Key Points
Beneficial effects of R-CHOP are sustained over a 10-year follow-up period in 60- to 80-year-old patients with DLBCL.
Relapse/progression led to very poor outcome, except for ∼10% of thoroughly selected patients who received autologous transplantation.
Abstract
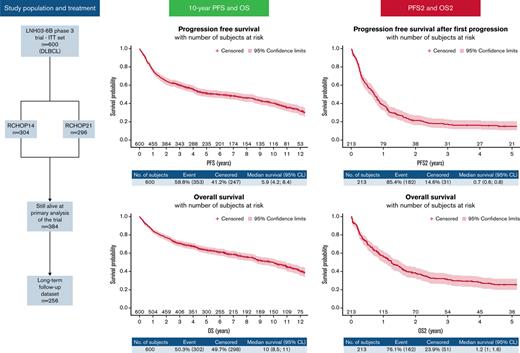
The LNH03-6B trial was a phase 3 randomized trial evaluating the efficacy of first-line rituximab, cyclophosphamide, doxorubicine, vincristine and prednisone (R-CHOP) delivered every 2 weeks (R-CHOP14) or 3 weeks (R-CHOP21) in patients with diffuse large B-cell lymphoma (DLBCL) aged 60 to 80 years with an aaIPI (age-adjusted International Prognostic Index) score ≥1 (registered as NCT00144755). We implemented a prospective long-term follow-up program at the end of this trial. The primary endpoints were progression-free survival (PFS) and overall survival (OS). Relapse patterns, PFS and OS after the first progression (PFS2 and OS2) were secondary endpoints. LNH03-6B was registered with ClinicalTrials.gov #NCT00144755. In the LNH03-6B trial, 304 and 296 patients were assigned to receive 8 cycles of R-CHOP14 or R-CHOP21, respectively. Long-term follow-up data were investigated for 256 of 384 (67%) patients still alive at the primary analysis. With a median follow-up of 10.1 years, 213 patients progressed, and 140 patients died without progression. The 10-year PFS was 40.4% (95% confidence interval, 35.9-44.9). Ten-year OS was based on 302 deaths and estimated at 50% (43-56). Of the 213 patients, 105 (49%) progressed after second-line therapy, and 77 patients died without a second progression (36%). The 1-year PFS2 and 1-year OS2 were estimated at 37.9% (95% confidence interval, 31.4-44.5) and 55.8% (95% confidence interval, 48.8-62.2), respectively. Ten years after randomization, the outcomes of patients treated for DLBCL were similar according to PFS and OS between the RCHOP-14 and R-CHOP21 groups. Progression or relapse led to poor prognosis after second-line chemotherapy in the pre CAR-T-cell era. Novel approaches in first-line and alternative treatments in second-line treatments are warranted in this population.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL), and its incidence is strongly related to increasing age, with a median age of occurrence of 70 years.1,2 The 60- to 80-year age class is the main DLBCL population in which the addition of rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), R-CHOP, was first explored in clinical trials.3-5 However, in this population, 3-year event-free survival or progression-free survival (PFS) remains relatively poor at ∼60% when treated with standard R-CHOP. In patients with refractory or relapsing disease, second-line response rates and outcomes are poor.6 Autologous stem cell transplant (ASCT) provides a survival benefit in relapsing chemosensitive patients (PARMA and ORCHARRD study), but generally, only a small fraction of patients >60 years old are considered eligible for ASCT, and those patients have a shorter survival than younger patients.7,8 More recently, several authors reported that older patients did as well as younger patients receiving chimeric antigen receptor (CAR) T cells as third-line therapy, indicating that age per se should not preclude CAR T-cell administration.9,10 Therefore, it seems of interest to report data concerning outcomes, relapse patterns, and second-line treatments of patients aged 60 to 80 years who received frontline R-CHOP in a pre–CAR-T era.
The LNH03-6B trial was a multicenter, phase 3, open-label, randomized trial that tested the efficacy of R-CHOP given every 14 days (RCHOP14) compared with R-CHOP given every 21 days (RCHOP21) in patients aged 60 to 80 years with previously untreated CD20+ DLBCL and ≥1 adverse prognostic factor of the aaIPI (age-adjusted International Prognostic Index). No survival difference was found between the PFS and overall survival (OS) rates in the treatment groups. At the time of publication of the results in 2013, the median follow-up was 56 months (27 to 60 months).11 Because the LNH03-6B trial included a very large and homogeneous cohort of patients, expanded follow-up was considered crucial to assess whether the results were maintained over time.
