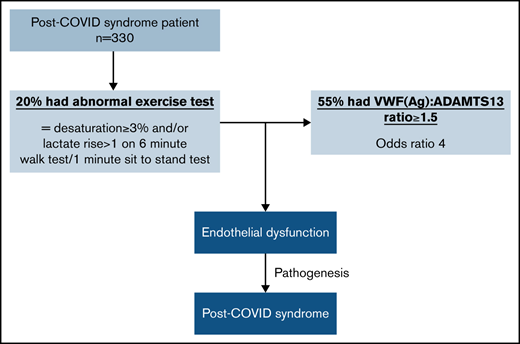
Key Points
A VWF(Ag)/ADAMTS13 ratio ≥1.5 was evident in 28% of the PCS cohort.
Of the patients with impaired exercise capacity, 55% had a VWF(Ag)/ADAMTS13 ratio ≥1.5 (odds ratio, 4).
Abstract
Post-COVID syndrome (PCS), or long COVID, is an increasingly recognized complication of acute SARS-CoV-2 infection, characterized by persistent fatigue, reduced exercise tolerance, chest pain, shortness of breath, and cognitive slowing. Acute COVID-19 is strongly linked with an increased risk of thrombosis, which is a prothrombotic state quantified by an elevated von Willebrand factor (VWF) antigen (Ag)/ADAMTS13 ratio that is associated with severity of acute COVID-19 infection. We investigated whether patients with PCS also had evidence of a prothrombotic state associated with symptom severity. In a large cohort of patients referred to a dedicated post-COVID-19 clinic, thrombotic risk, including VWF(Ag)/ADAMTS13 ratio, was investigated. An elevated VWF(Ag)/ADAMTS13 ratio (≥1.5) was present in nearly one-third of the cohort and was 4 times more likely to be present in patients with impaired exercise capacity, as evidenced by desaturation ≥3% and/or an increase in lactate level >1 from baseline on a 1-minute sit-to-stand test and/or a 6-minute walk test (P < .0001). Of 276 patients, 56 (20%) had impaired exercise capacity, of which 55% (31/56) had a VWF(Ag)/ADAMTS13 ratio ≥1.5 (P < .0001). Factor VIII and VWF(Ag) were elevated in 26% and 18%, respectively, and support a hypercoagulable state in some patients with PCS. These findings suggest possible ongoing microvascular/endothelial dysfunction in the pathogenesis of PCS and suggest a role for antithrombotic therapy in the treatment of these patients.
Introduction
The emergence of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has led to significant morbidity and mortality on a global level, with 257 662 742 confirmed cases of COVID-19 and 5 166 671 deaths1 at the time of this writing. Significant advances have been made in the clinical management of severe COVID-19 disease in the hospitalized population.2 However, just under 10% of patients with COVID-19 are admitted to the hospital.3 A proportion of patients experience ongoing symptoms, dominated by fatigue, chest pain, shortness of breath, and cognitive slowing. This persistence of symptoms has been named long COVID by patient groups, and termed post-COVID syndrome (PCS) by the National Institute for Health and Care Excellence (NICE).4 Estimates from the Office for National Statistics suggest that 1.1 million people in the United Kingdom have PCS.5 The NICE guidance on the long-term effects of COVID-19 provide definitions for illness and a framework for clinical management in PCS clinics, recently endorsed by the World Health Organization.6 It has been defined as a condition that “occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis.”4
Acute SARS-CoV-2 infection generates a hypercoagulable state, particularly evident in hospitalized patients.7 An increased D-dimer level, which has been associated with a poor outcome,7 as well as elevated factor VIII (FVIII) and von Willebrand factor (VWF) levels further support the hypercoagulability hypothesis.8 VWF plays a major role in primary hemostasis, and multimeric size is regulated by ADAMTS13 (a disintegrin and metalloprotease with thrombospondin 1 repeats, number 13). In acute COVID-19–infected patients, an increase in VWF:Ag (antigen), which may be associated with a relative reduction in ADAMTS13 and therefore an increase in VWF(Ag)/ADAMTS13 ratio is recognized, and this increase is likely to contribute to the hypercoagulable state and risk of microvascular thrombosis in these patients.8,9 Enhanced thrombin generation, decreased fibrinolytic activity, raised levels of FVIII and plasminogen-activator inhibitor type 1,10,11 high endothelin-1 levels,12 and increased D-dimer13 have also been identified in patients with PCS, suggesting a role for endothelial cell dysfunction and hypercoagulability.
We hypothesized that the prothrombotic state is involved in the pathogenesis of PCS. We investigated coagulation parameters associated with reduced exercise capacity in patients with PCS and identified the utility of these parameters in PCS, to determine ongoing disease activity. We further investigated whether an association exists between an elevated VWF(Ag)/ADAMTS13 ratio and impaired exercise capacity in patients with PCS treated at a dedicated clinical service for PCS.
