Key Points
Most carriers with CD40L deficiency have a normal immune profile with differential high CD40L expression in the cTfh compartment.
HSCT for CD40L deficiency using carrier donors results in robust engraftment, stable CD40L expression, and excellent immune reconstitution.
Abstract
Data are limited regarding the immune status of CD40 ligand (CD40L)–deficient carriers and hematopoietic stem cell transplantation (HSCT) outcomes using them as donors for CD40L-deficient patients. Therefore, we studied the immune profiles of 7 carriers, 4 of whom were HSCT donors for family members with CD40L deficiency, and we characterized their HSCT outcomes. Immunoglobulin profiles, CD4, CD8, circulating T-follicular helper (cTfh) cells, and regulatory T cells (Tregs) in carriers were comparable to those in healthy controls. CD40L expression in carriers ranged from 37% to 78%. cTfh cells from carriers expressed higher CD40L compared with total CD4 cells or the memory CD4 compartment, suggesting a potential advantage to CD40L-expressing cTfh cells. Tregs had minimal CD40L expression in carriers and healthy controls. So we postulated that HSCT using donors who were CD40L carriers may result in excellent immune reconstitution without immune dysregulation. Four CD40L-deficient patients underwent HSCT from carriers who had CD40L expression from 37% to 63%. All patients engrafted, achieved excellent immune reconstitution with lack of opportunistic infections, graft-versus-host disease, and immune dysregulation; stable CD40L expression mimicked that of donors 1 to 5 years after HSCT. Immunoglobulin independence was achieved in 3 of the 4 patients. We demonstrated higher CD40L expression in the cTfh compartment of carriers and excellent immune reconstitution using donors who were CD40L carriers in CD40L-deficient patients.
Introduction
Patients with CD40 ligand (CD40L) deficiency have a combined immunodeficiency with an increased risk for recurrent bacterial, viral, and Pneumocystisjirovecii infections.1,2 Hematopoietic stem cell transplantation (HSCT) is currently the only known curative option.3-5 Preexisting lung and liver comorbidities and donors other than HLA-identical siblings are important risk factors.3-5 The suitability of carrier family donors (HLA-identical or haploidentical) has not been previously addressed. With few reports of skewed lyonization and increased infection risk, little is known about the clinical or immune profiles of carriers of CD40L deficiency (CD40L carriers),6-8 unlike other X-linked immune defects such as chronic granulomatous disease for which there is more information.9
Skewed lyonization, especially with mixed donor chimerism, raises concerns for incomplete disease phenotype correction and immune dysregulation after HSCT. There are concerns regarding whether further skewing of lyonization after HSCT could have an additional impact on long-term disease correction. There are no data on using CD40L carriers as HSCT donors. Therefore, to address this knowledge gap, we evaluated the immune profiles of CD40L carriers and, for the first time, we report the use of CD40L carriers as HSCT donors, which results in excellent long-term immune reconstitution.
Methods
Patients
Four patients with CD40L deficiency underwent HSCT between 2016 and 2020 from related X-linked carriers at 4 institutions: Children’s Healthcare of Atlanta, Cincinnati Children’s Hospital Medical Center, Duke University (all in the United States), and Heinrich Heine University, Düsseldorf, Germany. Clinical and laboratory data were obtained from medical records of each patient. We studied the immune profiles (CD3, CD4, CD8, CD4-naïve, regulatory T cells [Tregs], circulating T-follicular helper [cTfh] cells, CD19, memory B cells, class-switched memory B cells, immunoglobulin G [IgG], IgA, IgM, vaccine titers) of these 4 patients (P1-P4) and carrier donors (C1-C4) and 3 additional carriers (C5-C7).
In addition, CD40L expression in different CD4 subsets was obtained for 5 carriers (C1, C2, C3, C5, C6) and 3 post-HSCT (P1-P3) patients. Details of clinical parameters have been abstracted, and immune studies are provided in Table 1 and supplemental Data.
