TO THE EDITOR:
To cope with unexpected life-threatening massive hemorrhage in situations in which prompt blood transfusion is not available, hemoglobin-based oxygen carriers (HBOCs) can be useful because they are pathogen-free, blood-type-free, and capable of long-term preservation. To date, HBOCs of various types have been developed.1,2 One type of polymerized hemoglobin has been approved for clinical use in some countries.3 First-generation acellular chemically modified HBOCs were shown to entail statistically higher risks of myocardial infarction and death in the clinical trials,4,5 which are presumably related to their small molecular sizes, high intrinsic affinities to nitric oxide, and their acellular structure, which collectively cause extravasation, vasoconstriction, hypertension, and oxidative lesion.5-8 To mitigate such risks, we have developed hemoglobin vesicles (HbVs), which are cellular-structured HBOCs encapsulating a purified and concentrated Hb solution in PEGylated phospholipid vesicles (liposomes; mean particle diameter, 225-285 nm), shielding all the toxic effects of molecular hemoglobin by means of a lipid bilayer membrane and by mimicking erythrocytes (Figure 1A).9-12 The efficacy of HbVs, when used as a resuscitative fluid, has been studied extensively using animal models.12-15 The safety profiles, including those of their pharmacokinetics, biodistribution, excretion, immunological response, and hematological responses, have also been clarified.16-21 In consultation with the Pharmaceuticals and Medical Devices Agency, Japan, preclinical toxicological studies were completed in accordance with Good Laboratory Practices. Finally, we conducted an academia-initiated first-in-human phase 1 clinical trial from 2020 to assess HbV safety and pharmacokinetics in healthy male adult volunteers.
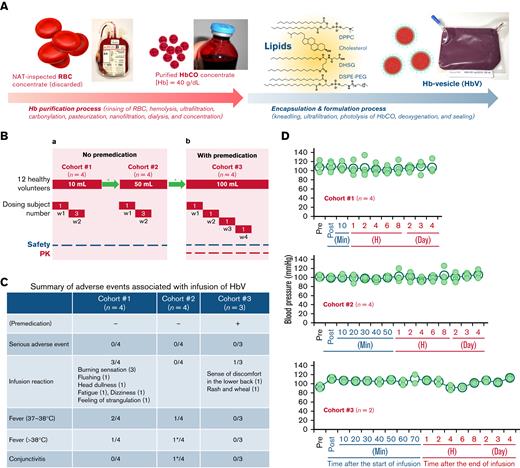
Production of HbV, phase 1 study design, adverse event and change of systemic blood pressure. (A) Outline of production scheme of HbV: carbonylhemoglobin (HbCO) solution was purified from nucleic acid amplification test (NAT)-inspected human red blood cell concentrate. The lipids for Hb encapsulation in liposomes comprised 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), cholesterol, 1,5-O-dihexadecyl-N-succinyl- l-glutamate (DHSG), and 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-poly(ethylene glycol) (DSPE-PEG). The HbCO encapsulation and particle size control were performed using the kneading method.11 The entire procedure was performed under Good Manufacturing Practice (GMP) controls (supplemental Methods). (B) Phase 1 study design: (a) Twelve healthy volunteers were scheduled to receive single doses of HbV with no premedication in 2 consecutive dosing cohorts 1 and 2 (10 mL and 50 mL). In each cohort (n = 4), HbV suspension was administered to 1 subject in week 1 and to 3 subjects in week 2. (b) For a subsequent cohort 3 (n = 4), administration was made of a single dose of 100 mL of HbV suspension to 1 subject per week with premedication. After completion of administration, subjects were housed for 4 days and were monitored as ambulatory on days 5, 8, and 15. In some cases, an additional follow-up study was conducted to confirm recovery from the deviated laboratory variable. ∗Review of safety and tolerability by the Data and Safety Monitoring Committee. (C) Summary of adverse events associated with the infusion of HbV: these adverse events were resolved without medication. Premedication: dexamethasone 6.6 mg (IV), famotidine 20 mg (PO), and acetaminophen 500 mg (PO) were premedicated 1 hour before infusion of the HbV suspension. ∗Same subject. (D) Change of systemic systolic blood pressure: the systemic systolic pressure was measured sequentially. No significant change was found among blood pressure measurements of the respective cohorts. P values (2-tailed) of cohorts 1, 2, and 3 were, respectively, 0.996, 0.866, and 0.073; post, immediately after the start of infusion (in cohorts 2 and 3, only saline was being infused at this time point); closed small circle, blood pressure of each subject; open large circle, mean blood pressure.