Methods
Of 1471 face-to-face appointments at a dedicated outpatient post-COVID clinic from July 2020 through May 2021, we assessed the baseline demographics retrospectively of 413 patients who had their VWF(Ag)/ADAMTS13 ratio tested. Of those episodes, 352 involved patients who were more than 3 months past acute infection with COVID-19. Twenty-two episodes were repeat measurements performed over time and were excluded from analysis unless specified, leaving 330 patients for our full analysis. Trends in patients with repeat measurements are shown separately. Patients followed up in the clinic had proven or presumed (when polymerase chain reaction testing was not widely available during the initial stages of pandemic) COVID-19 infection. A clinical syndrome compatible with COVID-19 infection was agreed on by 2 independent senior clinicians in those with presumed COVID-19 infection. Referral to the post-COVID clinic included patients discharged from the hospital and referrals from community cases. Patients attending the clinic did not have blood tests performed if they were completed by their general practitioner as part of the referral to the post-COVID clinic or if recent blood test results were available from the hospital emergency or other departments. Blood tests were not repeated in patients reporting symptom improvement; this decision was not influenced by any other baseline characteristics, such as age.
ADAMTS13 activity was measured using the fluorescence resonance energy transfer-VWF73 assay.14 Von Willebrand screening (VWF antigen and VWF activity) was conducted with a standard automated immunoturbidimetric assay in a Sysmex CS-2500 analyzer with a Siemens kit (VWF:Ag and INNOVANCE VWF activity; Siemens Healthcare Diagnostics, Marburg, Germany), and a clotting assay using factor VIII (FVIII)–deficient plasma (reference OTXW17); Dade Actin FS Activated PTT reagent (reference B4218-100) was used to measure FVIII. All analyses were performed on plasma derived from peripheral blood samples collected in sodium citrate tubes as part of a routine assessment in the post-COVID clinic that included D-dimer level.
The VWF(Ag)/ADAMTS13 ratio was calculated on 50 voluntary healthy controls (Medical Research Ethics Committee approvals 08/H0810/54 and 08/H0716/72). The VWF(Ag)/ADAMTS13 ratio was also calculated in the cohort of patients with PCS and correlated with symptoms including exercise tolerance, as assessed by 1-minute sit-to-stand (STS) test and/or 6-minute walk test (6MWT). The 6MWT and 1-minute STS test were performed to assess abnormalities in components of exercise testing and therefore as an indicator of impaired exercise capacity. The 1-minute STS test involves going from siting to standing as many times as possible in 1 minute. Desaturation and number of repetitions completed as part of the STS test were examined. The 6MWT, as outlined in the American Thoracic Society guidelines,15 is a simple practical test that requires a 100-ft hallway but without the requirement for complex or additional equipment. It measures the distance that a patient can quickly walk on a flat surface in 6 minutes. Partial pressure of oxygen, pH, and capillary lactate were measured from an ear-prick blood test before and after exercise during the 6MWT, in parallel with continuous peripheral oxygen saturation measurement. Peripheral oxygen desaturation ≥3% for 6MWT and STS test, as well as an increase in lactate >1 from baseline during 6MWT were taken as markers of impaired exercise capacity. Reference values for the 6MWT has been assessed in healthy subjects aged 20 to 50 years, and the mean oxygen saturation after walking was 97 ± 1.3%, with a mean change in saturation of 1 ± 1.1%.16
Of the 330 patients, 293 had the STS test performed, and the results were analyzed in detail with a focus on the number of repetitions completed. Results of 1-minute STS tests in patients with COPD were a mean of 19 ± 6 repetitions,17 and similarly, a mean of 21 ± 6 repetitions in patients with interstitial lung disease.18 This test has also been used to evaluate physical capacity and exertional desaturation 1 month after discharge in patients who survived COVID-19 pneumonia. The mean number of repetitions was 20.9 ± 4.8 in those patients.19 Repetitions ≤20 were therefore considered to be an indicator of fatigue. The correlation between these components of the STS test and VWF(Ag)/ADAMTS13 ratio was assessed. Potential associations with D-dimer results were also investigated.
Statistics
All statistical analysis was performed in GraphPad Prism 9. Continuous data were summarized as the median and interquartile range (IQR), with the use of the Mann-Whitney test to compare ranks across 2 groups, and the Kruskal-Wallis test was used to compare ranks across 3 groups. Gaussian distribution was not assumed for the comparison between groups, as variables showed signs of nonnormality, in particular a long right tail for the VWF(Ag)/ADAMTS13 ratio (Figure 1A). The VWF(Ag)/ADAMTS13 ratio was calculated by (VWF(Ag)/ADAMTS13 activity) × 100 in each set of results for patients and healthy controls. The number and percentage were used to summarize categorical data. A χ2/Fisher’s exact test was used to assess statistical significance in categorical variables. The odds ratio (OR) and 95% confidence interval (CI) was estimated using logistic regression models. P < .05 indicated statistical significance.