Immune profiles of carriers and patients with CD40L deficiency and HSCT characteristics
| Patient ID . | Carrier characteristics . | Patient and transplant characteristics . | Transplant outcomes . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) . | CD40L expressed in CD4 cells (%) . | B cells . | Class-switched memory B cells (%) . | Patient age at diagnosis/transplant . | Clinical features† . | Donor match and type . | Preparative regimen/ busulfan‡ . | CD34 × 106 cells per kilogram . | Myeloid/CD3 chimerism (when last checked) . | Outcome (at last follow-up) . | After HSCT, CD40L at time of evaluation . | Memory B/class-switched memory B cells (%) . | Ig replacement . | IgG/IgA/IgM, mg/dL . | Vaccine response . | Immune function phytohemagglutinin assay . | |
| 1 | 45 | 63.2 | 583 | 5.9 | 3 y/15 y | Neutropenia, Pneumocystis jirovecii pneumonia | 4/8 mismatched related donor, peripheral blood stem cells | busulfan (70)‡/fludarabine/thiotepa/anti-thymocyte globulin-r | 6.15 | 100/100 (49 mo) | Alive (49 mo) | 64.8% at 30 mo | 17/5.4 | No | 1020/158/106 (49 mo) | Protective pneumococcus, tetanus, diphtheria | Normal |
| 2 | 3 | 37 | 693 | 3.2 | 5 mo/8 mo | Neutropenia, Pneumocystis jirovecii pneumonia | 8/8 matched sibling donor, bone marrow | busulfan (64)‡/fludarabine/alemtuzumab | 23.1 | 100/91 (62 mo) | Alive (62 mo) | 34% at 62 mo | 21/3.2 | No | 1060/<7/114 (62 mo) | Protective pneumococcus, tetanus, diphtheria | Normal |
| 3 | 3.2 | 45 | 1596 | 2.6 | 5 mo/1.6 y | Neutropenia, adenovirus, cytomegalovirus, respiratory syncytial virus | 8/8 matched sibling donor, bone marrow | busulfan (64)‡/ fludarabine/anti-thymocyte globulin-r§ | 17.3 | 95/100 (28 mo) | Alive (45 mo) | 45% at 12 mo | 10.6/1.8 (L) | No | 551/75/140 (36 mo) | Protective tetanus, diphtheria, (pneumococcus not tested) | Normal |
| 4 | 9 | 54 | 164 (L) | 5 | 16 mo/3 y | Neutropenia, recurrent lower respiratory tract infections | 8/8 matched sibling donor, bone marrow | busulfan (90)‡/ fludarabine/anti-thymocyte globulin-gǁ | 16.2 | 99/92 (20 mo) | Alive (20 mo) | 35% at 20 mo | 6.3/0.5 (L) | Yes¶ | 400/<5/42# (22 mo) | Not tested | Not tested |
| 5 | 32 | 67 | 265 | Not applicable | |||||||||||||
| 6 | 35 | 78 | 43 (L) | 24.5 | |||||||||||||
| 7 | 37 | 41 | 42 (L) | 13 | |||||||||||||
| Patient ID . | Carrier characteristics . | Patient and transplant characteristics . | Transplant outcomes . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) . | CD40L expressed in CD4 cells (%) . | B cells . | Class-switched memory B cells (%) . | Patient age at diagnosis/transplant . | Clinical features† . | Donor match and type . | Preparative regimen/ busulfan‡ . | CD34 × 106 cells per kilogram . | Myeloid/CD3 chimerism (when last checked) . | Outcome (at last follow-up) . | After HSCT, CD40L at time of evaluation . | Memory B/class-switched memory B cells (%) . | Ig replacement . | IgG/IgA/IgM, mg/dL . | Vaccine response . | Immune function phytohemagglutinin assay . | |