Production of HbV, phase 1 study design, adverse event and change of systemic blood pressure. (A) Outline of production scheme of HbV: carbonylhemoglobin (HbCO) solution was purified from nucleic acid amplification test (NAT)-inspected human red blood cell concentrate. The lipids for Hb encapsulation in liposomes comprised 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), cholesterol, 1,5-O-dihexadecyl-N-succinyl- l-glutamate (DHSG), and 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-poly(ethylene glycol) (DSPE-PEG). The HbCO encapsulation and particle size control were performed using the kneading method.11 The entire procedure was performed under Good Manufacturing Practice (GMP) controls (supplemental Methods). (B) Phase 1 study design: (a) Twelve healthy volunteers were scheduled to receive single doses of HbV with no premedication in 2 consecutive dosing cohorts 1 and 2 (10 mL and 50 mL). In each cohort (n = 4), HbV suspension was administered to 1 subject in week 1 and to 3 subjects in week 2. (b) For a subsequent cohort 3 (n = 4), administration was made of a single dose of 100 mL of HbV suspension to 1 subject per week with premedication. After completion of administration, subjects were housed for 4 days and were monitored as ambulatory on days 5, 8, and 15. In some cases, an additional follow-up study was conducted to confirm recovery from the deviated laboratory variable. ∗Review of safety and tolerability by the Data and Safety Monitoring Committee. (C) Summary of adverse events associated with the infusion of HbV: these adverse events were resolved without medication. Premedication: dexamethasone 6.6 mg (IV), famotidine 20 mg (PO), and acetaminophen 500 mg (PO) were premedicated 1 hour before infusion of the HbV suspension. ∗Same subject. (D) Change of systemic systolic blood pressure: the systemic systolic pressure was measured sequentially. No significant change was found among blood pressure measurements of the respective cohorts. P values (2-tailed) of cohorts 1, 2, and 3 were, respectively, 0.996, 0.866, and 0.073; post, immediately after the start of infusion (in cohorts 2 and 3, only saline was being infused at this time point); closed small circle, blood pressure of each subject; open large circle, mean blood pressure.
The protocol (HbV-101) was approved by Pharmaceuticals and Medical Devices Agency and the local ethical committee (institutional review board) of Hokkaido University (registration number, jRCT2011200004) and was performed according to the Declaration of Helsinki. The trial was an open-label, single intravenous injection, including dose-escalation trials, with investigation of 3 cohorts 1, 2, and 3 (n = 4), respectively, receiving 10-, 50-, and 100-mL dosages (Figure 1B). To avoid effects of volume overload on healthy volunteers, the maximum dose used for this trial was set as 100 mL. Enrolled participants were healthy male volunteers, aged 20 to 50 years old and each weighing 50 to 85 kg, with a body mass index of 18.5 to 25.0 kg/m2, for whom no exclusion criterion was applicable (supplemental Table 1).
The HbVs were produced at the Cell Processing Center, Nara Medical University Hospital.11 The HbV final product was suspended in saline to give Hb content of 8.5 to 11.6 g/dL (Figure 1A; supplemental Methods). The HbV suspension was administered at a rate of 1 mL/min in cohort 1. In cohorts 2 and 3, the HbV suspension was administered at 1 mL/min for 10 minutes, followed by 2.5 mL/min. Electrocardiographic analysis, vital signs, and arterial blood oxygen saturation (only in cohort 3) were checked. Blood chemistry tests, hematology-related laboratory tests, and urinalyses were performed before and after administration. In cohort 3, complement system parameters (C3, C4, 50% hemolytic complement activity; CH50), C-reactive protein, and anti-PEG antibody were added to laboratory tests. In addition, the Hb concentration in plasma was measured sequentially for pharmacokinetic analysis of HbVs because HbVs remain in the plasma fraction after centrifugation of blood samples (supplemental Methods).