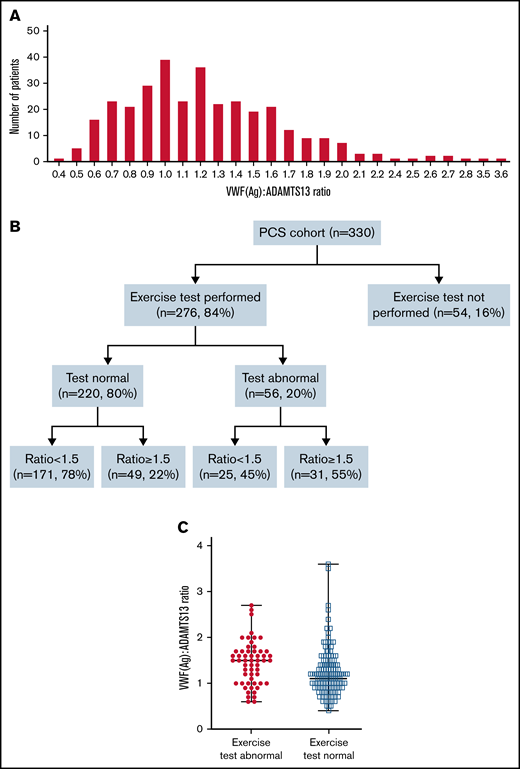
Spread of VWF(Ag)/ADAMTS13 ratio across patients with PCS, and overview of exercise testing and associated VWF(Ag)/ADAMTS13 ratio. (A) Histogram showing distribution of the VWF(Ag)/ADAMTS13 ratio across the post-COVID patient cohort. (B) Flowchart showing performance in exercise testing and associated VWF(Ag)/ADAMTS13 ratio. (C) Median VWF(Ag)/ADAMTS13 ratio in patients with normal and abnormal exercise test results. Median VWF(Ag)/ADAMTS13 ratio was 1.5 in patients with an abnormal result, compared with a median ratio of 1.1 in patients with a normal result. P < . 0001.
Spread of VWF(Ag)/ADAMTS13 ratio across patients with PCS, and overview of exercise testing and associated VWF(Ag)/ADAMTS13 ratio. (A) Histogram showing distribution of the VWF(Ag)/ADAMTS13 ratio across the post-COVID patient cohort. (B) Flowchart showing performance in exercise testing and associated VWF(Ag)/ADAMTS13 ratio. (C) Median VWF(Ag)/ADAMTS13 ratio in patients with normal and abnormal exercise test results. Median VWF(Ag)/ADAMTS13 ratio was 1.5 in patients with an abnormal result, compared with a median ratio of 1.1 in patients with a normal result. P < . 0001.
Results
The baseline demographics of 330 patients were analyzed: 60% were women and 40% were men, with a median age of 46 years (range, 18-88 years). Patient follow-up in the clinic varied from 3 to 15 months after proven or presumed COVID-19 infection. Of the 330 patients, 320 (97%) had symptoms for >3 months after acute COVID-19 infection, of which respiratory symptoms and fatigue were the commonest (Table 1).
Baseline demographics and proportion of patients reporting symptoms in a follow-up clinic visit, >3 months after acute COVID-19-infection
| . | Patients analyzed . |
|---|---|
| Female/male | 197 (60); 133 (40) |
| Median age | 46 y (range, 18-88) |
| Median time (mo) from acute COVID-19 infection | 6 (range, 4-10) |
| Symptomatic patients | 319 (97) |
| Respiratory symptoms (breathlessness, cough) | 223 (68) |
| Fatigue | 239 (72) |
| Neurological symptoms (headaches, numbness), cognitive impairment (poor memory/concentration), postural symptoms (disturbed sleep, dizziness/light headedness) | 153 (46) |
| Cardiac symptoms (chest discomfort, palpitations) | 155 (47) |
| Rheumatological symptoms (arthralgia, myalgia) | 79 (24) |
| Abdominal symptoms (abdominal pain, nausea/vomiting, diarrhea/constipation) | 39 (12) |
| Other (rash, anosmia, sore throat, splinter hemorrhages) | 45 (14) |
| . | Patients analyzed . |
|---|---|
| Female/male | 197 (60); 133 (40) |
| Median age | 46 y (range, 18-88) |
| Median time (mo) from acute COVID-19 infection | 6 (range, 4-10) |
| Symptomatic patients | 319 (97) |
| Respiratory symptoms (breathlessness, cough) | 223 (68) |
| Fatigue | 239 (72) |
| Neurological symptoms (headaches, numbness), cognitive impairment (poor memory/concentration), postural symptoms (disturbed sleep, dizziness/light headedness) | 153 (46) |
| Cardiac symptoms (chest discomfort, palpitations) | 155 (47) |
| Rheumatological symptoms (arthralgia, myalgia) | 79 (24) |
| Abdominal symptoms (abdominal pain, nausea/vomiting, diarrhea/constipation) | 39 (12) |
| Other (rash, anosmia, sore throat, splinter hemorrhages) | 45 (14) |
Data are the number (percentage) of patients in the total study group (N = 330), unless otherwise indicated.
Of the 330 patients with PCS, 85 (26%) had high FVIII, ranging from 2.1 to 5.1 IU/mL (normal range [NR], 0.5-2 IU/mL), and 60 (18%) had a high level of VWF(Ag), ranging from 1.7 to 3.3 IU/mL (NR, 0.5-1.6 IU/mL). ADAMTS13 activity ranged from 68.2 to 159.3 IU/dL (NR, 60-146 IU/dL).
