TO THE EDITOR:
T-cell lymphomas are a heterogeneous group of malignancies involving T lymphocytes, with a poor prognosis. Mycosis fungoides (MF) and Sézary syndrome (SS) are the most frequent cutaneous T-cell lymphomas (CTCL). The circulating clonal tumor T cells (Sézary cells) express CD4 and may lose expression of CD7 and CD26, while exhibiting in most cases aberrant expression of CD158k (KIR3DL2).1,2 Long-term responses are rare in advanced-stage CTCL, and new treatments are needed. Recently, treatment with anti-CCR4 monoclonal antibody (mogamulizumab) has improved progression-free survival (PFS) in CTCL.3 CCR4 is expressed by Sézary cells and peripheral blood activated regulatory T cells (Treg)4 ; depletion of CCR4+ skin-infiltrating and circulating Treg by mogamulizumab5 is associated with autoimmune adverse events.6,7 Moreover, resistance to mogamulizumab may occur through loss of CCR4 expression by tumor T cells.8,9 Besides CCR4, Sézary cells express several Treg markers and immune checkpoints, such as PD1,10 CD39,11 and T cell Immunoreceptor with Ig and ITIM domains.12 This led us to investigate the expression of CCR8 (CD198), a chemokine receptor involved in the homing of lymphocytes to skin.13 CCR8 is expressed by skin resident memory T cells,14 which are suspected to be the tumor cells of origin in MF.15 CCR8 is also strongly expressed by tumor-infiltrating Treg involved in immune escape while expression on peripheral blood Treg is lower.16,17 Depletion of CCR8+ Treg exerted a strong antitumor effect independently or in combination with PD-1 inhibitor in tumor mouse models.18 Anti-CCR8 monoclonal antibody depleted fewer peripheral Tregs than anti-CCR4 monoclonal antibody,18 suggesting that treatment with therapeutic anti-CCR8 antibody may avoid immune side effects associated with mogamulizumab while sparing antitumor immunity.18 Therapeutic depletion of CCR8-expressing cells could thus eliminate tumor cells and activate the antitumor immunity in T-cell lymphomas while avoiding immune side effects.
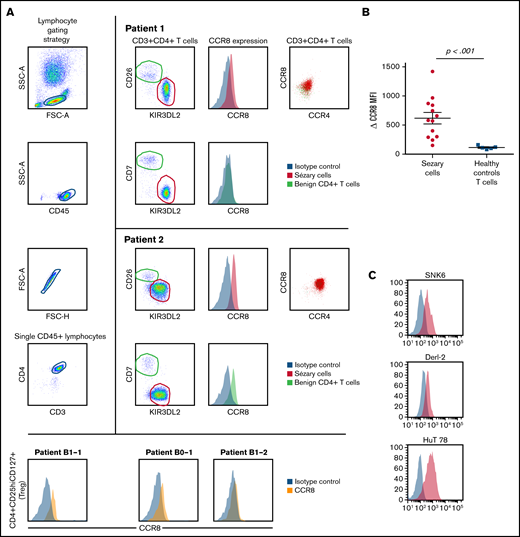
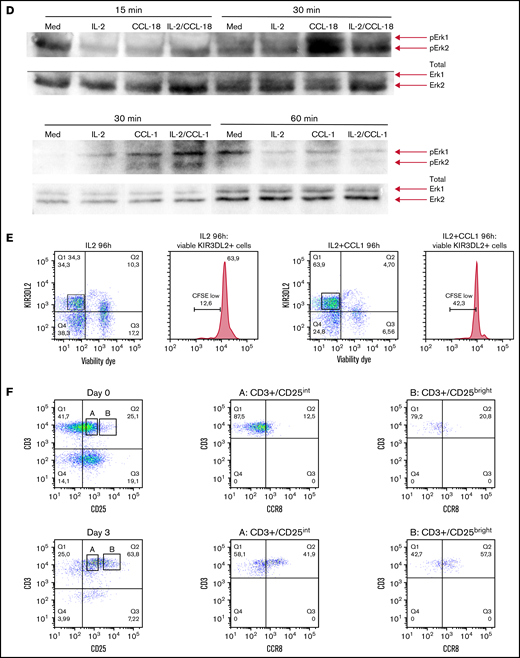
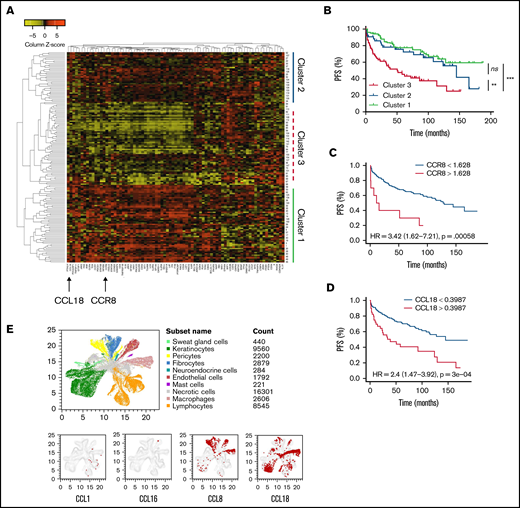
We performed flow cytometry analysis of peripheral blood leukocytes in 13 patients with SS and persistent blood involvement. The study received the approval of the Institutional Review Board, the Comité de Protection des Personnes, and was conducted in accordance with the Declaration of Helsinki. Sézary cells were identified as CD3+CD4+CD26− and/or CD7− KIR3DL2+ lymphocytes as previously described19,20 (Figure 1A). CCR8 expression was measured using the L263G8 monoclonal antibody and control immunoglobulin Ig G2a isotype and compared with that of healthy donors’ T cells. CCR8+ tumor T cells coexpressed CCR4 in all cases (Figure 1A). Peripheral blood CD4+ CD25hi CD127lo Tregs from Sézary patients did not express high levels of CCR8 (Figure 1A). CCR8 δ median mean fluorescence intensity (CCR8 mAb, control isotype) was 580 (range, 150-1420) in Sézary cells vs 110 (80-160) in healthy controls (P < .001, Figure 1B). Interestingly, not only CTCL HuT78 (SS) cell line but also the natural killer/T-cell lymphoma SNK6 and hepatosplenic γ-δ T-cell lymphoma DERL-2 cell lines expressed CCR8 (Figure 1C), suggesting CCR8 as a potential therapeutic target in different T-cell lymphoma subtypes. CCR8 engagement by its ligands CCL18 and CCL1 induced a significant Erk1/2 phosphorylation at 30 minutes and was independent on IL-2 in tumor cells of Sézary patients (Figure 1D). Moreover, it seems in some patients that CCL1 together with IL-2 induced a higher Sézary cell proliferation as compared with IL-2 alone (42% vs 12% CFSElo Sézary cells) (Figure 1E). The CCR8 expression was also analyzed after in vitro activation of healthy controls’ freshly isolated peripheral blood lymphocytes before (day 0) or after CD3/28 activation (day 3). CCR8 expression by T cells was significantly increased after 3 days of in vitro activation and was higher in CD25bright-activated T cells compared with CD25int T cells (Figure 1F). As previously reported,21–23 nonsupervised hierarchical clustering of Nanostring gene expression analyses in lesional skin of 157 patients with CTCL followed at Brigham and Women’s Hospital revealed the existence of a cluster of patients with a peculiar gene expression pattern (cluster 1, Figure 2A) and significantly shorter PFS compared with the 2 other clusters (Figure 2B). Of note, CCR8 was also expressed in the skin of patients with tumor and/or transformed disease. Among the overexpressed genes in skin of cluster 1 patients were CCR8 and CCL18, which is a CCR8 ligand. In the whole group of CTCL patients, PFS was shorter in patients with high expression of CCR8 messenger RNA (hazard ratio, 3.4 (95% confidence interval (CI), 1.6-7.2, P < .001, Figure 2C) and CCL18 messenger RNA in skin (hazard ratio, 2.4, 95% CI, 1.5-3.9, P < .0001, Figure 2D). These data are in accordance with the study from Miyagaki et al reporting that CCL18 expression in patients with CTCL was associated with disease severity and prognosis.21 This is of interest as CCL18 is a chemokine promoting skin infiltration by Th2 cells.24
CCR8 is overexpressed at the cell surface of CTCL peripheral blood tumor cells and is involved in Sézary cell activation and proliferation. Flow cytometric analyses of CCR8 expression by peripheral blood Sézary cells, regulatory T cells, healthy controls T cells, and T-cell lymphoma cell lines. (A) Gating strategy of peripheral blood Sézary cells (left panels) and mean fluorescence intensity of CCR8 expression in Sézary cells (vs control isotype) of 2 Sézary patients (medium panels). CCR8/CCR4 coexpression by Sézary cells is shown on the right panels. CCR8 was not significantly expressed by peripheral blood CD4+CD25hiCD127lo Tregs from Sézary patients (lower histograms). (B) CCR8 δ mean fluorescence intensity (CCR8 mAb, control isotype) in fresh Sézary cells from Sézary patients compared with healthy controls T cells. (C) Expression of CCR8 by SNK6, DERL-2, and HuT78 cell lines. (D) CCR8 stimulation by its ligands CCL18 (upper blots) and CCL1 (lower blots) induces Sézary cell activation. Freshly isolated Sézary cells were cultured for 15 or 30 minutes in medium alone or in the presence of IL-2 (100 IU/mL), CCL1 (10 ng/mL), CCL-18 (300 ng/mL), IL-2/CCL1, or IL-2/CCL18. The cells were lysed in NP40 (the content of the nuclei not visible), and expression of pErk1/2 was analyzed by western blot. (E) CCR8 stimulation by its CCL1 ligand induces Sézary cell proliferation. Fresh PBMC were incubated in CFSE and cultured over 96 hours in IL-2 (100 IU/mL), CCL-1 (10 ng/mL), or IL-2/CCL1 and the percentage of CFSElo cells calculated among live KIR3DL2+ Sézary cells. (F) CCR8 expression in freshly isolated healthy controls peripheral blood mononuclear cells before and after 3 days of in vitro CD3/28 activation. CFSE, carboxyfluorescein diacetate succinimidyl ester; mAb, monoclonal antibody; PBMC, peripheral blood mononuclear cell.