Here, we detail the long-term follow-up of the LNH03-6B study with a median follow-up of 10.1 years to depict the long-term evolution of patients with DLBCL aged 60 to 80 years treated with standard first-line immunochemotherapy with a particular interest in the treatment and outcomes of patients whose disease relapsed or progressed.
Patients and methods
Study design and patients
LNH03-6B was a phase 3, multicenter, randomized trial (NCT00144755) that compared the efficacy of 2 schedules of immunochemotherapy in older patients with untreated DLBCL. The study was undertaken at 83 centers in France, Belgium, Switzerland, and Portugal between December 2003 and December 2012. Eligible participants underwent 2 randomization procedures. In the first, we allocated 1 of 2 chemotherapy regimens, R-CHOP14 or R-CHOP21. In the second, we randomly assigned patients to an experimental arm with prophylactic darbepoetin alfa or to a standard arm with conventional “symptomatic” management of chemotherapy-induced anemia. We judged people eligible if they were aged 60 to 80 years and had untreated DLBCL. Furthermore, patients also needed ≥1 adverse prognostic factor on the aaIPI and a good performance status (Eastern Cooperative Oncology Group 0 to 2). Additional inclusion criteria were a life expectancy of ≥3 months and negative serological tests for HIV and hepatitis B and C virus in the past 4 weeks (except after vaccination for hepatitis B virus). Exclusion criteria were central nervous system or meningeal involvement by lymphoma, contraindication to any drug in the chemotherapy regimens, any serious comorbid active disease (investigator’s decision), or any history of cancer during the past 5 years, with the exception of nonmelanoma skin tumors or in situ cervical carcinoma. Unless these abnormalities were related to lymphoma, we also excluded patients with poor renal function (creatinine concentration >150 μmol/L), hepatic disorders (total bilirubin >30 mmol/L or aminotransferases >2.5 times the maximum normal amount), or poor bone marrow reserve (neutrophil count <1.5 × 109/L or platelet count <100 × 109/L). Local or national ethics committees approved the study protocol according to the laws of each country. The study was performed in accordance with the Declaration of Helsinki. Patients provided written informed consent before inclusion.
Randomization and masking
We used computer-assisted permuted-block randomization (block size of 4, allocation ratio 1:1) to assign treatment. Randomization was stratified by participating center and aaIPI (1 vs 2 or 3). A statistician located centrally supervised the randomization procedure. The treatment allocation was sent to the investigator by fax. Investigators and patients were not masked to treatment assignment.
Procedures
We planned for patients to receive 8 cycles of the R-CHOP regimen, which is a combination of IV rituximab (375 mg/m2), cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), and vincristine (1.4 mg/m2, up to 2 mg) all on day 1, and oral prednisone 40 mg/m2 daily for 5 days every 14 or 21 days. All patients received neuromeningeal prophylaxis of 4 consecutive intrathecal injections of methotrexate (15 mg) every 14 or 21 days. We administered granulocyte colony-stimulating factor (pegylated or not) according to the decision of the treating physician, fulfilling existing guidelines, and product labeling at that time. Radiotherapy was not allowed.
The response to treatment was assessed by the local investigator after 4 cycles and at the end of treatment. The response was defined according to International Workshop 1999 criteria (Cheson 99).12 The patients were followed (physical examination, laboratory tests, and computerised tomography scan) every 6 months during the first 2 years and yearly thereafter until the study completion date (December 2012).
Based on data from the literature, we defined “refractory” DLBCL as early disease progression within the first year after randomization and “relapsed” DLBCL as disease progression occurring >1 year after randomization.13-16
Long-term follow-up program
At the cutoff date of the primary analysis of the LNH03-6B trial in December 2012, we implemented a long-term follow-up program in French centers willing to participate in the long-term follow-up program. The inclusion criteria were as follows: patients included in the LNH03-6B trial who were still alive at the end of the trial and were not opposed to long-term data collection. The long-term follow-up program started at the end of LNH03-6B protocol-specified mandatory follow-up.
In this program, the primary endpoints were PFS, as measured from the date of random assignment to either progression or relapse or death from any cause, and OS. Secondary endpoints were second progression-free survival (PFS2) and second overall survival (OS2) measured from the date of first progression or relapse for the patients concerned. During this program, patient follow-up was assessed according to the habits of each center. We collected the status of the disease as judged by the investigator (complete response, partial response, stable disease, and progressive disease) at the date of last visit or contact. We collected secondary malignancy data and causes of death. We also collected second-line treatment for patients whose disease progressed or relapsed. For this analysis, we distinguished 2 chemotherapy treatment groups: (1) intensive treatments, considered “intensive” if usually given in a hospital (in-patient setting) and could usually cause profound cytopenia and other severe side effects, included the following combinations: ifosfamide, carboplatin, etoposide and dexamethasone, cytosine arabinoside and either cisplatin, oxaliplatin, or carboplatin, with or without rituximab; and (2) nonintensive treatments (usually administered in an outpatient setting) included the following combinations: gemcitabine, oxaliplatin, bendamustine, ifosfamide plus etoposide, and different single-agent therapies, with or without rituximab (supplemental Table 1). Progressions after second-line therapy were captured by a collection of “disease status” at the date of the last contact. The collection of long-term follow-up data was performed via a specific electronic case report form on a regular basis (at least once a year) and for a minimum period of 10 years for each patient (or less if the patient died or was lost to follow-up). Patients living without progression or relapse or lost to follow-up were censored on their date of last visit or contact.