The median VWF(Ag)/ADAMTS13 ratio was 0.98, with an IQR of 0.76 to 1.34 in 50 historical stored healthy control samples. Based on this IQR, a VWF(Ag)/ADAMTS13 ratio of ≥1.5 was considered to show an increase. The median VWF(Ag)/ADAMTS13 ratio was 1.2 (IQR, 0.9-1.5) across the 330 patients. Of the 330 patients in the cohort, 92 (28%) had a VWF(Ag)/ADAMTS13 ratio ≥1.5.
The D-dimer was increased (>550 μg/L fibrinogen equivalent units [FEU]) in 42 (13%) of the 330 patients tested. Elevated D-dimer was associated with a median VWF(Ag)/ADAMTS13 ratio of 1.3 (IQR, 1.0-1.6) compared with a ratio of 1.1 (IQR, 0.9-1.5) in 240 of the 330 (73%) who had a normal D-dimer level (P = .08); 48 patients did not have D-dimer level determined.
The VWF(Ag)/ADAMTS13 ratio was divided into 2 groups: <1.5 (normal) and ≥1.5 (abnormal). The groups were assessed separately against patient symptoms and results of exercise tolerance testing via the 1-minute STS exercise test and/or 6MWT. Of the 97% of symptomatic patients, 72% (230 of 320) had a VWF(Ag)/ADAMTS13 ratio <1.5, and 28% (90 of 320) had a ratio ≥1.5. Of the total cohort, 3% (10 of 330) was asymptomatic and had a median VWF(Ag)/ADAMTS13 ratio of 1.1. Therefore, the VWF(Ag)/ADAMTS13 ratio did not correlate with presenting clinical symptoms.
However, a VWF(Ag)/ADAMTS13 ratio ≥1.5 did correlate with abnormal exercise intolerance. Of the 330 patients assessed, 54 (16%) did not undergo exercise testing or failed to complete the test. Of those, 276 (84%) completed exercise testing (Figure 1B), and 220 (80%) were able to perform the tasks without impairment. Within this group, 171 of 220 (78%) had a normal VWF(Ag)/ADAMTS13 ratio <1.5, and 49 of 220 (22%) had a VWF(Ag)/ADAMTS13 ratio >1.5. Of the 276 patients, 56 (20%) had evidence of abnormal exercise tolerance, as confirmed by desaturation ≥3% and/or an increase in lactate levels >1 above baseline. Of those 56 patients, 31 (55%) had a high ratio (≥1.5; Table 2), which was equivalent to 11% of the whole cohort. Abnormal exercise tolerance was associated with a higher VWF(Ag)/ADAMTS13 ratio with a median of 1.5 (IQR, 1.2-1.7) compared with 1.1 (IQR, 0.9-1.4) in those with normal tolerance (P < .0001; Figure 1C). The risk of an increased VWF(Ag)/ADAMTS13 ratio was found to be 4 times more likely in those with abnormal exercise test results (OR, 4.3; 95% CI, 2.3-7.9; P < .0001), demonstrating a strong association by univariate analysis. The OR of 4 (95% CI, 2.1-7.8) was maintained according to multivariate analysis after adjustment for age, sex, and other comorbidities as potential confounders.
Proportion of patients with normal and raised VWF (Ag)/ADAMTS13 ratios, according to exercise test result
| . | Normal (n = 219), n (%) . | Abnormal (n = 56), n (%) . |
|---|---|---|
| VWF(Ag)/ADAMTS13 ratio <1.5 | 170 (78) | 25 (45) |
| VWF(Ag)/ADAMTS13 ratio ≥1.5 | 49 (22) | 31 (55) |
| . | Normal (n = 219), n (%) . | Abnormal (n = 56), n (%) . |
|---|---|---|
| VWF(Ag)/ADAMTS13 ratio <1.5 | 170 (78) | 25 (45) |
| VWF(Ag)/ADAMTS13 ratio ≥1.5 | 49 (22) | 31 (55) |
Significantly more patients with an abnormal exercise test result had a VWF(Ag)/ADAMTS13 ratio ≥1.5 (P < .0001; OR, 4).
Analysis of the 1-minute STS test result was performed focusing particularly on the number of repetitions completed. A repetition rate ≤20 was considered indicative of fatigue. Of the 293 STS test results analyzed, 228 were from patients more than 3 months past acute COVID-19 infection and were included in the analysis. Twelve patients did not have repetitions recorded, leaving full analysis to be completed in 216 patients (no repeat measurements were included). Of the 216 patients, 156 completed more than 20 repetitions, and of those, only 35 (22%) had a VWF(Ag)/ADAMTS13 ratio ≥1.5. Conversely, 60 patients completed ≤20 repetitions, and 19 (32%) of those had a VWF(Ag)/ADAMTS13 ratio ≥1.5. However, no significant difference was found between the groups (P = .17).
