDS) were defined according to strict preset criteria.15,16 The plausibility of an infection was assessed daily using a 4-point scale (ascending from none, possible, probable, to definite) as described in detail previously.17 Only definite and probable infections were included in the study. ICU-acquired complications were only counted when occurring after RBC transfusion and before ICU discharge.
Donor data
Data on donor sex were obtained from Sanquin Blood Supply in Amsterdam. Transfusion information was linked to their recipients via a product identification number. RBC units were prepared using the red cell filtration method. Briefly, RBCs were separated from plasma and platelets by centrifugation. An additive solution containing saline adenine glucose mannitol was added to RBCs. RBCs were then leucoreduced by filtration and stored at 1 to 6°C for a maximum of 35 days. Platelets were pooled from the buffy coats of 5 donors and stored in platelet additive solution. Plasma products were derived from males only.
Statistical analysis
Statistical analyses were performed using SPSS software version 25.00. Baseline data were summarized using medians and interquartile ranges (IQR) for continuous data or percentages and frequencies for categorical data. Differences between study groups were assessed by a Kruskal-Wallis test for continuous data and Pearson's χ2 test for categorical data. Survival was analyzed by the Kaplan-Meier method, and the 4 study groups were compared by the Mantel-Cox log-rank test with the female-to-female group as a reference group. The primary outcome was a 30-day ICU mortality measured from the ICU admission date. Patients who did not reach the primary outcome of ICU mortality at 30 days of admission or who were discharged from the ICU were considered alive and censored. Forward stepwise multivariate logistic model building was performed to evaluate associations between baseline characteristics and 30-day ICU mortality. First, univariate logistic regression was performed between each variable of baseline characteristics and mortality. Then variables that were significant at P < .05 were included in the multiple logistic regression model. Confounding effect was assessed for all variables that were not included in the regression by assessing the changes in regression coefficients (βs) of variables included in the model. If any of the βs of variables included in the model changes by 15% (Δβ >15%), then this variable was considered as a confounder and therefore included in the final logistic model.18 Parameters that gave P < .05 were included in the multivariate logistic analysis were patient age, sepsis, length of ICU stay, and the number of RBC units (supplemental Table 1). None of the dropped variables have confounding effect (supplemental Table 2). The patient age variable was standardized before adding it to the model in accordance to mean and standard deviation. Three variables were included in the model to estimate the odds ratio (OR) (95% confidence interval [CI]) of the study groups (female-to-female, female-to-male, male-to-female, and male-to-male). These variables are donor sex and donor-recipient sex mismatch in addition to the interaction between these 2 factors (supplemental Table 3). Computed ORs of the study groups was then verified by including the study groups as a variable in the logistic model instead of the previous mentioned 3 variables (supplemental Table 4). The advantages of including the 3 variables (donor sex, donor-recipient sex mismatch, and the interaction between these variables) are to estimate the effect of receiving blood from female donors and sex-mismatched donors in addition to using those to compute ORs for the study groups. The explained variation in this model is 17% based on the Nagelkerke R2 method. Association of transfusion with ICU-acquired complications was tested by Pearson's χ2 test. All statistical tests were 2-sided, and P < .05 was considered statistically significant.
Results
Study population
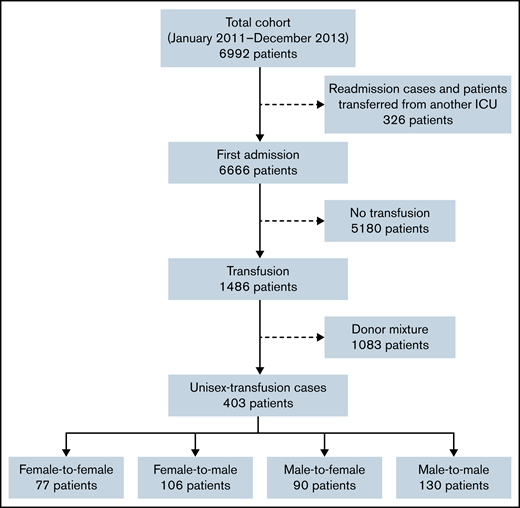
Of the 6992 patients included in the MARS project, 6666 patients were first admissions (Figure 1). Of these, 5180 patients were excluded because they did not receive RBC transfusions during their ICU stay. A further 1083 patients were excluded because they received RBC transfusions from both female and male donors (donor-mixed cases). Therefore, the current study included 403 patients who received RBC transfusions from only 1 sex group (unisex-transfusion cases), of which 77 female patients received RBC transfusions from female donors (female-to-female), 106 male patients received RBC transfusions from female donors (female-to-male), 90 female patients received RBC transfusions from male donors (male-to-female), and 130 male patients received RBC transfusions from male donors (male-to-male).