Statistical analysis
Categorical variables were described in terms of numbers and percentages, and continuous variables were described with the median and the range. The different survival functions (PFS, OS, PFS2, and OS2) were obtained with the Kaplan-Meier estimator using the randomization date as the index date for PFS and OS and using the date of progression or relapse as the index date for PFS2 and OS2. Comparisons between groups defined by a prognostic factor of interest were reported using the log-rank test, and a Cox proportional hazard model was used to complement these comparisons with an estimated hazards ratio. With the Aalen-Johansen estimator, we obtained (1) the cumulative risk (that is, the probability) of progression or relapse (treating deaths without progression as competing events); and (2) the probability of deaths without progression (treating progression or relapse as competing events). Statistical analyses were performed with SAS 9.3 software (SAS Institute, Cary, NC) and R software 4.0.2.
Role of the funding source
LYSARC (Lymphoma Study Association Academic Research organization) undertook data monitoring, study coordination, and data analysis. They performed the randomization, undertook distribution and collection of case report forms, assisted with data entry and validation, coordinated monitoring procedures, helped with elaboration and mailing of queries, reported serious adverse events, coordinated histological review, maintained relations with investigators, transmitted enrolment status to the sponsor, performed the statistical analysis, and wrote the report. Amgen had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient characteristics
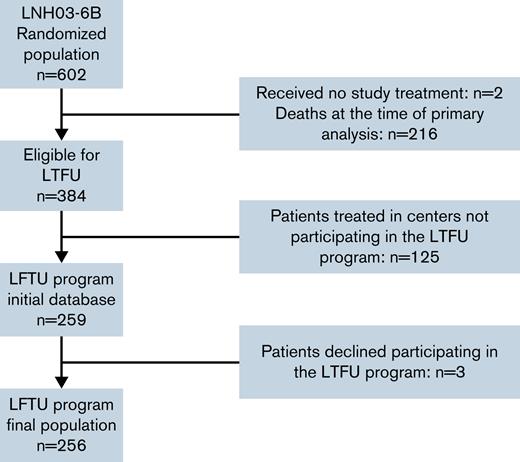
Between December 2003 and December 2012, 602 patients were randomized in the LNH03-6B trial in 4 countries; 600 were included in the intention-to-treat (ITT) population, 304 were included in the R-CHOP14 group, and 296 were included in the R-CHOP21 group. At the time of primary analysis, 384 patients were alive and therefore eligible for the long-term follow-up program.11 Among these 384 patients, 259 were considered for inclusion in the long-term follow-up database in participating centers. Three patients declined to participate in this program (these patients were censored for PFS and OS at the time of consent withdrawal), resulting in a final long-term follow-up subpopulation of 256 patients (Figure 1). The patient characteristics of the whole population are listed in Table 1. The median age at diagnosis was 70 (interquartile range, 66-74) years, and most of the patients were male (n = 332 [55.3%]) and presented at baseline with a good performance status (ECOG [Eastern Cooperative Oncology Group] 0 to 1: n = 465 [77.5%]), Ann Arbor stage III to IV (n = 530 [88.3%]), elevated lactate dehydrogenase (n = 411 [68.5%]), and aaIPI 2 to 3 (n = 381 [63.5%]). One hundred and twenty-five patients (41.1%) received darbepoetin alfa for chemotherapy-induced anemia in the R-CHOP14 arm vs 113 patients (38.2%) in the R-CHOP21 arm.