VWF(Ag)/ADAMTS13 ratio and significant desaturation (≥3%) were analyzed in relation to the number of repetitions completed in the STS test (Table 3). Of the 60 patients, 9 (15%) of those with ≤20 repetitions had a significant decrease in oxygen saturation (≥3%), of which 67% (6 of 9) had a VWF(Ag)/ADAMTS13 ratio ≥1.5. The median ratio was 1.6 (IQR, 1-1.8), compared with a normal ratio of 1.2 (IQR, 0.9-1.5) in the 85% (51 of 60) who did not have a significant decrease in saturation (P = .07). Comparably, of those who completed more than 20 repetitions, 13% (21 of 156) had a decline in oxygen saturation of ≥3%, with a median ratio of 1.5 (IQR, 1.2-1.9), compared with 1.1 (IQR, 0.8-1.4) in the 87% (135 of 156) with no significant desaturation (P < .0001). Overall, 63% (19 of 30) of those who had desaturation ≥3% had a high VWF(Ag)/ADAMTS13 ratio (P < .0001). Desaturation ≥3% during STS testing was associated with a high VWF(Ag)/ADAMTS13 ratio.
Proportion of patients with evidence of desaturation, median ratios in those groups, and percentage with VWF(Ag)/ADAMTS13 ratio ≥1.5
| . | Repetitions ≤20 (n = 60) . | . | Repetitions >20 (n = 156) . | . | ||
|---|---|---|---|---|---|---|
| Desaturation ≥3% 9/60 (15%) . | Desaturation <3% 51/60 (85%) . | P . | Desaturation ≥3% 21/156 (13%) . | Desaturation <3% 135/156 (87%) . | P . | |
| Median ratio (IQR) | 1.6 (1-1.8) | 1.2 (0.9-1.5) | .07 | 1.5 (1.2-1.9) | 1.1 (0.8-1.4) | <.0001 |
| Ratio ≥1.5, n (%) | 6/9 (67) | 13/51 (25) | .02 | 13/21 (62) | 22/135 (16) | <.0001 |
| . | Repetitions ≤20 (n = 60) . | . | Repetitions >20 (n = 156) . | . | ||
|---|---|---|---|---|---|---|
| Desaturation ≥3% 9/60 (15%) . | Desaturation <3% 51/60 (85%) . | P . | Desaturation ≥3% 21/156 (13%) . | Desaturation <3% 135/156 (87%) . | P . | |
| Median ratio (IQR) | 1.6 (1-1.8) | 1.2 (0.9-1.5) | .07 | 1.5 (1.2-1.9) | 1.1 (0.8-1.4) | <.0001 |
| Ratio ≥1.5, n (%) | 6/9 (67) | 13/51 (25) | .02 | 13/21 (62) | 22/135 (16) | <.0001 |
The split of abnormalities in STS and 6MWT results were assessed. Desaturation ≥3% was the most frequent abnormality in 29 of 56 (52%) patients, followed by lactate increase in 16 (29%) and desaturation plus lactate increase in 11 (20%). Median VWF(Ag)/ADAMTS13 ratios were 1.5 (IQR, 1-1.7), 1.3 (IQR, 0.9-1.5), and 1.6 (IQR, 1.6-1.9) in those groups, respectively. Nine of 11 (82%) of those with desaturation >3% and an increase in lactate had a VWF(Ag)/ADAMTS13 ratio ≥1.5, compared with 17 of 29 (59%) of those who had desaturation and 6 of 16 (38%) of those who had an increase in lactate alone (P = .07). Elevated D-dimer level (>550 μg/L FEU) was not associated with desaturation or an increase in lactate (P = .50).
VWF(Ag)/ADAMTS13 ratio and exercise test results were analyzed according to 3 age groups: ≤40 years, 41 to 60 years, and >60 years. The median VWF(Ag)/ADAMTS13 ratio was found to be 1 (IQR, 0.8-1.3), 1.2 (IQR, 1-1.5), and 1.6 (IQR, 1.2-2), respectively (P < .0001), showing a difference in ratio across the age groups. Exercise testing within the age groups showed that, whereas fewer patients had evidence of abnormal compared with normal exercise test results (abnormal test results in 16% [14 of 86] of patients <40 years, 17% [26 of 150] of patients 40-60 years, and 41% (16 of 39) of patients >60 years), results were abnormal in a greater proportion of patients >60 years of age, suggesting an age-related effect (P = .003).
Preexisting lung comorbidities were also analyzed in relation to the VWF(Ag)/ADAMTS13 ratio and the exercise test results. Of the 330 patients, 45 (14%) had a preexisting diagnosis of lung disease. No difference in median VWF(Ag)/ADAMTS13 ratio was found in those with or without preexisting lung disease: 1.1 (IQR, 0.9-1.7) and 1.2 (IQR, 0.9-1.5), respectively (P = .80). Of the 56 patients with abnormal exercise test results, only 7 (13%) had preexisting lung disease and 49 (88%) did not. Of the 7 patients with known lung disease, 4 had asthma (all with good control except 1 patient), 2 had chronic obstructive pulmonary disease (both were receiving treatment), and 1 had recent respiratory tuberculosis infection. Of the 220 patients with normal exercise test results, 28 (13%) had known lung disease compared with 192 (87%) who did not (P > .99). Therefore, no significant effect of preexisting lung disease was associated with abnormal exercise test results.
