Key Points
CD19 CAR therapy for R/R EM disease, particularly CNS, offers a beneficial option with similar toxicity and survival to BM disease.
There was no increased cytokine release syndrome or neurotoxicity in patients with R/R EM disease, including active CNS disease at infusion.
Abstract
Chimeric antigen receptor (CAR) T cells have transformed the therapeutic options for relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia. Data for CAR therapy in extramedullary (EM) involvement are limited. Retrospective data were abstracted from the Pediatric Real World CAR Consortium (PRWCC) of 184 infused patients from 15 US institutions. Response (complete response) rate, overall survival (OS), relapse-free survival (RFS), and duration of B-cell aplasia (BCA) in patients referred for tisagenlecleucel with EM disease (both central nervous system (CNS)3 and non-CNS EM) were compared with bone marrow (BM) only. Patients with CNS disease were further stratified for comparison. Outcomes are reported on 55 patients with EM disease before CAR therapy (CNS3, n = 40; non-CNS EM, n = 15). The median age at infusion in the CNS cohort was 10 years (range, <1-25 years), and in the non-CNS EM cohort it was 13 years (range, 2-26 years). In patients with CNS disease, 88% (35 of 40) achieved a complete response vs only 66% (10 of 15) with non-CNS EM disease. Patients with CNS disease (both with and without BM involvement) had 24-month OS outcomes comparable to those of non-CNS EM or BM only (P = .41). There was no difference in 12-month RFS between CNS, non-CNS EM, or BM-only patients (P = .92). No increased toxicity was seen with CNS or non-CNS EM disease (P = .3). Active CNS disease at time of infusion did not affect outcomes. Isolated CNS disease trended toward improved OS compared with combined CNS and BM (P = .12). R/R EM disease can be effectively treated with tisagenlecleucel; toxicity, relapse, and survival rates are comparable to those of patients with BM-only disease. Outcomes for isolated CNS relapse are encouraging.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy occurring in children, and survival with risk-stratified therapy for B-cell ALL (B-ALL) approaches ≥90%.1-3 However, for pediatric/young adult patients with relapsed or refractory (R/R) disease, the prognosis remains dismal.4-6 CD19-specific chimeric antigen receptor (CAR) T cells have transformed the therapeutic options for patients with R/R B-ALL.7-14 Historically, relapsed extramedullary (EM) disease has been treated with a combination of chemotherapy, radiation, and often allogeneic stem cell transplantation (SCT), depending on the time interval from diagnosis to relapse.15-19 Experience using CD19-specific CAR T-cell therapy for patients with R/R B-ALL presenting with central nervous system (CNS)3 disease, either isolated or in combination with bone marrow (BM) disease, or non-CNS EM is limited. This is in part because of exclusion of these patients from pivotal clinical trials, including ELIANA (Study of Efficacy and Safety of CTL019 in Pediatric ALL Patients), due to concerns regarding increased toxicity with EM disease and subthreshold BM burden.9,10
The tolerability and efficacy of CAR T cells in R/R ALL patients with EM disease are still undefined; however, a few reports expanding the treatment paradigm beyond BM disease and pivotal trials have shown effective trafficking of CAR T cells to sites of EM disease, encouraging its use for these patients.20-23 Increased neurotoxicity in an adult cohort of patients with EM disease treated with a 1928z CAR construct has been reported.13 Another report of 27 pediatric patients described a higher rate of neurotoxicity in patients with CNS disease before lymphodepletion, although this cohort included a combination of patients who received either a 41BB or CD28z based CAR construct.24 However, a report on the infusion of 7 patients with tisagenlecleucel who had isolated EM disease (negative or BM disease of <1%) showed no increased neurotoxicity and achievement of a complete response (CR) in all patients.25 Patients receiving either CTL019 and CTL119 for relapsed CNS disease similarly show clearance of CNS disease without increased risk of neurotoxicity and similar survival rates compared with patients without CNS disease at infusion.26 Herein, we report the results of patients who received tisagenlecleucel for R/R EM B-ALL disease as part of the multi-institutional Pediatric Real World CAR Consortium (PRWCC) and describe how patients with R/R EM disease have similar rates of toxicity and survival compared with patients with BM-only disease.
Methods
Study design
We conducted a retrospective analysis of pediatric and young adult patients with R/R B-ALL who were referred for tisagenlecleucel treatment for either isolated EM disease or combined BM + EM disease as part of the PRWCC multi-institutional consortium. Independent institutional review board approval was obtained by all centers, and data were collected by using the REDCap database, which is compliant with the Health Insurance Portability and Accountability Act of 1996. The study was conducted in accordance with the Declaration of Helsinki.
All patients with EM disease at tisagenlecleucel referral were comparatively analyzed against the remaining patients in the PRWCC consortium with BM-only disease. Patients with R/R CNS3 (CNS) disease or new-onset CNS3 disease at the time of referral for CAR T-cell therapy, as evident by the presence of leukemic blasts and ≥5 white blood cells/µL on flow cytometry or cytology from spinal fluid, were further stratified, with patients with isolated CNS3 (iCNS) separated from those with combined CNS + BM disease. To study the impact of active CNS3 disease at time of infusion on both toxicity and outcomes, the total CNS3 cohort (n = 40) was subdivided into active CNS3 disease at infusion (CNS+) vs CNS3 disease that cleared before infusion (CNS–). Patients with CNS2 disease were not included in this CNS cohort and are instead included in the non-EM disease cohort (BM only). Patients with non-CNS EM disease, all of whom had active EM disease at time of lymphodepletion, were distinctly analyzed. The identification of non-CNS EM disease varied by center, imaging, and physician investigation.
Study end points
The primary outcomes of interest were overall survival (OS), relapse-free survival (RFS), and duration of B cell aplasia (BCA). Additional outcomes of interest were response rate assessed at day 28, toxicity, relapse rates, and CD19 antigen expression at relapse. CR was defined as ≤5% BM blasts by morphology, absence of circulating lymphoblasts, and no evidence of EM disease. Minimal residual disease negativity was defined as <0.01% abnormal B cells assessed by using flow cytometry. Relapse was defined as any (medullary or EM) evidence of morphologic, immunophenotypic, cytogenetic, and/or molecular recurrence of primary disease. CD19-positive B-cell recovery was defined as any detectable CD19-positive B cells (>1 cell/µL) on a peripheral blood lymphocyte flow cytometry panel. Toxicity grading was described according to guideline criteria standard to each institution, with cytokine release syndrome (CRS) graded by American Society for Transplantation and Cellular Therapy27 and neurotoxicity (immune effector cell-associated neurotoxicity syndrome) graded by American Society for Transplantation and Cellular Therapy,27 CRES (CAR T cell related encephalopathy syndrome),28 and other.