Baseline characteristics of patients from the ITT set and according to long-term follow-up program enrolment
| . | Patients enrolled in the LTFU program . | All (ITT set) n = 600 . | ||
|---|---|---|---|---|
| No (center not participating in LTFU) n = 125 . | No (patients dead at cutoff date of primary analysis or refused data collection) n = 219 . | Yes n = 256 . | ||
| Treatment received, n (%) | ||||
| R-CHOP14 | 66 (52.8) | 108 (49.3) | 130 (50.8) | 304 (50.7) |
| R-CHOP21 | 59 (47.2) | 111 (50.7) | 126 (49.2) | 296 (49.3) |
| Sex, n (%) | ||||
| Male | 62 (49.6) | 127 (58.0) | 143 (55.9) | 332 (55.3) |
| Female | 63 (50.4) | 92 (42.0) | 113 (44.1) | 268 (44.7) |
| Age, yr | ||||
| n | 125 | 219 | 256 | 600 |
| Missing | 0 | 0 | 0 | 0 |
| Mean (SD) | 70 (4.94) | 71 (5.01) | 69 (4.96) | 70 (5.09) |
| Median | 70 | 72 | 69 | 70 |
| Q1;Q3 | 67;74 | 67;76 | 65;73 | 66;74 |
| Min;max | 60;79 | 59;80 | 60;79 | 59;80 |
| ECOG in class, n (%) | ||||
| 0-1 | 109 (87.2) | 147 (67.1) | 209 (81.6) | 465 (77.5) |
| ≥2 | 16 (12.8) | 72 (32.9) | 47 (18.4) | 135 (22.5) |
| Ann Arbor stage in class, n (%) | ||||
| 1-2 | 21 (16.8) | 20 (9.1) | 29 (11.3) | 70 (11.7) |
| 3-4 | 104 (83.2) | 199 (90.9) | 227 (88.7) | 530 (88.3) |
| LDH, n (%) | ||||
| ≤normal | 46 (36.8) | 47 (21.5) | 96 (37.5) | 189 (31.5) |
| >normal | 79 (63.2) | 172 (78.5) | 160 (62.5) | 411 (68.5) |
| Number of extranodal sites in class, n (%) | ||||
| 0-1 | 65 (52.0) | 94 (42.9) | 135 (52.7) | 294 (49.0) |
| ≥2 | 60 (48.0) | 125 (57.1) | 121 (47.3) | 306 (51.0) |
| Bone marrow biopsy, n (%) | ||||
| Not involved | 96 (76.8) | 145 (66.2) | 197 (77.0) | 438 (73.0) |
| Involved | 20 (16.0) | 60 (27.4) | 48 (18.8) | 128 (21.3) |
| Unspecified | 3 (2.4) | 4 (1.8) | 5 (2.0) | 12 (2.0) |
| Not done | 6 (4.8) | 10 (4.6) | 6 (2.3) | 22 (3.7) |
| IPI in class, n (%) | ||||
| 0-2 | 40 (32.0) | 35 (16.0) | 73 (28.5) | 148 (24.7) |
| 3 | 41 (32.8) | 68 (31.1) | 92 (35.9) | 201 (33.5) |
| 4-5 | 44 (35.2) | 116 (53.0) | 91 (35.5) | 251 (41.8) |
| Bulky mass >10 cm, n (%) | ||||
| No | 105 (84.0) | 176 (80.4) | 215 (84.0) | 496 (82.7) |
| Yes | 20 (16.0) | 43 (19.6) | 41 (16.0) | 104 (17.3) |
| B symptoms | ||||
| No | 80 (64.0) | 124 (56.6) | 173 (67.6) | 377 (62.8) |
| Yes | 45 (36.0) | 95 (43.4) | 83 (32.4) | 223 (37.2) |
| . | Patients enrolled in the LTFU program . | All (ITT set) n = 600 . | ||
|---|---|---|---|---|
| No (center not participating in LTFU) n = 125 . | No (patients dead at cutoff date of primary analysis or refused data collection) n = 219 . | Yes n = 256 . | ||
| Treatment received, n (%) | ||||
| R-CHOP14 | 66 (52.8) | 108 (49.3) | 130 (50.8) | 304 (50.7) |
| R-CHOP21 | 59 (47.2) | 111 (50.7) | 126 (49.2) | 296 (49.3) |
| Sex, n (%) | ||||
| Male | 62 (49.6) | 127 (58.0) | 143 (55.9) | 332 (55.3) |
| Female | 63 (50.4) | 92 (42.0) | 113 (44.1) | 268 (44.7) |
| Age, yr | ||||
| n | 125 | 219 | 256 | 600 |
| Missing | 0 | 0 | 0 | 0 |
| Mean (SD) | 70 (4.94) | 71 (5.01) | 69 (4.96) | 70 (5.09) |
| Median | 70 | 72 | 69 | 70 |
| Q1;Q3 | 67;74 | 67;76 | 65;73 | 66;74 |
| Min;max | 60;79 | 59;80 | 60;79 | 59;80 |
| ECOG in class, n (%) | ||||