Several adverse events (AEs) occurred (Figure 1C). The AEs observed in cohort 1 can be regarded as liposome-induced infusion reactions.22,23 The fact that HbV is a liposomal agent, that AEs appeared soon after the start of HbV administration, and that they then disappeared spontaneously within a few minutes with no medication all support this inference. No such infusion reaction was observed for cohort 2, possibly for the following reason. In contrast to cohort 1, in which the HbV suspension was infused directly via a syringe pump at a rate of 1 mL/min from the beginning of infusion, an infusion pump was used for cohort 2. Therefore, the mixture of HbV suspension and saline which prefulfilled the infusion route was infused in the initial phase.24,25 Elevated body temperatures were observed in 3 of the 4 subjects in cohort 1 and in 2 of the 4 subjects in cohort 2. The highest fever was 38.1°C, with shivering. Although it is difficult to ascertain whether a dose escalation effect exists, these results suggest that HbVs induce mild to moderate fever. In cohort 3, assigning top priority to subject safety, dexamethasone 6.6 mg (IV), famotidine 20 mg (PO), and acetaminophen 500 mg (PO) were premedicated 1 hour before HbV administration. Such premedication is often used for liposomal drugs.24 For the first 2 subjects, a 100-mL infusion was completed with no AE. However, the third subject reported discomfort in the lower back, followed by manifestation of a rash with a wheal on the anterior chest wall after approximately 10 mL of HbV suspension had been infused. For that reason, the infusion was halted immediately. The rash resolved soon without medication. Before infusion to the fourth subject, cohort 3 had to be terminated because the coronavirus pandemic affected project rescheduling and because the investigational agent had expired.
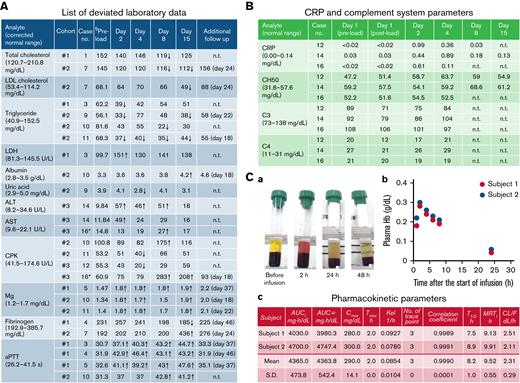
Except for body temperature, no clinically significant change was found in the vital signs, including systemic systolic blood pressure (Figure 1D). This finding suggests that, compared with the first generation of HBOCs, HbVs have less potential, if any, for raising risks of myocardial infarction. Some analytes used for hematological and biochemical tests showed values outside of normal ranges (supplemental Table 2). Among them, those that were ±20% or more of the corrected preload value are presented in Figure 2A. They reverted to the normal range by day 15 or on the day of the additional follow-up study with no clinical sign or symptom. In cohort 3, slight increases of C-reactive protein were observed in 3 of 3 subjects, with the highest peak of 0.99 mg/dL on day 2 (Figure 2B). Coupled with the manifestation of fever, this finding suggests that a certain amount of interleukin-6 was secreted. No significant decrease in CH50, C3, or C4 was found in any subject in cohort 3, except for 1 (case number 12), in whom a slight decrease in C3 was observed on day 1. Nevertheless, no AE was reported or observed. The subject who developed the rash with wheal, but also this subject (case number 12), was found to be positive only for anti-PEG IgG antibody: not for IgM antibody (data not shown). From these data, it was difficult to conclude whether the complement system or anti-PEG antibody was involved in the manifestation of rash with wheal.
Biochemical, immunological, and pharmacological parameters. (A) List of deviated laboratory data. Arrows indicate the values of analytes which were ±20% or more of the corrected preload value. §Preload values were corrected using a correction coefficient (supplemental Table 3; supplemental Methods). ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; n.t., not tested. ∗Only approximately 10 mL was infused because of the manifestation of rash with wheal. (B) C-reactive protein (CRP) and complement system parameters of the subjects in cohort 3. Slightly increased CRP was found in 3 subjects regardless of the infusion volume of HbV suspension. No significant change in complement system parameters was found. CH50, 50% hemolytic complement activity. (C) Plasma Hb concentration profile of subjects in cohort 3 (100-mL dosing). In cohort 3, 2 subjects completed a 100-mL infusion of HbV suspension: (a) sequential change of plasma color and transparency; (b) plasma Hb concentrations; and (c) pharmacokinetic parameters. AUC∞, area under the plasma concentration – time curve from time zero until infinity; AUCt, area under the plasma concentration – time curve from time zero until t; CL/F, apparent total body clearance; Cmax, maximum plasma concentration; MRTt, mean residence time from time zero until t; Kel, elimination rate constant; T1/2, elimination half-life; Tmax, time of maximum plasma concentration.