Median FVIII and VWF antigen levels were higher in patients with abnormal exercise tolerance (Table 4). No significant difference in D-dimer levels >550 μg/L FEU was found between those with normal and abnormal exercise tolerance (780 and 780 μg/L FEU, respectively; P = .5).
Table showing differences in FVIII, VWF(Ag), VWF activity, and D-dimer levels in patients with normal and abnormal exercise test results
| Median levels, median (IQR) . | Normal (n = 220) . | Abnormal (n = 56) . | P . |
|---|---|---|---|
| FVIII (NR 0.5-2 IU/mL) | 1.5 (1.2-2) | 2 (1.6-2.4) | <.0001 |
| VW Ag (NR 0.5-1.6 IU/mL) | 1.3 (1.0-1.5) | 1.5 (1.2-1.7) | .0003 |
| VW activity (NR 0.5-1.87 IU/mL) | 1.2 (1-1.4) | 1.2 (1-1.5) | .30 |
| D-dimer >550 (NR 0-550 μg/L FEU) | 780 (633-1128) | 780 (640-1315) | .50 |
| Median levels, median (IQR) . | Normal (n = 220) . | Abnormal (n = 56) . | P . |
|---|---|---|---|
| FVIII (NR 0.5-2 IU/mL) | 1.5 (1.2-2) | 2 (1.6-2.4) | <.0001 |
| VW Ag (NR 0.5-1.6 IU/mL) | 1.3 (1.0-1.5) | 1.5 (1.2-1.7) | .0003 |
| VW activity (NR 0.5-1.87 IU/mL) | 1.2 (1-1.4) | 1.2 (1-1.5) | .30 |
| D-dimer >550 (NR 0-550 μg/L FEU) | 780 (633-1128) | 780 (640-1315) | .50 |
Of the 330 patients, 273 had acute COVID-19 infection managed in the community. Community-managed (83%) and hospitalized (16%) patients had a comparable median VWF(Ag)/ADAMTS13 ratio of 1.2 (IQR, 1-1.4 and 0.9-1.5, respectively; P = .71). There was no significant difference in median VWF(Ag)/ADAMTS13 ratio in the hospitalized patients who required oxygen support, noninvasive ventilation, or intubation and ventilation, with median follow-up ratios of 1.2 (IQR, 1-1.4), 1.1 (IQR, 0.7-1.4), and 1.3 (1.2-1.6), respectively (0.22). Of the 53 hospitalized patients, 21 received dexamethasone. The median VWF(Ag)/ADAMTS13 ratios at follow-up were 1.3 and 1.1 (P = .10), respectively, in patients who received dexamethasone and those who did not.
Finally, changes in VWF(Ag)/ADAMTS13 ratio and exercise test results in 18 patients who had repeat assessments performed over time were analyzed (Table 5). Findings were variable from a stable VWF(Ag)/ADAMTS13 ratio to a reduction in ratio. Exercise testing was not repeated in all patients.
VWF(Ag)/ADAMTS13 ratios and exercise test results in 18 patients who had repeat assessments performed over time
| Patient . | Assessment . | VWF(Ag)/ ADAMTS13 ratio . | Exercise test result . |
|---|---|---|---|
| 1 | Baseline | 1.5 | Lactate increase 0.8-2.4 |
| 1 mo | 1.5 | Lactate increase and desaturation 3% | |
| 2 | Baseline | 1.7 | Lactate increase 0.5-2.4 and desaturation 8% |
| 1 mo | 1.5 | Desaturation 3% | |
| 3 | Baseline | 1.2 | Not done |
| 2 mo | 1.3 | Not done | |
| 4 | Baseline | 1.2 | Normal |
| 1 mo | 1.0 | Desaturation 3% | |
| 5 | Baseline | 1.3 | Normal |
| 5 mo | 1.4 | Not done | |
| 6 | Baseline | 0.6 | Lactate increase 0.9-6.9 |
| 4 mo | 0.7 | Not done | |
| 7 | Baseline | 1.2 | Normal |
| 1 mo | 1.0 | Normal | |
| 8 | Baseline | 1.3 | Normal |
| 1 mo | 1.5 | Desaturation 3% | |
| 4 mo | 1.3 | Not done | |
| 9 | Baseline | 1.7 | Desaturation 13% and lactate increase 2.6-7.3 |
| 1 mo | 1.7 | Desaturation 9% and lactate increase 0.5-8.7 | |
| 10 | Baseline | 1.5 | Normal |
| 6 mo | 1.3 | Normal | |
| 11 | Baseline | 1.1 | Normal |
| 3 mo | 1.1 | Not done | |
| 12 | Baseline | 0.9 | Normal |
| 1 mo | 0.9 | Not done | |
| 13 | Baseline | 3.5 | Normal |
| 3 mo | 3.6 | Not done | |
| 14 | Baseline | 1.9 | Normal |
| 1 mo | 0.7 | Normal | |
| 2 mo | 0.9 | Not done | |
| 15 | Baseline | 2.0 | Desaturation 3% and lactate increase 0.8-2.4 |
| 2 mo | 1.7 | Normal | |
| 3 mo | 1.6 | Not done | |
| 5 mo | 1.7 | Not done | |