| 0-1 | 109 (87.2) | 147 (67.1) | 209 (81.6) | 465 (77.5) |
| ≥2 | 16 (12.8) | 72 (32.9) | 47 (18.4) | 135 (22.5) |
| Ann Arbor stage in class, n (%) | ||||
| 1-2 | 21 (16.8) | 20 (9.1) | 29 (11.3) | 70 (11.7) |
| 3-4 | 104 (83.2) | 199 (90.9) | 227 (88.7) | 530 (88.3) |
| LDH, n (%) | ||||
| ≤normal | 46 (36.8) | 47 (21.5) | 96 (37.5) | 189 (31.5) |
| >normal | 79 (63.2) | 172 (78.5) | 160 (62.5) | 411 (68.5) |
| Number of extranodal sites in class, n (%) | ||||
| 0-1 | 65 (52.0) | 94 (42.9) | 135 (52.7) | 294 (49.0) |
| ≥2 | 60 (48.0) | 125 (57.1) | 121 (47.3) | 306 (51.0) |
| Bone marrow biopsy, n (%) | ||||
| Not involved | 96 (76.8) | 145 (66.2) | 197 (77.0) | 438 (73.0) |
| Involved | 20 (16.0) | 60 (27.4) | 48 (18.8) | 128 (21.3) |
| Unspecified | 3 (2.4) | 4 (1.8) | 5 (2.0) | 12 (2.0) |
| Not done | 6 (4.8) | 10 (4.6) | 6 (2.3) | 22 (3.7) |
| IPI in class, n (%) | ||||
| 0-2 | 40 (32.0) | 35 (16.0) | 73 (28.5) | 148 (24.7) |
| 3 | 41 (32.8) | 68 (31.1) | 92 (35.9) | 201 (33.5) |
| 4-5 | 44 (35.2) | 116 (53.0) | 91 (35.5) | 251 (41.8) |
| Bulky mass >10 cm, n (%) | ||||
| No | 105 (84.0) | 176 (80.4) | 215 (84.0) | 496 (82.7) |
| Yes | 20 (16.0) | 43 (19.6) | 41 (16.0) | 104 (17.3) |
| B symptoms | ||||
| No | 80 (64.0) | 124 (56.6) | 173 (67.6) | 377 (62.8) |
| Yes | 45 (36.0) | 95 (43.4) | 83 (32.4) | 223 (37.2) |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; LTFU, long-term follow-up; PS, performance status.
Outcomes
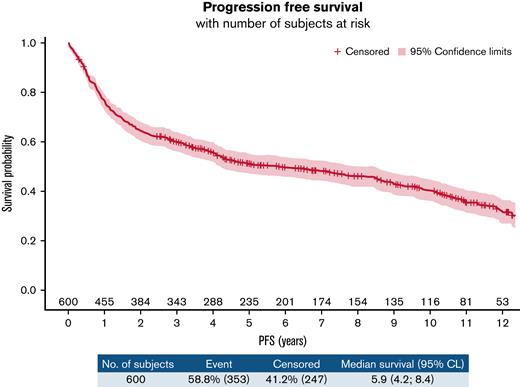
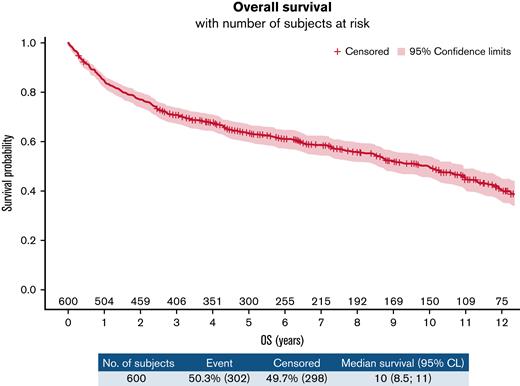
In the ITT population, we observed 353 PFS events (87 refractory diseases, 126 relapses, and 140 deaths). The PFS at 10 years was 40.4% (95% confidence interval [CI], 35.9-44.9) (Figure 2). According to treatment arms, the 10-year PFS was 41.2% (95% confidence interval, 34.9-47.4) for the R-CHOP14 group compared with 39.5% (95% confidence interval, 33.1-45.9) for the R-CHOP21 group (hazard ratio [HR] for R-CHOP21: 0.990 [95% confidence interval, 0.803-1.219]) (supplemental Figure 1A). Three hundred and two deaths occurred in the entire population. The 10-year OS was 49.8% (95% CI, 45.1-54.3) (Figure 3), and the 10-year OS estimates were almost identical between the 2 arms (49.8% [43.1-56.2] vs 49.7% [43.1-56] for R-CHOP14 and R-CHOP21, respectively; the hazard ratio for R-CHOP21: 0.999 (0.797-1.251) (supplemental Figure 1B). Death from study treatment (n = 28 [9.3%]) was death related to R-CHOP direct or indirect toxicity as judged by the investigator. According to the darbepoetin alfa or symptomatic treatment groups, the 10-year PFS was 41.5% (34.1-48.7) for the darbepoetin alfa group compared with 39.7% (34-45.3) for the symptomatic treatment group (HR for darbepoetin alfa, 0.889 [0.717-1.102]) (supplemental Figure 2A). The 10-year OS was 50.3% (42.7-57.5) for the darbepoetin alfa group compared with 49.3% (43.3-55) for the symptomatic treatment group (HR for darbepoetin alfa, 0.939 (0.744-1.184) (supplemental Figure 2B). Survival outcome data of high-risk patients IPI 3 to 5, who represented 75.3% of enrolled patients in this study, are presented in supplemental Figure 3A-B.