Statistical analysis
For all analyses, the tisagenlecleucel infusion date was considered time 0, with a data cutoff of March 6, 2020. OS was defined as the time from infusion to death from all causes. Patients alive were censored at their last follow-up date. Time to relapse was defined as the time from the date of infusion to the date of disease relapse. RFS was defined as the time from infusion to any relapse or death. Patients who died before day 28 or did not achieve a CR were excluded from the RFS analysis. The duration of BCA was defined as time from infusion to loss of BCA, with SCT, if prior to loss of BCA or SCT without relapse, as a censored event.
Continuous and categorical data are summarized as median (interquartile range) and frequency. Differences among groups were assessed with Fisher’s exact test and Kruskal-Wallis tests for categorical and continuous data, respectively. Kaplan-Meier analysis was used to estimate OS, RFS, and duration of BCA. A comparative analysis was performed between the CNS, EM cohort, and rest of the cohort with BM-only disease. Differences in survival end points between groups were tested by using a log-rank test.
Results
Patient characteristics
From approval by the US Food and Drug Administration approval to March 6, 2020, a total of 184 evaluable patients were treated with tisagenlecleucel for R/R B-ALL and reported upon in the PRWCC. Fifty-five patients had R/R EM disease (CNS3 disease, n = 40; non-CNS EM disease, n = 15). Baseline characteristics of these cohorts are shown in Table 1. Patients with CNS disease were further stratified according to iCNS disease (n = 23) or combined CNS + BM (n = 17). The entire CNS cohort was also analyzed for presence of active CNS disease at time of infusion: CNS+ (n = 9) and CNS– (n = 31). Sites of non-CNS EM disease at time of infusion included craniofacial (n = 6), bone (n = 3), testes (n = 3), soft tissue (n = 3), renal (n = 2), skin (n = 1), ocular (n = 1), and lung (n = 1) (Table 2). Patients with non-CNS EM disease had either no BM involvement (n = 6) or BM involvement (n = 9). The median duration of follow-up for the entire cohort was 335 days (range, 6-863 days).
Pre-tisagenlecleucel demographic characteristics of patients with R/R CNS disease, non-CNS EM disease, and the remaining cohort with isolated BM disease
| Characteristic . | CNS disease . | P . | CNS disease . | Non-CNS EM . | BM-only disease . | P . | |
|---|---|---|---|---|---|---|---|
| iCNS (n = 23) . | CNS + BM (n = 17) . | iCNS + CNS + BM (n = 40) . | (n = 15) . | (n = 129) . | |||
| Age at CAR infusion, y | 11 (2-24) | 10 (<1-25) | .85 | 10 (<1-25) | 13 (2-26) | 13 (<1-26) | .087 |
| Sex (male/female) | 18/5 | 13/4 | 1.0 | 31/9 | 9/6 | 71/58 | .036 |
| Initial NCI risk stratification | .72 | .013 | |||||
| Low/standard | 11 | 5 | 16 | 4 | 23 | ||
| High/very high | 9 | 7 | 16 | 6 | 74 | ||
| Unknown | 3 | 5 | 8 | 5 | 32 | ||
| Initial cytogenetics risk | .70 | .3 | |||||
| Favorable/intermediate | 11 | 7 | 18 | 5 | 50 | ||
| Unfavorable | 7 | 3 | 10 | 3 | 53 | ||
| Unknown | 5 | 7 | 12 | 7 | 26 | ||
| CNS status at diagnosis | .510 | .17 | |||||
| CNS1 | 16 | 11 | 27 | 13 | 85 | ||
| CNS2 | 4 | 4 | 8 | 1 | 32 | ||
| CNS | 2 | 0 | 2 | 0 | 1 | ||
| Unknown | 1 | 2 | 3 | 1 | 11 | ||
| No. of relapses before CAR therapy | 1.0 | .003 | |||||
| 0 | 1 | 0 | 1 | 1 | 29 | ||
| ≥1 | 22 | 17 | 39 | 14 | 100 | ||
| No. of lines of treatment before CAR therapy | .69 | .07 | |||||
| 1 | 1 | 0 | 1 | 0 | 8 | ||
| 2 | 5 | 2 | 7 | 1 | 39 | ||
| 3 | 7 | 8 | 15 | 3 | 39 | ||
| >4 | 10 | 7 | 17 | 11 | 43 | ||
| Prior SCT | 0 | 0 | 1.0 | 0 | 1 | 10 | .16 |
| Previous CRT/CSI | 3 | 2 | 1.0 | 5 | 3 | 6 | .037 |
| Time from diagnosis to CAR infusion, mo | 45 (4-135) | 52 (5-94) | .76 | 51 (4-135) | 50 (4-171) | 27 (3-164) | .01 |
| Time from relapse to CAR infusion, mo | 3 (2-19) | 2 (1-13) | .50 | 3 (1-19) | 3 (1-12) | 3 (0-12) | .10 |
| Prophylactic levetiracetam | 1.0 | .13 | |||||
| Yes | 16 | 11 | 27 | 6 | 67 | ||
| No | 7 | 6 | 13 | 9 | 60 | ||
| Unknown | 0 | 0 | 0 | 0 | 2 | ||
| Characteristic . | CNS disease . | P . | CNS disease . | Non-CNS EM . | BM-only disease . | P . | |
|---|---|---|---|---|---|---|---|
| iCNS (n = 23) . | CNS + BM (n = 17) . | iCNS + CNS + BM (n = 40) . | (n = 15) . | (n = 129) . | |||
| Age at CAR infusion, y | 11 (2-24) | 10 (<1-25) | .85 | 10 (<1-25) | 13 (2-26) | 13 (<1-26) | .087 |
| Sex (male/female) | 18/5 | 13/4 | 1.0 | 31/9 | 9/6 | 71/58 | .036 |
| Initial NCI risk stratification | .72 | .013 | |||||
| Low/standard | 11 | 5 | 16 | 4 | 23 | ||
| High/very high | 9 | 7 | 16 | 6 | 74 | ||
| Unknown | 3 | 5 | 8 | 5 | 32 | ||
| Initial cytogenetics risk | .70 | .3 | |||||