| 16 | Baseline | 1.5 | Normal |
| 6 mo | 1.4 | Normal | |
| 17 | Baseline | 0.5 | Normal |
| 6 mo | 0.4 | Not done | |
| 18 | Baseline | 2.8 | Not done |
| 4 mo | 1.8 | Not done |
| Patient . | Assessment . | VWF(Ag)/ ADAMTS13 ratio . | Exercise test result . |
|---|---|---|---|
| 1 | Baseline | 1.5 | Lactate increase 0.8-2.4 |
| 1 mo | 1.5 | Lactate increase and desaturation 3% | |
| 2 | Baseline | 1.7 | Lactate increase 0.5-2.4 and desaturation 8% |
| 1 mo | 1.5 | Desaturation 3% | |
| 3 | Baseline | 1.2 | Not done |
| 2 mo | 1.3 | Not done | |
| 4 | Baseline | 1.2 | Normal |
| 1 mo | 1.0 | Desaturation 3% | |
| 5 | Baseline | 1.3 | Normal |
| 5 mo | 1.4 | Not done | |
| 6 | Baseline | 0.6 | Lactate increase 0.9-6.9 |
| 4 mo | 0.7 | Not done | |
| 7 | Baseline | 1.2 | Normal |
| 1 mo | 1.0 | Normal | |
| 8 | Baseline | 1.3 | Normal |
| 1 mo | 1.5 | Desaturation 3% | |
| 4 mo | 1.3 | Not done | |
| 9 | Baseline | 1.7 | Desaturation 13% and lactate increase 2.6-7.3 |
| 1 mo | 1.7 | Desaturation 9% and lactate increase 0.5-8.7 | |
| 10 | Baseline | 1.5 | Normal |
| 6 mo | 1.3 | Normal | |
| 11 | Baseline | 1.1 | Normal |
| 3 mo | 1.1 | Not done | |
| 12 | Baseline | 0.9 | Normal |
| 1 mo | 0.9 | Not done | |
| 13 | Baseline | 3.5 | Normal |
| 3 mo | 3.6 | Not done | |
| 14 | Baseline | 1.9 | Normal |
| 1 mo | 0.7 | Normal | |
| 2 mo | 0.9 | Not done | |
| 15 | Baseline | 2.0 | Desaturation 3% and lactate increase 0.8-2.4 |
| 2 mo | 1.7 | Normal | |
| 3 mo | 1.6 | Not done | |
| 5 mo | 1.7 | Not done | |
| 16 | Baseline | 1.5 | Normal |
| 6 mo | 1.4 | Normal | |
| 17 | Baseline | 0.5 | Normal |
| 6 mo | 0.4 | Not done | |
| 18 | Baseline | 2.8 | Not done |
| 4 mo | 1.8 | Not done |
Discussion
The long-term impact of COVID-19 infection is now recognized to be associated with ongoing symptomatology beyond 3 months after acute infection; 22.1% of patients experience at least 1 symptom at 5 weeks after COVID-19 infection and 9.8% at 12 weeks,20 and high symptom burden after infection in nonhospitalized as well as posthospitalized patients have been shown.21 A thromboinflammatory process has been implicated in the pathogenesis of COVID-19 infection. Increased FVIII, VWF, and D-dimer levels are the primary hemostasis factors supportive of a hypercoagulable state. An analysis of the coagulation parameters led to investigation of the VWF(Ag)/ADAMTS 13 ratio, and a higher ratio was particularly seen in the most severe COVID-19 cases.8,9 We extended analysis of the VWF(Ag)/ADAMTS13 ratio to patients with PCS and identified nearly one-third of the cohort as having an increased ratio.
The pathophysiology of PCS has not been adequately addressed. This is the first report, to our knowledge, to identify and report an association between a high VWF(Ag)/ADAMTS13 ratio and impaired exercise capacity, specifically related to desaturation ≥3% and an increase in lactate levels >1 above baseline. We found an association between a higher VWF(Ag)/ADAMTS13 ratio and limited exercise capacity, in both hospitalized and nonhospitalized patients. A high ratio was found to be 4 times more likely in patients with abnormal exercise tolerance. These findings suggest an ongoing prothrombotic state in PCS, demonstrated by measurable laboratory and clinical parameters, therefore adding confirmation to the hypothesis of microvascular endothelial dysfunction in the pathogenesis of PCS. We hypothesize the persistence of a hypercoagulable state that may be associated with endothelial dysfunction and microthrombi in the capillary bed of large muscles, causing a reduction in oxygen capacity during exercise and resulting in anaerobic respiration and fatigue.