Characteristics of the patient groups are summarized in Table 1. Groups in this study did not differ in terms of baseline characteristics. In addition, there were no significant differences between groups in the transfusion-related parameters. The majority of patients received between 1 and 3 RBC units within the first 3 days of ICU admission. Transfusion of FFP products during the ICU stay was marginally higher in female-to-male and male-to-female groups (24.5% and 22.2%, respectively; P = .05).
Baseline characteristics of patients who received unisex transfusion
| . | Female-to-female (n = 77) . | Female-to-male (n = 106) . | Male-to-female (n = 90) . | Male-to-male (m = 130) . | P value . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y), median (IQR) | 61 (52-73) | 60 (51-72) | 59 (49-71) | 64 (53-73) | .67 |
| Length of hospital stay before ICU admission (d), median (IQR) | 1 (0-3) | 0 (0-2) | 1 (0-3) | 1 (0-6) | .43 |
| Chronic comorbidity | |||||
| Cardiovascular insufficiency, n (%) | 5 (6.5) | 14 (13.2) | 4 (4.4) | 16 (12.3) | .10 |
| Renal insufficiency, n (%) | 3 (3.9) | 3 (2.8) | 0 (0) | 3 (2.3) | .36 |
| COPD, n (%) | 8 (10.4) | 8 (7.5) | 6 (6.7) | 17 (13.1) | .35 |
| Malignancy, n (%) | 5 (6.5) | 9 (8.5) | 10 (11.1) | 4 (3.1) | .12 |
| Severity of disease on ICU admission | |||||
| APACHE IV score, median (IQR) | 75 (55.5-105) | 76.5 (58-110) | 73 (60-101.3) | 75 (52.7-97.3) | .49 |
| SOFA score, median (IQR) | 7 (5-10) | 7 (5-9) | 6 (5-10) | 7 (5-9) | .26 |
| AKI, n (%) | 1 (1.3) | 0 (0) | 0 (0) | 1 (0.8) | .54 |
| ARDS, n (%) | 1 (1.3) | 0 (0) | 1 (1.1) | 0 (0) | .41 |
| AMI, n (%) | 1 (1.3) | 1 (0.9) | 0 (0) | 0 (0) | .47 |
| Sepsis, n (%) | 31 (40.3) | 38 (35.8) | 43 (47.8) | 51 (39.2) | .39 |
| Mechanical ventilation, n (%) | 68 (88.3) | 100 (94.3) | 75 (83.3) | 119 (91.5) | .07 |
| Length of ICU stay (d), median (IQR) | 6 (4-11) | 6 (3-10) | 5 (3-10) | 6 (3-10) | .57 |
| Transfusion related parameters | |||||
| Number of RBC units, median (IQR) | 1 (1-2) | 2 (1-3) | 2 (1-2) | 2 (1-3) | .13 |
| Time to transfusion (d), median (IQR) | 1 (0-3) | 1 (0-3) | 0 (0-2) | 1 (0-3) | .31 |
| Number of RBC units received by donors’ age group, median (IQR) | |||||
| 18-40 y old | 1 (0-1) | 1 (0-1) | 0 (0-1) | 0 (0-1) | .21 |
| >40 y old | 1 (0-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | .24 |
| Non-RBC product transfusion, n (%) | |||||
| Platelet | 15 (19.5) | 21 (19.8) | 15 (16.7) | 16 (12.3) | .40 |
| FFP | 11 (14.3) | 26 (24.5) | 20 (22.2) | 16 (12.3) | .05 |