| Favorable/intermediate | 11 | 7 | 18 | 5 | 50 | ||
| Unfavorable | 7 | 3 | 10 | 3 | 53 | ||
| Unknown | 5 | 7 | 12 | 7 | 26 | ||
| CNS status at diagnosis | .510 | .17 | |||||
| CNS1 | 16 | 11 | 27 | 13 | 85 | ||
| CNS2 | 4 | 4 | 8 | 1 | 32 | ||
| CNS | 2 | 0 | 2 | 0 | 1 | ||
| Unknown | 1 | 2 | 3 | 1 | 11 | ||
| No. of relapses before CAR therapy | 1.0 | .003 | |||||
| 0 | 1 | 0 | 1 | 1 | 29 | ||
| ≥1 | 22 | 17 | 39 | 14 | 100 | ||
| No. of lines of treatment before CAR therapy | .69 | .07 | |||||
| 1 | 1 | 0 | 1 | 0 | 8 | ||
| 2 | 5 | 2 | 7 | 1 | 39 | ||
| 3 | 7 | 8 | 15 | 3 | 39 | ||
| >4 | 10 | 7 | 17 | 11 | 43 | ||
| Prior SCT | 0 | 0 | 1.0 | 0 | 1 | 10 | .16 |
| Previous CRT/CSI | 3 | 2 | 1.0 | 5 | 3 | 6 | .037 |
| Time from diagnosis to CAR infusion, mo | 45 (4-135) | 52 (5-94) | .76 | 51 (4-135) | 50 (4-171) | 27 (3-164) | .01 |
| Time from relapse to CAR infusion, mo | 3 (2-19) | 2 (1-13) | .50 | 3 (1-19) | 3 (1-12) | 3 (0-12) | .10 |
| Prophylactic levetiracetam | 1.0 | .13 | |||||
| Yes | 16 | 11 | 27 | 6 | 67 | ||
| No | 7 | 6 | 13 | 9 | 60 | ||
| Unknown | 0 | 0 | 0 | 0 | 2 | ||
CRT, cranial radiation; CSI, craniospinal radiation; iCNS, isolated CNS disease; NCI, National Cancer Institute.
Sites of the other non-CNS EM disease (n = 15)
| Sites of EM disease at infusion* . | . |
|---|---|
| Craniofacial | 6 |
| Bone | 3 |
| Testes | 3 |
| Soft tissue | 3 |
| Renal | 2 |
| Skin | 1 |
| Ocular | 1 |
| Lung | 1 |
| Sites of EM disease at infusion* . | . |
|---|---|
| Craniofacial | 6 |
| Bone | 3 |
| Testes | 3 |
| Soft tissue | 3 |
| Renal | 2 |
| Skin | 1 |
| Ocular | 1 |
| Lung | 1 |
Patients can have >1 site of EM disease.
The median age at infusion of the CNS cohort was 10 years (range, <1-25 years), and in the non-CNS EM cohort it was 13 years (range, 2-26 years). Three patients with EM were diagnosed with high-risk infant ALL (CNS, n = 2; non-CNS EM, n = 1), but only one received their tisagenlecleucel infusion at <1 year of age to treat an on-therapy combined CNS + BM relapse. Disease refractory to upfront therapy was reported in only 2 patients treated with EM (CNS, n = 1; non-CNS EM, n = 1). The majority of patients had multiple lines of prior therapy, with 80% (32 of 40) of the CNS cohort having >3 lines of therapy before infusion with a median of 51 months (range, 4-135 months) between diagnosis and infusion. Similarly, 93% (14 of 15) of patients with non-CNS EM had >3 lines of therapy before infusion, with a median of 50 months (range, 4-171 months) between diagnosis and infusion. One patient from the entire EM cohort had pre-tisagenlecleucel SCT (non-CNS EM, n = 1; CNS, n = 0). Notably, only 12% of patients with CNS disease (n = 5) had prior cranial or craniospinal radiation before infusion. Remaining patients with BM-only disease had a shorter time from diagnosis to infusion, with a median of 27 months (range, 3-164 months) (P = .01). There was no difference in time from the most recent relapse to infusion between patients with either CNS disease (range, 2-5 months), non-CNS EM disease (range, 2-5 months), or BM-only disease (range, 2-3 months; all median, 3 months). Ninety-six percent of patients (53 of 55) with any EM disease received lymphodepletion with fludarabine (30 mg/m2 per day × 4 days) and cyclophosphamide (500 mg/m2 per day × 2 days). One patient had renal toxicity requiring a 50% dose reduction of lymphodepletion, and another patient did not receive any lymphodepletion due to pancytopenia (white blood cell count <0.2 K/µL) from preceding bridging chemotherapy
CNS3 disease
Response and toxicity.
Comparable to the response rate from the PRWCC infused cohort of 85%,29 88% (35 of 40) of patients with CNS disease achieved a CR (Table 3). Sixty-three percent (25 of 40) and 35% (12 of 40) of patients with CNS disease experienced any grade CRS or neurotoxicity, respectively. There was no difference in CRS grade between patients with iCNS compared with CNS + BM (P = .71) as well as patients with CNS+ compared with CNS– (P = .55) (Table 4). Grade 3 or 4 neurotoxicity was not observed in any patients with iCNS, compared with 3 patients with CNS + BM disease (P = .22). Notably, no patients with active CNS+ disease at infusion experienced grade 3 or 4 neurotoxicity. Sixty-eight percent of patients with CNS disease (27 of 40) were given levetiracetam as prophylaxis before CAR T-cell therapy. There was no difference in the use of prophylactic levetiracetam between patients with iCNS (70% [16 of 23]) vs combined CNS + BM (65% [11 of 17]; P = 1.0). Treatment of toxicity (either CRS or neurotoxicity) in patients with CNS3 disease included tocilizumab (n = 10), steroids (n = 6), anakinra (n = 2), and siltuximab (n = 1).