| 1 | 45 | 63.2 | 583 | 5.9 | 3 y/15 y | Neutropenia, Pneumocystis jirovecii pneumonia | 4/8 mismatched related donor, peripheral blood stem cells | busulfan (70)‡/fludarabine/thiotepa/anti-thymocyte globulin-r | 6.15 | 100/100 (49 mo) | Alive (49 mo) | 64.8% at 30 mo | 17/5.4 | No | 1020/158/106 (49 mo) | Protective pneumococcus, tetanus, diphtheria | Normal |
| 2 | 3 | 37 | 693 | 3.2 | 5 mo/8 mo | Neutropenia, Pneumocystis jirovecii pneumonia | 8/8 matched sibling donor, bone marrow | busulfan (64)‡/fludarabine/alemtuzumab | 23.1 | 100/91 (62 mo) | Alive (62 mo) | 34% at 62 mo | 21/3.2 | No | 1060/<7/114 (62 mo) | Protective pneumococcus, tetanus, diphtheria | Normal |
| 3 | 3.2 | 45 | 1596 | 2.6 | 5 mo/1.6 y | Neutropenia, adenovirus, cytomegalovirus, respiratory syncytial virus | 8/8 matched sibling donor, bone marrow | busulfan (64)‡/ fludarabine/anti-thymocyte globulin-r§ | 17.3 | 95/100 (28 mo) | Alive (45 mo) | 45% at 12 mo | 10.6/1.8 (L) | No | 551/75/140 (36 mo) | Protective tetanus, diphtheria, (pneumococcus not tested) | Normal |
| 4 | 9 | 54 | 164 (L) | 5 | 16 mo/3 y | Neutropenia, recurrent lower respiratory tract infections | 8/8 matched sibling donor, bone marrow | busulfan (90)‡/ fludarabine/anti-thymocyte globulin-gǁ | 16.2 | 99/92 (20 mo) | Alive (20 mo) | 35% at 20 mo | 6.3/0.5 (L) | Yes¶ | 400/<5/42# (22 mo) | Not tested | Not tested |
| 5 | 32 | 67 | 265 | Not applicable | |||||||||||||
| 6 | 35 | 78 | 43 (L) | 24.5 | |||||||||||||
| 7 | 37 | 41 | 42 (L) | 13 | |||||||||||||
Patient ID: 1-4 indicates carriers C1-C4 and patients P1-P4; 5-7 indicates carriers C5-C7. Memory B cells are defined as CD27+ B cells; class-switched memory B cells are defined as IgD-CD27+ B cells.
IgG levels were normal in 5 of 7 carriers and borderline low in 2 carriers based on age and laboratory-specific norms.
g, Grafalon; L, low; r, rabbit.
CD40L expression was 0 in all patients, except patient 4 in whom it was 4%; IgG and IgA were undetectable in all patients; IgM was normal in 2 patients and elevated in 2 patients.
Area under the curve for busulfan (mg*h/L).
Genzyme rabbit ATG.
Grafalon rabbit ATG.
Restarted during winter months because of insufficient production.
#Ig profile before re-initiation of Ig replacement.
Results and discussion
CD40L carrier characteristics
We evaluated immune parameters as stated above in 7 CD40L carriers (C1-C7) ranging in age from 3 to 45 years. Four of them (C1-C4) were HSCT donors for family members with CD40L deficiency. All except carrier C3 were asymptomatic. Carrier C3 had a history of oral ulcers, periodontitis, and staphylococcal skin infections with absolute neutrophil count ranging from 730 to 2940 cells per microliter. Serum IgG levels were normal in 5 of 7 carriers, and low IgA was noted in 1 carrier (C3). All carriers had a normal T-cell profile, including normal proportions of naïve CD4, cTfh cells, and Tregs. CD40L expression in the CD4 T cells of carriers ranged from 37% to 78.2%. Details are provided in Table 1 and supplemental Table 1.