Biochemical, immunological, and pharmacological parameters. (A) List of deviated laboratory data. Arrows indicate the values of analytes which were ±20% or more of the corrected preload value. §Preload values were corrected using a correction coefficient (supplemental Table 3; supplemental Methods). ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; n.t., not tested. ∗Only approximately 10 mL was infused because of the manifestation of rash with wheal. (B) C-reactive protein (CRP) and complement system parameters of the subjects in cohort 3. Slightly increased CRP was found in 3 subjects regardless of the infusion volume of HbV suspension. No significant change in complement system parameters was found. CH50, 50% hemolytic complement activity. (C) Plasma Hb concentration profile of subjects in cohort 3 (100-mL dosing). In cohort 3, 2 subjects completed a 100-mL infusion of HbV suspension: (a) sequential change of plasma color and transparency; (b) plasma Hb concentrations; and (c) pharmacokinetic parameters. AUC∞, area under the plasma concentration – time curve from time zero until infinity; AUCt, area under the plasma concentration – time curve from time zero until t; CL/F, apparent total body clearance; Cmax, maximum plasma concentration; MRTt, mean residence time from time zero until t; Kel, elimination rate constant; T1/2, elimination half-life; Tmax, time of maximum plasma concentration.
Pharmacokinetic analysis results revealed that HbVs circulate in the bloodstream with a half-life of approximately 8 hours (Figure 2C). The half-life of HbVs is known to be dose-dependent. Therefore, it can be prolonged when using a higher dosage. Results strongly suggest that HbVs can function as oxygen carriers for several hours in the bloodstream of patients with hemorrhagic shock until a transfusion of packed red blood cells can be prepared.
Collectively, all AEs including liposome-induced infusion reactions observed were tolerable and spontaneously resolved. For the safety of volunteers, premedication was preferred. No clinically significant change was observed in any vital sign, including blood pressure. All deviated laboratory test results recovered without clinical sign or symptom associated with them. Moreover, the half-life of HbV in the bloodstream was approximately 8 hours. We plan to conduct dose escalation studies starting from 100 mL, with premedication using a larger number of subjects in a phase 2 trial.
Acknowledgments: The authors thank Norihiro Sato, Teruyo Arato, and Toshiyuki Isoe (Clinical Research and Medical Innovation Center, Hokkaido University Hospital) and Seiji Matsumoto (Clinical Research Support Center, Asahikawa Medical University) for expert advice on Good Clinical Practices, Jun Teragaki and Masanori Umeyama (Safety Research Institute for Chemical Compounds Co., Ltd.) for statistical analyses of collected data, and Yayoi Umeki and Mayumi Hasegawa (Department of Blood Transfusion Medicine, Nara Medical University), and Kouichiro Tada (Technopro R&D) for technical assistance with HbV production.
This study was supported by the Japan Agency for Medical Research and Development (AMED, project numbers JP18lk1403022, JP21ym0126034).
Contribution: H.A., T.A., N. Kamiyama, and N.T. were involved in designing the study and data acquisition; T.A., N. Kamiyama, N.T., and T.S. were involved in collecting and assembling the study data; T.A., M.J., and H.T. coordinated this trial; N. Kobayashi, T.K., and H.S. prepared the investigational agent; O.S. and M.M. confirmed the quality of the investigational agent; H.A., T.A., T.I., and H.S. wrote the manuscript; and all authors reviewed the manuscript and gave final approval to submission of the manuscript.
Conflict-of-interest disclosure: H.S. is a holder of patents related to the production and utilization of HbV. The remaining authors declare no competing financial interests.
Correspondence: Hiromi Sakai, Department of Chemistry, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8521, Japan; e-mail: hirosakai@naramed-u.ac.jp.
References
Author notes
Study design is available at Japan Registry of Clinical Trials (https://jrct.niph.go.jp/en-latest-detail/jRCT2011200004). For original data, contact the corresponding author.
The full-text version of this article contains a data supplement.