Outcomes of patients with R/R CNS disease, non-CNS EM disease, and the rest of the cohort after tisagenlecleucel infusion
| Outcome . | CNS disease (n = 40) . | Non-CNS EM disease (n = 15) . | BM-only disease (n = 129) . | P . |
|---|---|---|---|---|
| CRS | .30 | |||
| Grade 1 | 15 | 3 | 27 | |
| Grade 2 | 5 | 4 | 23 | |
| Grade 3 | 3 | 2 | 14 | |
| Grade 4 | 2 | 3 | 14 | |
| Grade 5 | 0 | 0 | 1 | |
| Unknown | 1 | 0 | 0 | |
| ICANS | .39 | |||
| Grade 1 | 9 | 2 | 8 | |
| Grade 2 | 2 | 0 | 5 | |
| Grade 3 | 1 | 0 | 7 | |
| Grade 4 | 2 | 0 | 2 | |
| Treatment of toxicity* | .14 | |||
| Tocilizumab | 10 | 5 | 31 | |
| Steroids | 6 | 2 | 18 | |
| Other | 2 | 0 | 4 | |
| Response | .35 | |||
| No CR | 4 | 4 | 15 | |
| CR (% MRD negative) | 35 (97%) | 10 (90%) | 111 (95%) | |
| Died before day 28 | 1 | 1 | 3 | |
| Relapsed post-CAR | 15 | 6 | 45 | .91 |
| Site of relapse | ||||
| CNS | 5 | 0 | 0 | |
| CNS + BM | 2 | 0 | 2 | |
| BM only | 8 | 2 | 37 | |
| BM + other EM | 0 | 2 | 4 | |
| Other EM disease | 0 | 2 | 2 | |
| CD19-negative relapse | 5/15 | 1/6 | 20/38 | .025 |
| Unknown | 2 | 3 | 3 | |
| Time from infusion to relapse, d | 101 (30-577) | 95 (30-245) | 90 (28-645) | .87 |
| SCT post CAR | 6 | 1 | 38 | .06 |
| Rationale for SCT post-CAR | .52 | |||
| Preemptive | 1 | 0 | 5 | |
| Loss of BCA | 0 | 0 | 11 | |
| Refractory/relapse | 4 | 1 | 16 | |
| MLL rearranged | 1 | 0 | 2 | |
| Alive/dead | 31/9 | 9/6 | 93/36 | .40 |
| Cause of death | .87 | |||
| Relapse | 7 | 5 | 26 | |
| Infection | 2 | 0 | 4 | |
| CRS | 0 | 0 | 1 | |
| Neurotoxicity | 0 | 0 | 1 | |
| Transplant related | 0 | 1 | 3 | |
| Cardiac related | 0 | 0 | 1 | |
| Loss of BCA | 10 | 8 | 39 | .14 |
| Time from infusion to loss of BCA, d | 174 (36-266) | 84.5 (29-396) | 93 (28-545) | .23 |
| Outcome . | CNS disease (n = 40) . | Non-CNS EM disease (n = 15) . | BM-only disease (n = 129) . | P . |
|---|---|---|---|---|
| CRS | .30 | |||
| Grade 1 | 15 | 3 | 27 | |
| Grade 2 | 5 | 4 | 23 | |
| Grade 3 | 3 | 2 | 14 | |
| Grade 4 | 2 | 3 | 14 | |
| Grade 5 | 0 | 0 | 1 | |
| Unknown | 1 | 0 | 0 | |
| ICANS | .39 | |||
| Grade 1 | 9 | 2 | 8 | |
| Grade 2 | 2 | 0 | 5 | |
| Grade 3 | 1 | 0 | 7 | |
| Grade 4 | 2 | 0 | 2 | |
| Treatment of toxicity* | .14 | |||
| Tocilizumab | 10 | 5 | 31 | |
| Steroids | 6 | 2 | 18 | |
| Other | 2 | 0 | 4 | |
| Response | .35 | |||
| No CR | 4 | 4 | 15 | |
| CR (% MRD negative) | 35 (97%) | 10 (90%) | 111 (95%) | |
| Died before day 28 | 1 | 1 | 3 | |
| Relapsed post-CAR | 15 | 6 | 45 | .91 |
| Site of relapse | ||||
| CNS | 5 | 0 | 0 | |
| CNS + BM | 2 | 0 | 2 | |
| BM only | 8 | 2 | 37 | |
| BM + other EM | 0 | 2 | 4 | |
| Other EM disease | 0 | 2 | 2 | |
| CD19-negative relapse | 5/15 | 1/6 | 20/38 | .025 |
| Unknown | 2 | 3 | 3 | |
| Time from infusion to relapse, d | 101 (30-577) | 95 (30-245) | 90 (28-645) | .87 |
| SCT post CAR | 6 | 1 | 38 | .06 |
| Rationale for SCT post-CAR | .52 | |||
| Preemptive | 1 | 0 | 5 | |
| Loss of BCA | 0 | 0 | 11 | |
| Refractory/relapse | 4 | 1 | 16 | |
| MLL rearranged | 1 | 0 | 2 | |
| Alive/dead | 31/9 | 9/6 | 93/36 | .40 |
| Cause of death | .87 | |||
| Relapse | 7 | 5 | 26 | |
| Infection | 2 | 0 | 4 | |
| CRS | 0 | 0 | 1 | |
| Neurotoxicity | 0 | 0 | 1 | |
| Transplant related | 0 | 1 | 3 | |
| Cardiac related | 0 | 0 | 1 | |
| Loss of BCA | 10 | 8 | 39 | .14 |
| Time from infusion to loss of BCA, d | 174 (36-266) | 84.5 (29-396) | 93 (28-545) | .23 |
ICANS, immune effector cell-associated neurotoxicity syndrome; MLL, mixed-lineage leukemia; MRD, minimal residual disease.
Toxicity refers to either CRS or ICANS.