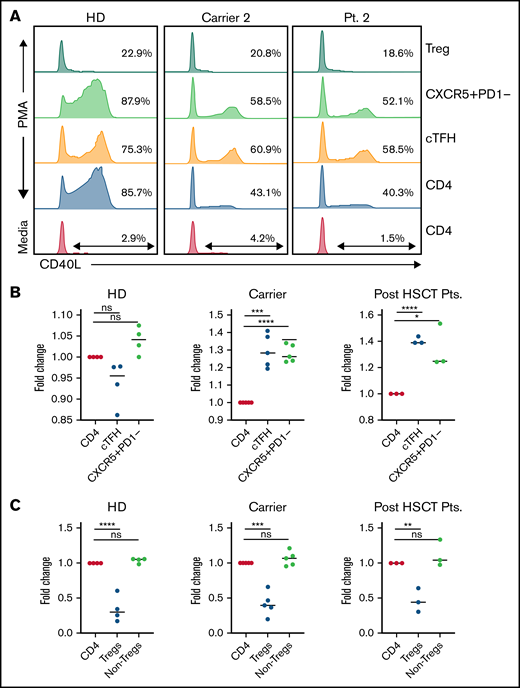
We evaluated CD40L expression in different CD4 T-cell compartments of carriers, including cTfh cells, Tregs, and the non-Treg memory CD4 compartment. Interestingly, we noted that CD40L expression was not uniform in different CD4 compartments. In the CD40L carriers, an increased proportion of cTfh cells expressed CD40L compared with total CD4 T cells (Figure 1) suggesting the preferential expansion or survival advantage of CD40L-expressing cTfh cells. Functional cTfh cells with CD40L expression are critical for T- and B-cell cross-talk, which leads to B-cell class switching, somatic hypermutation, and affinity maturation of antibodies.10 Another important observation was that Tregs of even healthy controls express very minimal or no CD40L (Figure 1), suggesting that CD40L may not be critical for Treg maintenance in humans. On the basis of these observations, we hypothesized that a higher proportion of CD40L expression in cTfh cells could facilitate better B-cell class switching and adequate humoral immune function, even in carriers. In addition, because of the normal Treg frequency in carriers and only minimal CD40L expression in Tregs, using carrier donors may not increase the risk of immune dysregulation and autoimmunity after HSCT.
Differential CD40L expression in different CD4 T cells compartments in carriers and recipients post-HSCT. (A) Representative flow cytometry plot demonstrating CD40L expression in different CD4 T-cell immune compartments at baseline and after stimulation; CD40L expression in cTfh cells is increased when compared with total CD4 of CD40L carriers and minimal expression of CD40L in Tregs. (B-C) Fold change in CD40L expression in cTfh, CXCR5+PD1-memory CD4, Tregs, and non-Treg CD4 cells relative to CD40L expression in total CD4 cells in healthy donors (HD), CD40L carriers, and recipients after HSCT. One-way analysis of variance with multiple comparison test was used for calculating the significant differences between groups. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, not significant.
Differential CD40L expression in different CD4 T cells compartments in carriers and recipients post-HSCT. (A) Representative flow cytometry plot demonstrating CD40L expression in different CD4 T-cell immune compartments at baseline and after stimulation; CD40L expression in cTfh cells is increased when compared with total CD4 of CD40L carriers and minimal expression of CD40L in Tregs. (B-C) Fold change in CD40L expression in cTfh, CXCR5+PD1-memory CD4, Tregs, and non-Treg CD4 cells relative to CD40L expression in total CD4 cells in healthy donors (HD), CD40L carriers, and recipients after HSCT. One-way analysis of variance with multiple comparison test was used for calculating the significant differences between groups. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, not significant.
Patient and HSCT characteristics
Details of the 4 patients and HSCTs are provided in Table 1 and supplemental Tables 1-4. Age at the time of HSCT ranged from 8 months to 15 years. Three patients received HLA-identical sibling bone marrow (P2-P4), and 1 patient received maternal haploidentical T-cell receptor alpha/beta (TCR alpha/beta)–depleted and CD19-depleted mobilized peripheral blood HSCT (P1). All patients received busulfan-fludarabine–based reduced-toxicity conditioning (Table 1; supplemental Table 3). Serotherapy consisted of rabbit anti-thymocyte globulin (rATG) (n = 3) and alemtuzumab (n = 1). Donor CD40L expression ranged from 37% to 63.2% on activated CD4 cells. CD34 dose ranged from 6.1 × 106 to 23.1 × 106 cells per kilogram. Graft-versus-host disease (GVHD) prophylaxis consisted of TCR alpha/beta and CD19 depletion only (P1) and treatment with cyclosporine-mycophenolate (P2, P3) or cyclosporine-methotrexate (P4).
All patients demonstrated neutrophil engraftment from 11 to 16 days after HSCT. Robust engraftment, even with a carrier donor and in 1 patient in a haploidentical setting, suggests that there may not be a significant disease intrinsic engraftment barrier in CD40L deficiency if adequate myeloablation is achieved. None of the patients developed acute or chronic GVHD. All patients maintained full donor myeloid chimerism (≥95%) and T-cell chimerism >90% at last testing (20-62 months). In addition, CD40L expression remained stable and mimicked that of donors 1 to 5 years after HSCT (Table 1). Despite massive hematopoietic stem cell stress from HSCT and new hormonal milieu (female to male), the CD40L expression did not seem to change after HSCT.