CCR8 is overexpressed at the cell surface of CTCL peripheral blood tumor cells and is involved in Sézary cell activation and proliferation. Flow cytometric analyses of CCR8 expression by peripheral blood Sézary cells, regulatory T cells, healthy controls T cells, and T-cell lymphoma cell lines. (A) Gating strategy of peripheral blood Sézary cells (left panels) and mean fluorescence intensity of CCR8 expression in Sézary cells (vs control isotype) of 2 Sézary patients (medium panels). CCR8/CCR4 coexpression by Sézary cells is shown on the right panels. CCR8 was not significantly expressed by peripheral blood CD4+CD25hiCD127lo Tregs from Sézary patients (lower histograms). (B) CCR8 δ mean fluorescence intensity (CCR8 mAb, control isotype) in fresh Sézary cells from Sézary patients compared with healthy controls T cells. (C) Expression of CCR8 by SNK6, DERL-2, and HuT78 cell lines. (D) CCR8 stimulation by its ligands CCL18 (upper blots) and CCL1 (lower blots) induces Sézary cell activation. Freshly isolated Sézary cells were cultured for 15 or 30 minutes in medium alone or in the presence of IL-2 (100 IU/mL), CCL1 (10 ng/mL), CCL-18 (300 ng/mL), IL-2/CCL1, or IL-2/CCL18. The cells were lysed in NP40 (the content of the nuclei not visible), and expression of pErk1/2 was analyzed by western blot. (E) CCR8 stimulation by its CCL1 ligand induces Sézary cell proliferation. Fresh PBMC were incubated in CFSE and cultured over 96 hours in IL-2 (100 IU/mL), CCL-1 (10 ng/mL), or IL-2/CCL1 and the percentage of CFSElo cells calculated among live KIR3DL2+ Sézary cells. (F) CCR8 expression in freshly isolated healthy controls peripheral blood mononuclear cells before and after 3 days of in vitro CD3/28 activation. CFSE, carboxyfluorescein diacetate succinimidyl ester; mAb, monoclonal antibody; PBMC, peripheral blood mononuclear cell.
Expression of CCR8 and ligands in CTCL skin and correlation with prognosis. (A) Heatmap of the unsupervised hierarchical clustering of 157 CTCL patients according to the Nanostring gene expression of 78 genes in lesional skin. Arrows indicate CCR8 and CCL18 gene expression. (B) Kaplan-Meier PFS curves of 157 CTCL patients according to their gene expression cluster. (C) Kaplan-Meier PFS curves of 157 CTCL patients according to CCR8 expression in lesional skin. (D) Kaplan-Meier PFS curves of 157 CTCL patients according to CCL18 expression in lesional skin. (E) Single-cell RNA sequencing data representing expression patterns of CCR8 ligands. Red dots show the localization of CCL1, CCL16, CCL8, and CCL18 among cell populations in the skin of CTCL patients.
Expression of CCR8 and ligands in CTCL skin and correlation with prognosis. (A) Heatmap of the unsupervised hierarchical clustering of 157 CTCL patients according to the Nanostring gene expression of 78 genes in lesional skin. Arrows indicate CCR8 and CCL18 gene expression. (B) Kaplan-Meier PFS curves of 157 CTCL patients according to their gene expression cluster. (C) Kaplan-Meier PFS curves of 157 CTCL patients according to CCR8 expression in lesional skin. (D) Kaplan-Meier PFS curves of 157 CTCL patients according to CCL18 expression in lesional skin. (E) Single-cell RNA sequencing data representing expression patterns of CCR8 ligands. Red dots show the localization of CCL1, CCL16, CCL8, and CCL18 among cell populations in the skin of CTCL patients.