Outcomes of patients with R/R CNS disease categorized into separate cohorts
| Outcome . | CNS disease (n = 40)* . | |||||
|---|---|---|---|---|---|---|
| iCNS (n = 23) . | CNS + BM (n = 17) . | P . | CNS+ (n = 9) . | CNS– (n = 31) . | P . | |
| CRS | .71 | .55 | ||||
| Grade 1 | 9 | 6 | 2 | 13 | ||
| Grade 2 | 3 | 2 | 1 | 4 | ||
| Grade 3 | 2 | 1 | 0 | 3 | ||
| Grade 4 | 0 | 2 | 0 | 2 | ||
| Unknown | 1 | 0 | 1 | 0 | ||
| ICANS | .22 | 1.0 | ||||
| Grade 1 | 5 | 4 | 3 | 6 | ||
| Grade 2 | 2 | 0 | 1 | 1 | ||
| Grade 3 | 0 | 1 | 0 | 1 | ||
| Grade 4 | 0 | 2 | 0 | 2 | ||
| Response | .07 | .54 | ||||
| No CR | 1 | 3 | 2 | 2 | ||
| CR (% MRD negative) | 22 (100%) | 13 (92%) | 7 (100%) | 28 (96%) | ||
| Died before day 28 | 0 | 1 | 0 | 1 | ||
| Relapsed post-CAR | 7 | 8 | .34 | 5 | 10 | .26 |
| Site of relapse | .18 | .32 | ||||
| CNS | 4 | 1 | 3 | 2 | ||
| CNS + BM | 0 | 2 | 0 | 2 | ||
| BM only | 3 | 5 | 2 | 6 | ||
| BM + other EM | 0 | 0 | 0 | 0 | ||
| Other EM disease | 0 | 0 | 0 | 0 | ||
| CD19-negative relapse | 2/7 | 3/8 | 1.0 | 1/5 | 4/10 | 1.0 |
| Unknown | 1 | 1 | 1 | 1 | ||
| Time from infusion to relapse, d | 178 (30-577) | 61 (27-266) | .20 | 174 (30-577) | 81.5 (27-206) | .54 |
| SCT post-CAR | 2 | 4 | 0.37 | 2 | 4 | .60 |
| Rationale for SCT post-CAR | 0.23 | .54 | ||||
| Preemptive | 0 | 1 | 0 | 1 | ||
| Loss of BCA | 0 | 0 | 0 | 0 | ||
| Refractory/relapse | 1 | 3 | 2 | 2 | ||
| MLL rearranged | 1 | 0 | 0 | 1 | ||
| Alive/dead | 20/3 | 11/6 | 0.13 | 7/2 | 24/7 | 1.0 |
| Cause of death | 1.0 | 1.0 | ||||
| Relapse | 2 | 5 | 2 | 5 | ||
| Infection | 1 | 1 | 0 | 2 | ||
| CRS | 0 | 0 | 0 | 0 | ||
| Neurotoxicity | 0 | 0 | 0 | 0 | ||
| Transplant related | 0 | 0 | 0 | 0 | ||
| Cardiac related | 0 | 0 | 0 | 0 | ||
| Loss of BCA | 5 | 5 | 0.72 | 3 | 7 | .67 |
| Time from infusion to loss of BCA, d | 146 (36-192) | 174 (148-266) | .48 | 114 (36-192) | 174 (101-266) | .56 |
| Outcome . | CNS disease (n = 40)* . | |||||
|---|---|---|---|---|---|---|
| iCNS (n = 23) . | CNS + BM (n = 17) . | P . | CNS+ (n = 9) . | CNS– (n = 31) . | P . | |
| CRS | .71 | .55 | ||||
| Grade 1 | 9 | 6 | 2 | 13 | ||
| Grade 2 | 3 | 2 | 1 | 4 | ||
| Grade 3 | 2 | 1 | 0 | 3 | ||
| Grade 4 | 0 | 2 | 0 | 2 | ||
| Unknown | 1 | 0 | 1 | 0 | ||
| ICANS | .22 | 1.0 | ||||
| Grade 1 | 5 | 4 | 3 | 6 | ||
| Grade 2 | 2 | 0 | 1 | 1 | ||
| Grade 3 | 0 | 1 | 0 | 1 | ||
| Grade 4 | 0 | 2 | 0 | 2 | ||
| Response | .07 | .54 | ||||
| No CR | 1 | 3 | 2 | 2 | ||
| CR (% MRD negative) | 22 (100%) | 13 (92%) | 7 (100%) | 28 (96%) | ||
| Died before day 28 | 0 | 1 | 0 | 1 | ||
| Relapsed post-CAR | 7 | 8 | .34 | 5 | 10 | .26 |
| Site of relapse | .18 | .32 | ||||
| CNS | 4 | 1 | 3 | 2 | ||
| CNS + BM | 0 | 2 | 0 | 2 | ||
| BM only | 3 | 5 | 2 | 6 | ||
| BM + other EM | 0 | 0 | 0 | 0 | ||
| Other EM disease | 0 | 0 | 0 | 0 | ||
| CD19-negative relapse | 2/7 | 3/8 | 1.0 | 1/5 | 4/10 | 1.0 |
| Unknown | 1 | 1 | 1 | 1 | ||
| Time from infusion to relapse, d | 178 (30-577) | 61 (27-266) | .20 | 174 (30-577) | 81.5 (27-206) | .54 |
| SCT post-CAR | 2 | 4 | 0.37 | 2 | 4 | .60 |
| Rationale for SCT post-CAR | 0.23 | .54 | ||||
| Preemptive | 0 | 1 | 0 | 1 | ||
| Loss of BCA | 0 | 0 | 0 | 0 | ||
| Refractory/relapse | 1 | 3 | 2 | 2 | ||
| MLL rearranged | 1 | 0 | 0 | 1 | ||
| Alive/dead | 20/3 | 11/6 | 0.13 | 7/2 | 24/7 | 1.0 |
| Cause of death | 1.0 | 1.0 | ||||
| Relapse | 2 | 5 | 2 | 5 | ||
| Infection | 1 | 1 | 0 | 2 | ||
| CRS | 0 | 0 | 0 | 0 | ||
| Neurotoxicity | 0 | 0 | 0 | 0 | ||
| Transplant related | 0 | 0 | 0 | 0 | ||
| Cardiac related | 0 | 0 | 0 | 0 | ||
| Loss of BCA | 5 | 5 | 0.72 | 3 | 7 | .67 |
| Time from infusion to loss of BCA, d | 146 (36-192) | 174 (148-266) | .48 | 114 (36-192) | 174 (101-266) | .56 |
ICANS, immune effector cell-associated neurotoxicity syndrome; MLL, mixed-lineage leukemia; MRD, minimal residual disease.
All 40 patients with R/R CNS disease were put into 2 separate cohorts to evaluate outcomes based on specific patient scenarios and important questions related to treating CNS disease.
Survival.