| Pre-ICU admission transfusion, n (%) | |||||
| RBC | 15 (19.5) | 22 (20.8) | 20 (22.2) | 23 (17.7) | .86 |
| Platelet | 7 (9.1) | 9 (8.5) | 12 (13.3) | 13 (10) | .55 |
| FFP | 6 (7.8) | 6 (5.7) | 6 (6.7) | 11 (8.5) | .86 |
| . | Female-to-female (n = 77) . | Female-to-male (n = 106) . | Male-to-female (n = 90) . | Male-to-male (m = 130) . | P value . |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y), median (IQR) | 61 (52-73) | 60 (51-72) | 59 (49-71) | 64 (53-73) | .67 |
| Length of hospital stay before ICU admission (d), median (IQR) | 1 (0-3) | 0 (0-2) | 1 (0-3) | 1 (0-6) | .43 |
| Chronic comorbidity | |||||
| Cardiovascular insufficiency, n (%) | 5 (6.5) | 14 (13.2) | 4 (4.4) | 16 (12.3) | .10 |
| Renal insufficiency, n (%) | 3 (3.9) | 3 (2.8) | 0 (0) | 3 (2.3) | .36 |
| COPD, n (%) | 8 (10.4) | 8 (7.5) | 6 (6.7) | 17 (13.1) | .35 |
| Malignancy, n (%) | 5 (6.5) | 9 (8.5) | 10 (11.1) | 4 (3.1) | .12 |
| Severity of disease on ICU admission | |||||
| APACHE IV score, median (IQR) | 75 (55.5-105) | 76.5 (58-110) | 73 (60-101.3) | 75 (52.7-97.3) | .49 |
| SOFA score, median (IQR) | 7 (5-10) | 7 (5-9) | 6 (5-10) | 7 (5-9) | .26 |
| AKI, n (%) | 1 (1.3) | 0 (0) | 0 (0) | 1 (0.8) | .54 |
| ARDS, n (%) | 1 (1.3) | 0 (0) | 1 (1.1) | 0 (0) | .41 |
| AMI, n (%) | 1 (1.3) | 1 (0.9) | 0 (0) | 0 (0) | .47 |
| Sepsis, n (%) | 31 (40.3) | 38 (35.8) | 43 (47.8) | 51 (39.2) | .39 |
| Mechanical ventilation, n (%) | 68 (88.3) | 100 (94.3) | 75 (83.3) | 119 (91.5) | .07 |
| Length of ICU stay (d), median (IQR) | 6 (4-11) | 6 (3-10) | 5 (3-10) | 6 (3-10) | .57 |
| Transfusion related parameters | |||||
| Number of RBC units, median (IQR) | 1 (1-2) | 2 (1-3) | 2 (1-2) | 2 (1-3) | .13 |
| Time to transfusion (d), median (IQR) | 1 (0-3) | 1 (0-3) | 0 (0-2) | 1 (0-3) | .31 |
| Number of RBC units received by donors’ age group, median (IQR) | |||||
| 18-40 y old | 1 (0-1) | 1 (0-1) | 0 (0-1) | 0 (0-1) | .21 |
| >40 y old | 1 (0-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | .24 |
| Non-RBC product transfusion, n (%) | |||||
| Platelet | 15 (19.5) | 21 (19.8) | 15 (16.7) | 16 (12.3) | .40 |
| FFP | 11 (14.3) | 26 (24.5) | 20 (22.2) | 16 (12.3) | .05 |
| Pre-ICU admission transfusion, n (%) | |||||
| RBC | 15 (19.5) | 22 (20.8) | 20 (22.2) | 23 (17.7) | .86 |
| Platelet | 7 (9.1) | 9 (8.5) | 12 (13.3) | 13 (10) | .55 |
| FFP | 6 (7.8) | 6 (5.7) | 6 (6.7) | 11 (8.5) | .86 |
AMI, acute myocardial infarction; APACHE, Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; FFP, fresh frozen plasma; SOFA, Sequential Organ Failure Assessment.