To confirm the expression of CCR8 ligands in lesional skin of CTCL patients and analyze their expression by the tumor microenvironment, publicly available single-cell RNA sequencing data were accessed from the Gene Expression Omnibus database (accession GSE128531). This contains gene expression profiles of skin biopsies from 4 healthy volunteers, 3 biopsies of tumor-stage MF, 1 biopsy of an erythrodermic MF patient, and 1 from a SS patient as described in Gaydosik et al.25 A median of 4467 cells (range, 2200-9272) were analyzed per sample.
As shown in Figure 2E, CCL8 and CCL18 were significantly expressed by human skin cells in CTCL samples, whereas CCL1 expression was low and almost restricted to lymphocytes and CCL16 virtually absent. No CCR8 expression was detected by this technique. CCL8 was predominantly expressed by pericytes, fibroblasts, macrophages, and endothelial cells, and to a lower extent by keratinocytes, whereas CCL18 was mainly expressed by macrophages, keratinocytes, lymphocytes, fibroblasts, and endothelial cells.
In conclusion, this study confirms the overexpression of the homing marker CCR8 by peripheral blood Sézary cells compared with healthy controls’ T cells and its expression in lesional skin of CTCL patients at high risk of progression. Because this molecule is also expressed at the cell surface on other T-cell lymphoma cell lines, our results suggest that CCR8 might be a therapeutic target in distinct aggressive T-cell lymphoma subtypes. Among CCR8 ligands, CCL8 and CCL18 were the main chemokines expressed in the skin of CTCL patients and could be involved in skin homing of CCR8-expressing lymphocytes in CTCL patients. Consistent with these data, CCL18 was shown to be expressed by CD163(+) CD209(+) macrophages at the invasive margin of the tumor, and by c-Kit+ dendritic cells,24 promoting CTCL cell chemotaxis and Th2 cell infiltration.
Our study is the first to analyze CCR8 as a potential therapeutic target in CTCL. Immunomodulatory treatments such as PD-1 inhibition10 or allogeneic stem cell transplantation have shown able to produce long-term responses in CTCL, suggesting that the activation of antitumor immune responses might provide long-lasting disease control. In mogamulizumab-treated patients, depletion of CCR4-expressing peripheral activated Tregs was associated with immune side effects, but these immune reactions were associated with disease response and long-term disease control.6,7 CCR8 has recently been proposed as optimal tumor Treg target.18 Unlike CCR4, CCR8 was selectively expressed on human tumor Tregs and minimally expressed on proinflammatory effector T-cells.17,18 Preclinical mouse tumor models showed that depletion of CCR8+ Tregs through an FcyR-engaging anti-CCR8 antibody enabled long-lasting antitumor immunity that synergized with PD-1 blockade.25 Fc-optimized, nonfucosylated anti-human CCR8 antibodies specifically depleted Tregs and not effector T cells in ex vivo tumor cultures from primary human specimens.25 The efficacy of anti-human CCR8 depleting antibodies in the treatment of CTCL warrants further investigation.
Acknowledgments: The authors thank the patients enrolled in the present study. They also thank the French Society of Dermatology for their financial support.
Part of this study was supported by charitable contributions from Edward. P. Lawrence and from the Lubin Family Foundation. Support was also obtained from the National Institutes of Health (NIH)/National Cancer Institue (NCI) Specialized Program of Research Excellence grant P50 CA9368305 (T.S.K.), NIH R01CA203721 (R.A.C. and T.S.K.), NIH T32 AR007098 (T.S.K.), NIH R01 AR063962 (R.A.C.), and NIH P30 AR069625 (R.A.C.).
Contribution: J.G., A.d.M., A.B., and M. Bagot supervised the study and analyzed the data; T.E. performed experiments, analyzed data, and approved the final manuscript; G.D. and H.M.-T. provided data and contributed to the preparation of the manuscript; R.A.C and T.S.K. provided patients samples and contributed to the preparation of the manuscript; and M. Battistella, N.O., C.R.W., J.-D.B., A.M.-C., and S.M. critically reviewed the manuscript.
Conflict-of-interest disclosure: J.G, A.d.M., A.B., M. Bagot, N.O., and M. Battistella are named as inventors on a pending patent application regarding the use of CCR8 as a diagnostic and therapeutic target for the treatment of cutaneous T-cell lymphomas (EP21305356.4 contributed on 23 March 2021). The remaining authors declare no competing financial interests.
Correspondence: Adèle de Masson, Dermatology Department, INSERM U976 “Human Immunology, Pathophysiology and Immunotherapy,” Saint-Louis Hospital, 1 avenue Claude Vellefaux, 75010 Paris, France, adele.demasson@aphp.fr
References
Author notes
A.B. and A.d.M. are joint last authors.
Requests for data sharing may be submitted to Adèle de Masson (adele.demasson@aphp.fr).