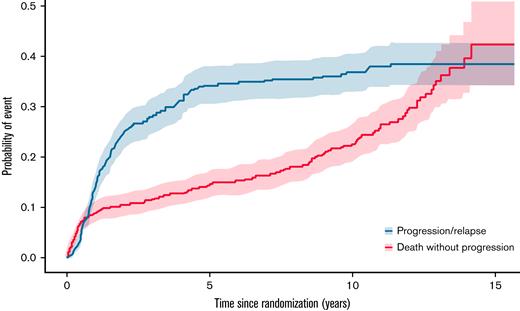
The causes of death were the following: lymphoma (n = 128 [42.5%]), unknown (n = 48 [15.9%]), concurrent illness (n = 39 [13%]), other cancer (n = 38 [12.6%]), toxicity of study treatment (n = 28 [9.3%]), other reason (n = 13 [4.3%], including suicide, alteration of performance status due to age, cardiac arrest, and pneumopathy), and toxicity of additional treatment or salvage treatment (n = 7 [2.3%]) (Table 2). The cause of death distribution was similar in the 2 randomization groups. The probability of death without progression up to 10 years after diagnosis, assessed on the ITT set (n = 600), was estimated as 22.7% (19.0-26.7), whereas the probability of progression or relapse up to 10 years was estimated as 36.8% (32.8-40.9). The probability of progression or relapse levels off from approximately 5 years after diagnosis, whereas the probability of death increases steadily (Figure 4).
Causes of death in the ITT set
| . | Arm . | All n = 302 . | |
|---|---|---|---|
| RCHOP14 n = 151 . | RCHOP21 n = 151 . | ||
| Cause of death, n (%) | |||
| Lymphoma | 62 (41.1) | 66 (44) | 128 (42.5) |
| Concurrent illness | 23 (15.2) | 17 (11.3) | 40 (13.2) |
| Other cancer | 19 (12.6) | 19 (12.7) | 38 (12.6) |
| Toxicity of study treatment | 14 (9.3) | 14 (9.3) | 28 (9.3) |
| Other reason | 8 (5.3) | 5 (3.3) | 13 (4.3) |
| Toxicity of additional treatment | 2 (1.3) | 5 (3.3) | 7 (2.3) |
| Unknown | 23 (15.2) | 25 (16.7) | 48 (15.9) |
| . | Arm . | All n = 302 . | |
|---|---|---|---|
| RCHOP14 n = 151 . | RCHOP21 n = 151 . | ||
| Cause of death, n (%) | |||
| Lymphoma | 62 (41.1) | 66 (44) | 128 (42.5) |
| Concurrent illness | 23 (15.2) | 17 (11.3) | 40 (13.2) |
| Other cancer | 19 (12.6) | 19 (12.7) | 38 (12.6) |
| Toxicity of study treatment | 14 (9.3) | 14 (9.3) | 28 (9.3) |
| Other reason | 8 (5.3) | 5 (3.3) | 13 (4.3) |
| Toxicity of additional treatment | 2 (1.3) | 5 (3.3) | 7 (2.3) |
| Unknown | 23 (15.2) | 25 (16.7) | 48 (15.9) |
Cumulative incidence function. The probability of death without progression up to 10 years after diagnosis is estimated as 22.7% (95% confidence interval, 19.0-26.7), whereas the probability of progression or relapse up to 10 years is estimated as 36.8% (95% confidence interval, 32.8-40.9). The probability of progression or relapse plateaus from approximately 5 years after diagnosis, while the probability of death constantly increases.
Cumulative incidence function. The probability of death without progression up to 10 years after diagnosis is estimated as 22.7% (95% confidence interval, 19.0-26.7), whereas the probability of progression or relapse up to 10 years is estimated as 36.8% (95% confidence interval, 32.8-40.9). The probability of progression or relapse plateaus from approximately 5 years after diagnosis, while the probability of death constantly increases.