The impact of different unisex groups on mortality
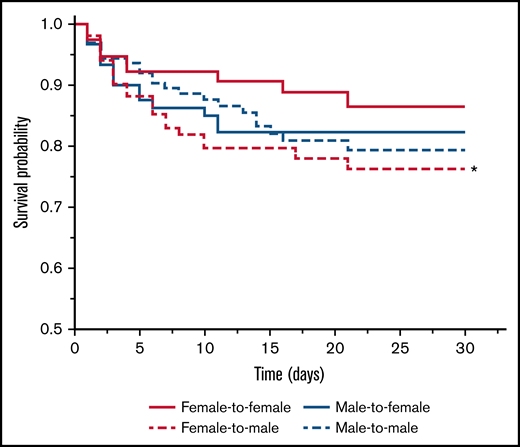
Figure 2 shows the Kaplan-Meier survival curve of the 4 groups. The group of females receiving female blood showed the best survival among other groups and served as the reference group. The Mantel-Cox Log-rank test revealed that the female-to-male group was associated with the highest mortality compared with the reference group (P < .05). Receipt of male blood was associated with an intermediate but not statistically significant difference for survival in both female and male recipients compared with the reference group.
Kaplan-Meier survival curve of the 4 groups of patients categorized according to unisex transfusion. Female-to-male group (long dash red line) had significantly shorter survival time compared with the female-to-female group (solid red line; P < .02 by Mantel-Cox Log-rank test).
Kaplan-Meier survival curve of the 4 groups of patients categorized according to unisex transfusion. Female-to-male group (long dash red line) had significantly shorter survival time compared with the female-to-female group (solid red line; P < .02 by Mantel-Cox Log-rank test).
The logistic model predicting ICU mortality
The multivariate logistic analysis shows that donor-recipient sex mismatch independently contributes to mortality with an OR 2.43 (95% CI, 1.02-5.77; P < .05) compared with the sex-matched transfused group (Table 2), which is equivalent to the OR (95% CI) of the female-to-male group (supplemental Tables 3 and 4). Patient age, sepsis, and length of ICU stay contributed significantly to the ICU mortality in this model (OR, 1.87; 95% CI, 1.34-2.63; OR, 2.25; 95% CI, 1.32-3.83; and OR, 0.94; 95% CI, 0.89-0.99, respectively; P < .05). In addition, the number of RBC units transfused marginally contributes to the prediction of mortality in this model (OR, 1.19; 95% CI, 0.98-1.43; P = .08).
The multivariate logistic model predicting ICU mortality
| Variable . | . | Descriptive* . | OR (95% CI) . |
|---|---|---|---|
| Donor sex† | Female (reference) | 183 (45.4) | 1.52 (0.64-3.60) |
| Male | 220 (54.6) | ||
| Donor-recipient sex mismatch‡ | Sex match (reference) | 207 (51.4) | 2.43 (1.02-5.77) |
| Sex mismatch | 196 (48.6) | ||
| Interaction between donor sex and donor-recipient sex mismatch§ | 0.36 (0.11-1.13) | ||
| Patient age | 63 (51-72) | 1.87 (1.34-2.63) | |
| Sepsis | No (reference) | 240 (59.6) | 2.30 (1.35-3.93) |
| Yes | 163 (40.4) | ||
| Length of ICU stay | 6 (3-10) | 0.94 (0.89-0.99) | |
| Number of RBC units | 2 (1-2) | 1.19 (0.98-1.43) | |
| Variable . | . | Descriptive* . | OR (95% CI) . |
|---|---|---|---|
| Donor sex† | Female (reference) | 183 (45.4) | 1.52 (0.64-3.60) |
| Male | 220 (54.6) | ||
| Donor-recipient sex mismatch‡ | Sex match (reference) | 207 (51.4) | 2.43 (1.02-5.77) |
| Sex mismatch | 196 (48.6) | ||
| Interaction between donor sex and donor-recipient sex mismatch§ | 0.36 (0.11-1.13) | ||
| Patient age | 63 (51-72) | 1.87 (1.34-2.63) | |
| Sepsis | No (reference) | 240 (59.6) | 2.30 (1.35-3.93) |
| Yes | 163 (40.4) | ||
| Length of ICU stay | 6 (3-10) | 0.94 (0.89-0.99) | |
| Number of RBC units | 2 (1-2) | 1.19 (0.98-1.43) | |
Frequency (%) for categorical variables and median (IQR) for contentious variables.
Equivalent to OR (95% CI) of male-to-male group.
Equivalent to OR (95% CI) of female-to-male group.
OR of male-to-female group can be estimated by multiplying of donor sex variable, donor-recipient sex-mismatch variable, and the interaction between donor sex and donor-recipient sex-match variable (supplemental Tables 3 and 4).