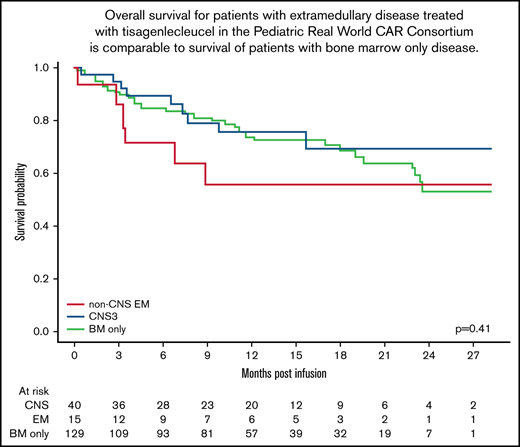
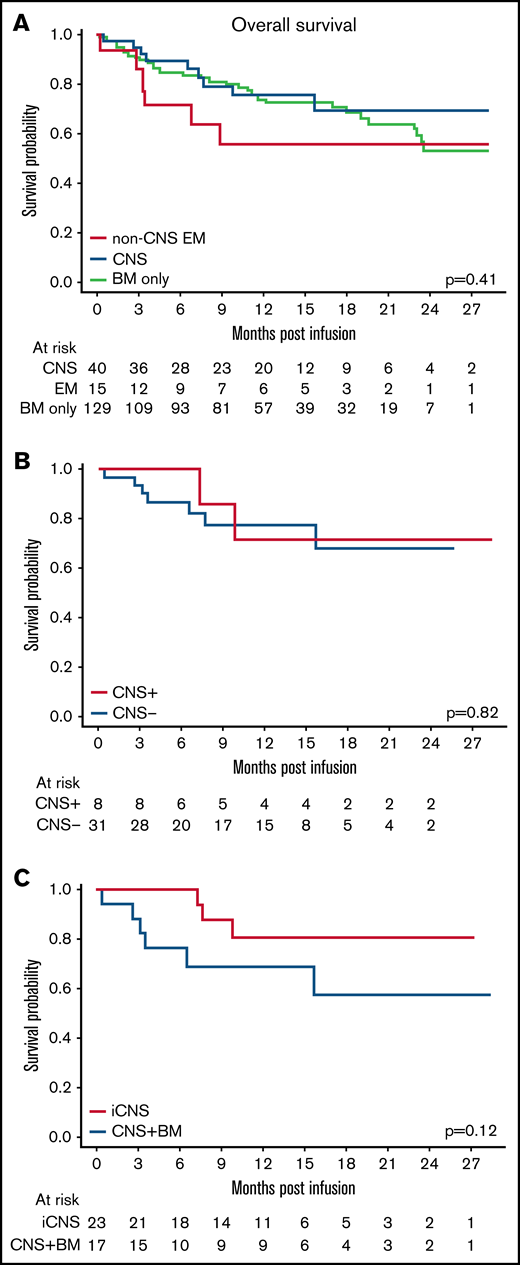
Twenty-three percent (9 of 40) of patients with CNS disease died after infusion. Causes of death included relapsed disease (n = 7) and infection (n = 2) (Table 3). No patients died of CRS or neurotoxicity. Patients with CNS disease had a 12- and 24-month OS of 75.7% (confidence interval [CI], 62.1-92.2) and 69.3% (CI, 53.4-90.1), respectively. Further comparison of patients with CNS disease revealed similar OS in patients with active CNS+ (71% [CI, 44.7-100] for both 12 and 24 months) and CNS– disease that cleared before infusion (77.6%; CI, 63.1-95.6) and 67.9% (CI, 48.6-94.9) at time of infusion (P = .82) (Figure 1B). The 12- and 24-month OS probabilities were 80.8% (CI, 63.4-100) and 80.8% (CI, 63.4-100) for the iCNS cohort and 68.8% (CI, 49.2-96.2) and 57.4% (CI, 35.1-93.6) for the CNS + BM cohort (P = .12) (Figure 1C).
EM disease did not affect OS. (A) Overall survival of patients with CNS3 disease, non-CNS EM disease, and BM-only disease (P = .41). (B) OS of patients with active CNS disease (CNS+) at time of infusion compared with those with CNS disease that cleared (CNS–) before infusion (P = .82). (C) OS of patients with isolated CNS (iCNS) disease compared with those with combined CNS and BM disease (CNS + BM) (P = .12).
EM disease did not affect OS. (A) Overall survival of patients with CNS3 disease, non-CNS EM disease, and BM-only disease (P = .41). (B) OS of patients with active CNS disease (CNS+) at time of infusion compared with those with CNS disease that cleared (CNS–) before infusion (P = .82). (C) OS of patients with isolated CNS (iCNS) disease compared with those with combined CNS and BM disease (CNS + BM) (P = .12).
Relapse.
Overall, 38% (15 of 40) of patients with CNS disease relapsed post-tisagenlecleucel, 5 of whom experienced a CD19-negative relapse (Table 3). Two of these CD19-negative relapses were CNS3, one combined with a BM relapse and the other an isolated CNS relapse; the other 3 CD19-negative relapses were BM only. The 12-month RFS probabilities for the CNS+ and CNS– cohorts were 68.6% (CI, 40.3-100) vs 57.5% (CI, 40.3-82.1) (P = .32) (Figure 2B). The 12-month RFS probabilities for the iCNS and CNS + BM cohorts were 66.1% (CI, 46.9-93.1) vs 49.5% (CI, 27.3-89.4), respectively (P = .63) (Figure 2C). The median time from infusion to relapse was 101 days (range, 30-577 days). Sites of relapse included iCNS (n = 5), combined CNS + BM (n = 2), and BM only (n = 8). A total of 6 patients with CNS disease underwent SCT post-CAR; 4 of 6 were due to relapsed disease. The 12-month RFS probability for all patients with CNS disease was 59.4% (CI, 43.7-80.7).
EM disease did not affect RFS. (A) RFS of patients with CNS3 disease, non-CNS EM disease, and BM-only disease (P = .92). (B) RFS of patients with active CNS disease (CNS+) at time of infusion compared with those with CNS disease that cleared (CNS–) before infusion (P = .32). (C) RFS of patients with isolated CNS disease (iCNS) compared with those with combined CNS and BM disease (CNS + BM) (P = .63).
EM disease did not affect RFS. (A) RFS of patients with CNS3 disease, non-CNS EM disease, and BM-only disease (P = .92). (B) RFS of patients with active CNS disease (CNS+) at time of infusion compared with those with CNS disease that cleared (CNS–) before infusion (P = .32). (C) RFS of patients with isolated CNS disease (iCNS) compared with those with combined CNS and BM disease (CNS + BM) (P = .63).
B-cell aplasia.
Twenty-eight percent (10 of 35) of patients with CNS disease lost BCA, with a median time from infusion to BCA loss of 174 days (range, 36-266 days) (Table 3). The 12-month duration of BCA for the CNS cohort was 66.4% (CI, 49.3-89.5). There was no difference in duration of BCA between patients with CNS+ (64.3%; CI, 33.8-100) and CNS– (67.3%; CI, 48.4-93.7) (P = .74) (Figure 3B). Similarly, no difference in the 12-month duration of BCA existed between patients with iCNS or CNS + BM disease compared with the rest of the cohort (67.7% [CI, 47.3-96.9] vs 62.9% [CI, 35.9-100]; P = .71) (Figure 3C).