The marginal associations between the treatment arm, main initial prognostic factors, and outcomes are summarized in forest plots (supplemental Figure 4A-B for PFS and OS, respectively). An age >70 years, a poor performance status, elevated lactate dehydrogenase, and a high aaIPI at baseline were strongly associated with PFS and OS.
Salvage treatments, PFS, and OS after the first progression
Second-line regimens were at the discretion of the investigators. Details from 194 of the 213 patients who progressed or relapsed were collected. One hundred seventy-two patients received second-line systemic therapy, including 99 (57.6%) patients who received intensive treatment, 72 (41.9%) patients who received nonintensive chemotherapy, and 1 (0.5%) patient who was included in a clinical trial (supplemental Table 1). Baseline characteristics of patients who received second-line intensive treatments are provided in supplemental Table 2.
Rituximab was combined with second-line chemotherapy in 138/194 (71.1%) patients, and radiotherapy was used in 28/194 (14.4%) patients. Twenty (10.3%) patients received ASCT (median age at randomization, 64 years). One hundred and five patients of the 213 (49%) progressed after second-line therapy, and 77 (36%) patients died without a second progression.
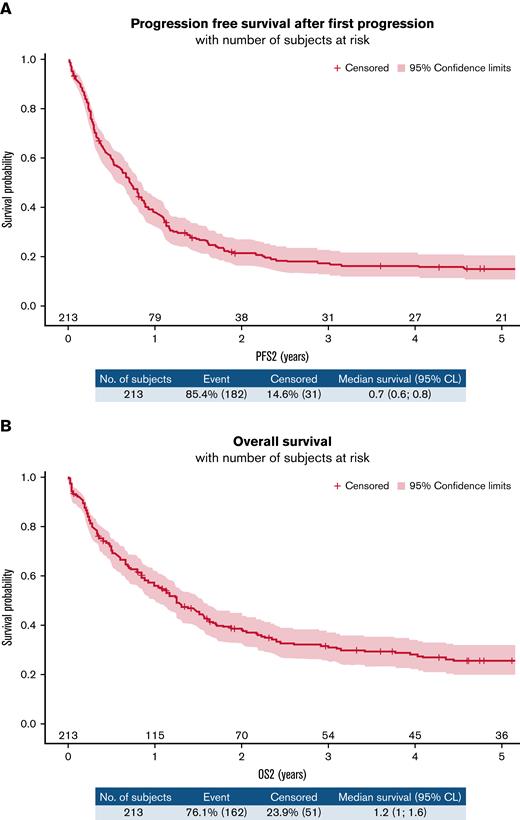
After a first progression, the 1-year PFS2 and 1-year OS2 were estimated as 37.9% (31.4-44.5) and 55.8% (48.8-62.2), respectively (Figure 5A-B). We did not observe any difference between randomized arms, but we highlighted a difference according to refractory status. The 1-year PFS2 was 19.5% (12-28.4) in the refractory group vs 50.8% (41.7-59.2) in the relapsed group (HR, 0.488 [0.363-0.656]; P < .001) (supplemental Figure 5A). The OS2 estimate at 1 year was 36.8% (26.8-46.8) in the refractory group vs 69.2% (60.2-76.5) in the relapsed group (HR, 0.513 [0.375-0.7]; P < .0001) (supplemental Figure 5B). In univariate analysis, receiving intensive treatment for the first treatment of relapse was associated with better PFS2 but similar OS2 compared with nonintensive treatments (supplemental Figure 6A-B). We also observed a better PFS2 and OS2 for patients receiving an autologous stem cell transplant (after salvage intensive chemotherapy) vs not (supplemental Figure 7A-B), but only very few selected and younger patients (n = 20) were in this group (baseline characteristics of these patients are provided in supplemental Table 3).
(A) PFS2 in the overall population. (B) OS2 in the overall population.
Secondary malignancies
Secondary malignancies appeared in 73 (12.2%) patients (R-CHOP14: n = 41 [13.5%]; R-CHOP21: n = 32 [10.8%]) (Table 3), including 18 (24.7%) cases of squamous cell carcinoma (R-CHOP14: n = 9; R-CHOP21: n = 9), 3 cases of acute myeloid leukemia (R-CHOP14: n = 2; R-CHOP21: n = 1), and 1 myelodysplastic syndrome (R-CHOP14 arm). Thirty-eight (12.6%) patients died of secondary malignancies.