The association of unisex transfusion on ICU-acquired complications
There were no statistically significant differences between the 4 groups of unisex transfusions and ICU-acquired complications (Table 3). AKI incidence was higher in donor-recipient sex-matched groups (female-to-female and male-to-male) when compared with the sex-mismatched groups (P = .05). The receipt of female blood was associated with a trend toward increased risk of developing ARDS (female-to-female group and female-to-male group) compared with the receipt of male blood, although not statistically significant (P = .06). The nosocomial infection rate did not differ between groups.
Association of unisex transfusion and ICU-acquired complications
| Variable . | Female-to-female (n = 77) . | Female-to-male (n = 106) . | Male-to-female (n = 90) . | Male-to-male (n = 130) . | P value . |
|---|---|---|---|---|---|
| AKI | |||||
| Yes, n (%) | 5 (6.5) | 2 (1.9) | 1 (1.1) | 10 (7.7) | .05 |
| ARDS | |||||
| Yes, n (%) | 6 (7.8) | 7 (6.6) | 1 (1.1) | 3 (2.3) | .06 |
| Infection | |||||
| Yes, n (%) | 8 (10.4) | 10 (9.4) | 7 (7.8) | 15 (11.5) | .83 |
| Variable . | Female-to-female (n = 77) . | Female-to-male (n = 106) . | Male-to-female (n = 90) . | Male-to-male (n = 130) . | P value . |
|---|---|---|---|---|---|
| AKI | |||||
| Yes, n (%) | 5 (6.5) | 2 (1.9) | 1 (1.1) | 10 (7.7) | .05 |
| ARDS | |||||
| Yes, n (%) | 6 (7.8) | 7 (6.6) | 1 (1.1) | 3 (2.3) | .06 |
| Infection | |||||
| Yes, n (%) | 8 (10.4) | 10 (9.4) | 7 (7.8) | 15 (11.5) | .83 |
Discussion
Critically ill male recipients of female blood had a higher risk of dying compared with female recipients of female blood. In previous studies, both receipt of female blood5,8,19 as well as donor-recipient sex-mismatched transfusions were found to be associated with mortality.6,7,20 The current study showed that ICU mortality was higher in male patients than in female patients, as has been found previously.21,22 It has been shown that male ICU patients require higher intensity ICU treatment than female patients.23 The present study adds to these findings, showing that transfusing blood from female donors to male recipients is the only form of sex-mismatched transfusion that is significantly associated with mortality.
The findings of the current study are contrary to other studies suggesting that female recipients of male blood have an increased risk of in-hospital mortality7 and studies that do not suggest an association between donor sex and patient survival.10,24,25 It is challenging to explain the inconsistency between studies investigating the effect of donor sex on recipient outcomes. The majority of the previous studies, especially those not showing an association between donor sex and the risk of mortality, have included patients who received blood from both female and male donors (donor-mixed cases). Edgren et al found that donor sex is not significantly associated with posttransfusion mortality. In their study, the reference group for receiving female blood were patients who received RBCs exclusively from male donors. However, for the study group, patients were included who received at least 1 unit from female donors but may also have received male blood as well.24 In the current study, we excluded such cases as donor-mixed transfusion may lower the chance of finding any effect related to donor sex. Another important possible explanation for inconsistency could be related to the fact that some previous studies have used all types of blood transfusions, including plasma, platelets, and RBCs,6,19 whereas others used only RBC transfusions.5,7,8,25
Currently, there is no established mechanism to explain how donor-related factors result in adverse outcomes of critically ill patients. Therefore, the current study examined the association between donor sex and the development of ICU-acquired complications. In general, there was no association between donor sex and posttransfusion morbidity. ARDS tended to be more prevalent in patients receiving female blood. Female sex of the donor is a well-established risk factor for transfusion related acute lung injury (TRALI) for plasma transfusion.26-28 Exclusion of females from plasma donation has led to a lower incidence of TRALI.28,29 However, RBCs products contain 10 to 20 mL residual plasma, which is sufficient to cause TRALI.30,31 In line with this explanation, a recent cohort study showed that male recipients of RBCs from female donors who had been pregnant had a higher risk of mortality than those receiving RBCs either from female donors who had never been pregnant or from male donors.8 In the current study, the mortality in the female-to-female group is low; therefore, ARDS or TRALI is not the only factor driving mortality. This study does not provide a clear alternative mechanism. On the contrary, AKI occurred more often in donor-recipient sex-matched groups of female-to-female and male-to-male. The explanation for this result is unclear. It is also important to note that the current study did not demonstrate an increased risk of nosocomial infection in groups receiving blood from female donors.