In samples taken from patients after HSCT, like their carrier donors, an increased proportion of CD40L-expressing cells was noted in the cTfh compartment. Because a functional cTfh compartment is critical for humoral immune function, an increased proportion of CD40L-expressing cTfh cells may have played a role in achieving functional B-cell immune reconstitution, even with a carrier donor. Consistent with these findings, 3 patients demonstrated class-switched memory B cells, achieved intravenous Ig independence 5 to 11 months after HSCT, and maintained normal IgG levels, for a median follow-up of 49 months (range, 45-62 months) (supplemental Table 4). The remaining patient stopped receiving Ig replacement for several months, but his IgG level was declining, albeit more slowly than expected, which indicated his own production. Ig replacement was resumed for prophylaxis against respiratory viruses during the winter months. None of the patients had any persistent viral infections or opportunistic infections. Although the exact mechanism of apparent increase in CD40L-expressing cTfh cells of carriers is not known, it is possible that CD40L-expressing cTfh cells engage lymph nodal B and dendritic cells better, which results in enhanced survival and proliferation compared with cTfh cells that do not express CD40L.11
Our data suggest that HSCT using a carrier with CD40L expression of ≥37% seems to be safe and results in excellent humoral and cellular immune reconstitution in patients with CD40L deficiency. Additional mixed donor T-cell chimerism data from allogeneic HSCT are needed to understand the minimum threshold necessary to achieve adequate immune reconstitution after HSCT. In addition, larger longitudinal studies in carriers are needed to determine dynamic changes in expression and the minimum CD40L threshold necessary to be infection free. These data could also help inform the minimal thresholds required for the success of gene editing strategies that are in development.12,13
The decision to use carriers as HSCT donors in X-linked immunodeficiency may need to be individualized and will be driven by the biology of the disease. If an inborn error of immunity (IEI) results in a predominantly immunodeficiency state and the deficient immune compartment does not drive immune dysregulation or autoimmunity, it might be reasonable to use carrier females if they are clinically well and have a normal immune profile with stable lyonization. With IEI, in which the deficient immune compartment has the potential to drive autoimmunity, more precaution should be exercised before considering people with this condition as HSCT donors. Understanding whether there is a potential survival advantage for the immune competent fraction in critical cellular compartments might also be helpful in making decisions regarding HSCT with X-linked carrier donors.
In summary, HSCT using X-linked carriers seems to be safe and results in excellent humoral and cellular immune reconstitution in patients with CD40L deficiency and carriers preferentially expressing CD40L in the cTfh fraction.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (1K08HL141635-01A1), from Atlanta Pediatric Scholars Program K12 Scholar, Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12HD072245), from the National Institute of Allergy and Infectious Diseases (U54AI082973) (S.C.), and from the HyperIGM Foundation.
Authorship
Contribution: S. Chandrakasan helped collect and analyze the data, researched CD40L and flow cytometry studies, created the figure, helped write the manuscript, and oversaw the project; C.P. helped collect and analyze the data; L.J.K., K.P., J.W., R.H.B., and R.M. provided clinical care, edited the manuscript, and provided critical input; L.L. provided clinical care and edited the manuscript; S.H.P., S. Chandra, and S.G provided clinical care, helped write and edit the manuscript, and provided critical input; S.H.P. provided project oversight and helped create the tables; and J.W., R.H.B., and R.M. provided clinical care and helped edit the manuscript.
Conflict-of-interest disclosure: S. Chandrakasan serves on the advisory committee of SOBI. The remaining authors declare no competing financial interests.
Correspondence: Shanmuganathan Chandrakasan, Emory University, Children's Healthcare of Atlanta, 1760 Haygood Drive, HSRB Bridge, 3rd floor, Room W368, Atlanta, GA 30322; e-mail: shanmuganathan.chandrakasan@emory.edu.
References
Author notes
S. Chandrakasan, S. Chandra, S.G., and S.H.P. contributed equally to this study.
Additional data related to this article are available by e-mail request to the Shanmuganathan Chandrakasan at shanmuganathan.chandrakasan@emory.edu.
The full-text version of this article contains a data supplement.