A markedly high VWF(Ag)/ADAMTS13 ratio with a median of 6.07 has been shown during the acute phase of COVID-19 infection.8 Measurement of VWF(Ag)/ADAMTS13 levels was not increased when analyzed against several clinical symptoms, including fatigue, headaches, and abnormalities in cognition, with a median VWF(Ag)/ADAMTS13 ratio of 1.2 in our PCS cohort, which was a median of 6 months past acute COVID-19 infection. This finding therefore suggests that the high VWF(Ag)/ADAMTS13 ratio observed in patients with acute COVID-19 infection may settle over time, despite ongoing symptoms of PCS, with a reversal of the ratio to normal in most patients. However, a limitation is that longitudinal measurements repeated over time are lacking in this study. The VWF(Ag)/ADAMTS13 ratio remained >1.5 in a proportion of patients with impaired exercise capacity measured objectively.
Virus dependent and independent mechanisms have been implicated in the pathogenesis of PCS, particularly in patients with respiratory symptoms. Evidence of myocardial inflammation in patients experiencing chest pain has been shown on magnetic resonance imaging. ACE2 downregulation, inflammation, and immunological responses affecting the structural integrity of the heart have been suggested as potential mechanisms of PCS. Direct viral invasion, as has been found in the heart tissue of patients with COVID-19 at autopsy, has also been implicated.22 A physiological basis for PCS, with measurable patient-reported outcomes and organ impairment shown through biochemical and imaging characterization of organ function with quantitative magnetic resonance imaging, has been shown in the ongoing COVERSCAN study.23
Results of whole-blood viscoelastic and thrombin-generation tests support a hypercoagulable state in acute COVID-19 infection. Raised FVIII and plasminogen-activator inhibitor type 1 levels and a decline in the previously high admission levels of plasmin-antiplasmin complexes at 4-month follow-up after COVID-19 infection suggest a hypercoagulable and hypofibrinolytic, and therefore prothrombotic, state in PCS.10 Pretorius et al24 have shown the presence of large amyloid fibrin deposits in the plasma of patients with acute COVID-19 infection. Importantly, the deposits were found to persist in patients with PCS and were resistant to fibrinolysis. A significant increase in serum amyloid A and α2-antiplasmin, which were trapped in the fibrinolysis-resistant pellet, was also found.
Mandal et al25 also reported persistent raised D-dimer levels in 30.1% of patients after hospital discharge for COVID-19 infection with symptom predominance of shortness of breath and fatigue, and Townsend et al13 showed that D-dimer levels remained increased in 25.3% of patients up to 4 months after acute COVID-19 infection. Increased D-dimer was found in only 13% of our PCS cohort who were more than 3 months past acute COVID-19 infection. The longer time since COVID-19 infection in our cohort may explain this difference. Fogarty et al26 demonstrated a role for sustained endotheliopathy in PCS, supported by increased endogenous thrombin potential and peak thrombin levels, as well as raised VWF antigen, VWF propeptide, and FVIII. They showed a significant inverse correlation between 6MWT distances and both VWF antigen and VWF propeptide, which was lost after adjustment for age, sex, and severity of initial infection. Our results provide further support for a role of endothelial dysfunction in the pathogenesis of PCS. However, in addition to this, we have provided evidence of an association between a VWF(Ag)/ADAMTS13 ratio ≥1.5 and impaired exercise capacity, as evidenced by desaturation ≥3% and/or an increase in lactate levels. Desaturation ≥3% during a 1-minute STS test was specifically associated with a high VWF(Ag)/ADAMTS13 ratio.
These findings support screening patients referred with PCS for the VWF(Ag)/ADAMTS13 ratio and performing exercise tests. In a time when the pathogenesis of this newly arisen phenomenon is not yet clear, it is important from a research perspective to do these screenings. The underlying mechanism that leads to abnormalities of exercise test results in this group of patients with a VWF(Ag)/ADAMTS13 ratio ≥1.5 remain undiscovered. Importantly, the findings also suggest a potential role for antithrombotic therapy in this cohort of patients and requires large-scale trials to evaluate the therapy as a treatment option in patients with PCS.
Authorship
Contribution: N.P. analyzed the data and wrote the manuscript; M.H., T.H., E.W., and R.B. designed the research, collected the data and reviewed the manuscript; A.K., A. Doyle, and A. Devaraj collected the data and reviewed the manuscript; L.N. and D.S. conducted the laboratory tests and reviewed the manuscript; H.-M.D. oversaw the statistical analysis and reviewed the manuscript; and as the senior author, M.S. designed the research and reviewed the manuscript.
Conflict-of-interest disclosure: M.S. has received speaker fees and has served on advisory boards for Alexion, Novartis, Takeda, Sanofi, and Octapharma and has received research grants from Shire and Alexion. The remaining authors declare no competing financial interests.
Correspondence: Nithya Prasannan, Haemostasis Research Unit, University College London, 51 Chenies Mews, London WC1E 6HX, United Kingdom; e-mail: n.prasannan@nhs.net.
References
Author notes
Requests for data sharing may be submitted to Nithya Prasannan (n.prasannan@nhs.net).