EM disease did not affect the loss of BCA. (A) The probability of BCA loss of patients with CNS3 disease, non-CNS EM disease, and BM-only disease (P = .14). (B) The probability of BCA loss of patients with active CNS disease (CNS+) at time of infusion compared with those with CNS disease that cleared (CNS–) before infusion (P = .74). (C) The probability of BCA loss of patients with isolated CNS disease (iCNS) compared with those with combined CNS and BM disease (CNS + BM) (P = .71).
EM disease did not affect the loss of BCA. (A) The probability of BCA loss of patients with CNS3 disease, non-CNS EM disease, and BM-only disease (P = .14). (B) The probability of BCA loss of patients with active CNS disease (CNS+) at time of infusion compared with those with CNS disease that cleared (CNS–) before infusion (P = .74). (C) The probability of BCA loss of patients with isolated CNS disease (iCNS) compared with those with combined CNS and BM disease (CNS + BM) (P = .71).
Non-CNS EM disease
Response and toxicity.
The response rate of patients with non-CNS EM disease was 66% (10 of 15) after tisagenlecleucel infusion (Table 3). Of the 4 patients who had refractory disease, none of them cleared the non-CNS EM site of disease. Three of the 4 nonresponders also had residual BM disease, suggesting true nonresponse vs pseudo-progression in the EM sites. Eighty percent (12 of 15) and 13% (2 of 15) of patients with non-CNS EM disease experienced any grade CRS or neurotoxicity, respectively. This was not different from patients with BM-only disease or CNS disease (P = .3 and .39). Levetiracetam as seizure prophylaxis was used in only 40% of patients with non-CNS EM disease (6 of 15) and 52% of patients with BM-only disease (67 of 129), compared with 68% in patients with CNS disease (P = .13). Treatment of toxicity included tocilizumab (n = 5) or steroids (n = 2).
Survival.
Forty percent (6 of 15) of patients with non-CNS EM disease died after infusion, and this finding was not different from those with CNS disease or BM-only disease (P = .4). Causes of death included relapsed disease (n = 5) and transplant-related mortality (n = 1) (Table 3). No patients with any EM disease in this cohort died of either CRS or neurotoxicity. Patients with non-CNS EM disease had a 12- and 24-month OS of 55.8% (CI, 34.6-90.1). This was not significantly different from those patients with BM-only disease (72.8% [CI, 64.8-81.9] and 53.3% [CI, 39.4-72.1]), nor those with CNS disease (P = .41) (Figure 1A).
Relapse.
Forty percent (6 of 15) of patients with non-CNS EM disease relapsed post-tisagenlecleucel, one of whom experienced a CD19-negative relapse. No differences in relapse rates were seen compared with CNS or BM-only disease (P = .91) (Table 3). The median time from infusion to relapse was 95 days (30-245 days). CD19-negative relapse was seen more frequently in BM-only disease (20 of 38) vs CNS3 (5 of 15). Sites of relapse included BM only (n = 2), combined BM + non-CNS EM (n = 2), and non-CNS EM (n = 2) relapses. One patient with non-CNS EM disease who relapsed post-CAR progressed to SCT. The 12-month RFS probability for all patients with non-CNS EM disease was 50% (CI, 26.9-92.9). This was not different when compared with the 12-month RFS for patients with BM-only disease of 59.4% (CI, 50.2-70.2) and CNS disease (P = .92) (Figure 2A).
B-cell aplasia.
A greater proportion of patients with non-CNS EM disease (80% [8 of 10]) lost BCA, at a shorter duration from infusion to BCA loss (median, 84.5 days; range, 29-396 days) (Table 3). The 12-month duration of BCA for the non-CNS EM disease was 38.9% (CI, 14.8-100) and 59.4% (CI, 49.7-71) for patients with BM-only disease, which did not differ significantly from those with CNS disease (P = .14) (Figure 3A).
Discussion
The therapeutic standard for R/R B-ALL with EM involvement depends on multiple factors, including timing and location of relapse. Historic treatment options including possible combinations of chemotherapy, radiation, surgery, and/or SCT; although these options can offer cure, they are accompanied by risk toxicity and potential short- and long-term complications.15-19,30,31 The role of CD19-specific CAR T-cell therapy in R/R EM disease has yet to be defined due to the paucity of data on the tolerability and outcomes of patients treated with this therapy for active EM disease. This report presents the outcomes of a cohort of patients who received tisagenlecleucel for R/R EM disease across a multi-institutional consortium and shows the feasibility and safety of using this therapy in these patients.
Early studies of CD19-specific CAR T-cell therapy, which were associated with severe neurotoxicity and cerebral edema in adults, appropriately led to caution in the use for children and young adults with CNS disease.32 Smaller institutional studies have shown efficacy and tolerability for patients with CNS disease, and commercial use of tisagenlecleucel for these patients continues to expand.24,26 The data presented here further support feasibility of treating patients with CNS leukemia with tisagenlecleucel without excess CRS or neurotoxicity. These results are consistent with another report of adult patients with CNS lymphoma who received tisagenlecleucel, with no evidence of greater than grade 1 neurotoxicity.33 A notable limitation of this retrospective study is the utilization of different bridging therapies that provide various degrees of disease control before infusion. CNS+ patients represent a group with residual CNS disease at time of infusion that did not experience grade 3 or 4 neurotoxicity, although our study does not incorporate direct measures of disease burden such as cells per high-power field, which can affect risk for toxicity. Furthermore, our results highlight significant antileukemia activity in CNS patients referred for CAR T-cell therapy, with CR and OS comparable to those with BM-only disease. The ability of CAR T cells to control CNS disease is highlighted by the subset of patients who have sustained remission without receiving cranial radiation, which warrants further study on the potential to limit radiation in these patients.
As more novel therapies for R/R B-ALL emerge, the selection and sequencing of these treatments become more challenging, particularly in smaller subgroups of patients. Because the treatment pathway of isolated EM relapses remains controversial, we were particularly interested in evaluating this group separately. Late isolated CNS relapses have improved outcomes over BM or combined relapses but at the cost of significant cumulative doses of chemotherapy and cranial radiation. Furthermore, early isolated CNS relapses <18 months from diagnosis have significantly inferior outcomes.19,30 Although not statistically significant (largely due to patient volumes), this cohort of patients with isolated CNS relapse had higher OS rates post-tisagenlecleucel and did not have inferior outcomes compared with patients with combined CNS and BM relapse or BM-only relapses. This finding is consistent with the recent report of improved OS in patients with isolated CNS disease compared with those with BM disease using tisagenlecleucel or humanized CD19 CAR T-cell therapy on clinical trials.26 This cohort of 23 patients included both patients with on-treatment/early relapses (n = 12) and late relapses (n = 11). These patients also did not receive more cranial or craniospinal radiation than those with combined BM disease. These results suggest that patients with isolated CNS relapses may represent a unique patient group and highlights this as a therapeutic option for these patients, whom physicians may not have been inclined to recommend for this therapy and are often excluded from clinical trials.