Our findings have potential clinical implications. Although our results are observational and need confirmation in controlled trials, results are hypothesis generating. If indeed donor sex is a relevant factor, this should be included during the crossmatching procedure, as recently suggested.5,7 In stem cell and organ transplantation, donor sex is a well-established factor that can impact donor selection.32-36 It is also possible that the reported findings are pathology-dependent outcomes in which certain blood units with specific conditions are harmful for specific patient groups but not for others. Therefore, future transfusion policies may move toward algorithms which, in addition to blood type, include factors such as donor-recipient sex in order to match donor blood with recipients, at least in the specific patient group of the critically ill. Obviously, feasibility would be a concern, as such a policy would be challenging for blood donation centers given the global concern on continuous blood shortage.
Aside from the use of unisex transfusion, a strength of the current study is that following current transfusion regulations, blood units were randomly allocated among recipients regardless of donor characteristics, rendering it a double-blinded study. Therefore, the chance of having systematic confounders among the study groups is minimal. However, this study also has limitations. This is an observational study, which could have some external confounders that were not captured. Most importantly, donor sex of non-RBC product transfusions (plasma and platelets) was not taken into account, which could be a major confounder. However, the impact of this factor may be low as plasma was collected from male donors only, and a previous study has reported that donor sex has no role in platelet transfusion outcomes.37 Furthermore, when tested as a confounder in the regression model, the effect of platelet and plasma was minimal. Another potential limitation of the current study is related to generalizability. Many patients receiving blood from both sexes needed to be excluded in order to study the impact of donor sex. Also, it was a study in only 2 centers in the Netherlands. Future studies to validate these findings in other sites and countries are therefore recommended.
In conclusion, this study shows significant associations between male patients who received RBCs from female donors and ICU mortality, which may in part be due to ARDS, although other, as yet undetermined, factors are likely to be present. The mechanism of the association of donor-recipient sex and transfusion-related outcomes requires further study. Results may also inform studies investigating whether matching of donor and recipient sex improves outcome of critically ill patients in need of transfusion, in particular male recipients.
Acknowledgments
The authors would like to thank statisticians at the Department of Epidemiology and Data Science-Methodology and Statistics Consultation at Amsterdam UMC for their help on verifying the statistical analyses. They would also like to acknowledge staff of Sanquin and blood banks of Amsterdam UMC and Utrecht UMC for their help on retrieving blood donor information.
A.A. is supported by the King Saud University and the Saudi Arabian Cultural Bureau in the Netherlands.
Authorship
Contribution: A.A., F.U., and N.P.J. conceived and planned the study; MARS consortium collected data for the original MARS study; A.A. performed data analyses and drafted the manuscript; F.U., O.L.C., M.J.S., K.M.K.d.V., R.v.B., J.P.A., and N.P.J. provided critical feedback and helped shape the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the MARS Consortium appears in “Appendix.”
Correspondence: Abdulrahman Alshalani, Laboratory of Experimental Intensive Care and Anesthesiology, Amsterdam UMC, location AMC, Room M0-210, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: a.j.alshalani@amsterdamumc.nl.
Appendix
The members of the MARS Consortium: Friso M. de Beer, Lieuwe D. J. Bos, Gerie J. Glas, Arie J. Hoogendijk, Roosmarijn T. M. van Hooijdonk, Janneke Horn, Mischa A. Huson, Laura R. A. Schouten, Marcus J. Schultz, Brendon P. Scicluna, Marleen Straat, Lonneke A. van Vught, Luuk Wieske, Maryse A. Wiewel, Esther Witteveen, Marc J. M. Bonten, Olaf M. Cremer, David S. Y. Ong, Jos F. Frencken, Peter M. C. Klein Klouwenberg, Maria E. Koster-Brouwer, Kirsten van de Groep, and Diana M. Verboom.
References
Author notes
Requests for data sharing may be submitted to Abdulrahman Alshalani (a.j.alshalani@amsterdamumc.nl).
The full-text version of this article contains a data supplement.