The cohort of patients with non-CNS EM B-ALL active at time of infusion represent a smaller mixed group of patients, with some having large disease burden with associated EM involvement and others with a particularly challenging EM location to treat. Six of the 15 did not have BM involvement. Although complete response was seen in 10 of 15 patients, only 4 remain alive without relapse. These small numbers are difficult to ascertain efficacy, as well as the limitation of heterogeneity in reporting and the fact that physician investigation typically is dependent on physical symptoms. CAR T cells show activity in areas of EM disease, such as testicular disease, but likely have higher risk of failure with bulky EM disease + BM, which represents high tumor burden. Although efficacy in this group was mixed, we found no increased incidence of CRS or neurotoxicity in patients with presence of non-CNS EM disease.
Despite the success of CD19-specific CAR T-cell therapy, the incidence of relapse after treatment with CAR T cells in all patients with R/R B-ALL is unknown and has been reported to exceed 50% in some series.8-10,13 No differences in RFS rates between patients with CNS disease and those without have been reported in patients who received tisagenlecleucel or humanized CD19 CAR T cell therapy.26 Similarly, we found that patients with EM disease, both CNS (relapse rate 38%) and non-CNS EM (relapse rate 40%) disease, have rates of relapse after tisagenlecleucel similar to those of patients with BM-only disease. CD19-negative relapse occurred more often in the BM-only group compared with the R/R EM group. These relapse rates display the continued need to improve this therapy, and consolidative options to treat these patients who relapse after CD19-specific CAR T-cell therapy is still under investigation. SCT has been shown to be favorable after CD19-specific CAR T-cell therapy but has not been evaluated in the setting of relapse post-CAR.34 Treatment with investigational humanized CD19-specific CAR T cells is an option for relapsed patients but is not widely available.35 Reinfusion of tisagenlecleucel when additional doses are manufactured is under clinical investigation (#NCT04225676), but the utility of this approach may be ineffective if the patient has already established an anti-CAR immunologic response.36
In summary, CD19-specific CAR T cells for R/R EM disease offer a beneficial, effective option with similar toxicity and OS rates compared with patients with BM-only disease. Limitations of this study include its retrospective nature with its smaller number of heterogeneous patients. As an additional limit to this study, no biological markers or data on expansion of CAR T cells are available to correlate with treatment response. A prospective study is therefore needed to determine if CAR T-cell therapy should be the standard of care for patients with R/R EM disease to further reduce long-term side effects of radiation and SCT. In addition to efficacy, it will be important to study biological markers that correlate with treatment response, expansion data as they relate to B-cell recovery, and toxicity in patients with EM disease. These encouraging results, particularly in isolated CNS relapse, warrant further study to determine where CAR T cells can best be sequenced in relapse platforms. Further expansion of study for patients in first relapse may reduce potential need for cranial radiation, especially in younger patients, with possible sparing of SCT.
Acknowledgments
The authors acknowledge the following individuals for their major roles in supporting successful execution of this multi-institutional study: regulatory support, Sharon Mavroukakis and Emily Egeler; administrative support, Anika Dove and Daisy Torres; legal counsel and contracting, Neil Morimoto; and data management, Anne Marcy, Michelle Fujimoto, Jennifer Sheppard, Jean Sosna, Victoria Koch, Katie Doherty, Emily Bakinowski, Elizabeth Klein, Daritzya Baraja, Courtney Newbold, Glenn McWillians, Maggie Dyer, Kasey Abrahamnson, Angie Peltz, Ahmed Tahoun, Mary Suarez, Megan Hanby, Stacy Cooper, and Brad Muller.
This work was supported by a St Baldrick's/StandUp2 Cancer Pediatric Dream Team Translational Cancer Research Grant (C.L.M.). StandUp2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.L.M is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research.
Authorship
Contribution: All authors were involved in conception and design and collection and assembly of patient data; L.M.S. performed administrative duties; C.B. and L.M.S. designed the data collection tool; V.A.F. and C.L.P. designed this study; A.L. performed statistical analysis; and all authors were involved in data analysis and interpretation, manuscript writing, and final manuscript approval and are accountable for all aspects of this work.
Conflict-of-interest disclosure: C.L.P. has served on an advisory committee for Novartis. H.E.S. has served on an advisory committee and speakers bureau for Novartis. S.P.M. has served on an advisory committee for Novartis and Jazz Pharmaceuticals. M.R.V. has served on an advisory committee for Novartis; has been a consultant for and current equity holder in Fate Therapeutics and B-Mogen Biotechnologies; and has been a consultant for UpToDate. G.D.M. has served on the ELIANA trial Steering Committee and speakers bureau; and has served as a consultant for and received honoraria from Novartis. P.A.B. has served on an advisory committee for Novartis, Kite, Takeda, Janssen, Kura, Servier, and Jazz. M.Q. has served as a consultant for Novartis and Mesoblast. M.H. has served on an advisory board for Sobi and Novartis. P.S. has served as a consultant for Takeda and Mesoblast. K.J.C. has served as a consultant and received research funding from Novartis; has served as a consultant for Mesoblast; and has received research funding from Celgene. C.L.M. has served as a consultant for and is a current equity holder in Lyell Immunopharma and Apricity Health; has served as a consultant for NeoImmune Tech, Nektar Therapeutics and BMS; and is a current equity holder in Allogene. T.W.L. reports consultancy relationships with Novartis, Cellectis, Bayer, Deciphera, Jumo Health, and Y-mAbs Therapeutics; and has received research funding from Pfizer, Novartis, and Bayer. The remaining authors declare no competing financial interests.
Correspondence: Liora M. Shultz, Stanford University School of Medicine, 1000 Welch Rd, Suite #300, Stanford, CA 94304; e-mail: lioras@stanford.edu.
References
Author notes
V.A.F. and C.L.P. are joint first authors.
Requests for data sharing may be submitted to Liora M. Schultz; e-mail: liora.schultz@gmail.com.