Secondary malignancies in the ITT set
| . | RCHOP14, n (%) . | RCHOP21, n (%) . | All, n (%) . |
|---|---|---|---|
| ≥1 secondary malignancy | 41 (13.5) | 32 (10.8) | 73 (12.2) |
| Squamous cell carcinoma of the skin | 9 (22) | 9 (28.1) | 18 (24.7) |
| Carcinoma of unknown primary origin (CUP) | 5 (12.2) | 3 (9.4) | 8 (11) |
| Prostatic adenocarcinoma | 3 (7.3) | 4 (12.5) | 7 (9.6) |
| Lung carcinoma | 1 (2.4) | 3 (9.4) | 4 (5.5) |
| Renal cell carcinoma | 3 (7.3) | 1 (3.1) | 4 (5.5) |
| Acute myeloid leukemia | 2 (4.9) | 1 (3.1) | 3 (4.1) |
| Breast adenocarcinoma | 2 (4.9) | 1 (3.1) | 3 (4.1) |
| Colorectal adenocarcinoma | 1 (2.4) | 2 (6.3) | 3 (4.1) |
| Gastric adenocarcinoma | 1 (2.4) | 2 (6.3) | 3 (4.1) |
| Hepatocellular carcinoma | 1 (2.4) | 2 (6.3) | 3 (4.1) |
| Kaposi sarcoma | 1 (2.4) | 1 (3.1) | 2 (2.7) |
| Melanoma | 1 (2.4) | 1 (3.1) | 2 (2.7) |
| Pancreatic carcinoma | 2 (4.9) | 0 | 2 (2.7) |
| Anal adenocarcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Bladder carcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Carcinomatous meningitidis | 1 (2.4) | 0 | 1 (1.4) |
| Cholangiocarcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Hodgkin lymphoma | 1 (2.4) | 0 | 1 (1.4) |
| Malignant peritoneal mesothelioma | 1 (2.4) | 0 | 1 (1.4) |
| Multifocal hepatic angiosarcoma | 0 | 1 (3.1) | 1 (1.4) |
| Sinonasal carcinoma | 0 | 1 (3.1) | 1 (1.4) |
| Neuroendocrine carcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Esophageal carcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Myelodysplastic syndrome | 1 (2.4) | 0 | 1 (1.4) |
| . | RCHOP14, n (%) . | RCHOP21, n (%) . | All, n (%) . |
|---|---|---|---|
| ≥1 secondary malignancy | 41 (13.5) | 32 (10.8) | 73 (12.2) |
| Squamous cell carcinoma of the skin | 9 (22) | 9 (28.1) | 18 (24.7) |
| Carcinoma of unknown primary origin (CUP) | 5 (12.2) | 3 (9.4) | 8 (11) |
| Prostatic adenocarcinoma | 3 (7.3) | 4 (12.5) | 7 (9.6) |
| Lung carcinoma | 1 (2.4) | 3 (9.4) | 4 (5.5) |
| Renal cell carcinoma | 3 (7.3) | 1 (3.1) | 4 (5.5) |
| Acute myeloid leukemia | 2 (4.9) | 1 (3.1) | 3 (4.1) |
| Breast adenocarcinoma | 2 (4.9) | 1 (3.1) | 3 (4.1) |
| Colorectal adenocarcinoma | 1 (2.4) | 2 (6.3) | 3 (4.1) |
| Gastric adenocarcinoma | 1 (2.4) | 2 (6.3) | 3 (4.1) |
| Hepatocellular carcinoma | 1 (2.4) | 2 (6.3) | 3 (4.1) |
| Kaposi sarcoma | 1 (2.4) | 1 (3.1) | 2 (2.7) |
| Melanoma | 1 (2.4) | 1 (3.1) | 2 (2.7) |
| Pancreatic carcinoma | 2 (4.9) | 0 | 2 (2.7) |
| Anal adenocarcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Bladder carcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Carcinomatous meningitidis | 1 (2.4) | 0 | 1 (1.4) |
| Cholangiocarcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Hodgkin lymphoma | 1 (2.4) | 0 | 1 (1.4) |
| Malignant peritoneal mesothelioma | 1 (2.4) | 0 | 1 (1.4) |
| Multifocal hepatic angiosarcoma | 0 | 1 (3.1) | 1 (1.4) |
| Sinonasal carcinoma | 0 | 1 (3.1) | 1 (1.4) |
| Neuroendocrine carcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Esophageal carcinoma | 1 (2.4) | 0 | 1 (1.4) |
| Myelodysplastic syndrome | 1 (2.4) | 0 | 1 (1.4) |
Discussion
In the present study, we reported the long-term outcomes of a homogeneous population of patients with DLBCL aged 60 to 80 years treated with R-CHOP in the LNH03-6B trial. The 10-year PFS and OS rates were 40.4% and 49.8%, respectively, slightly higher than those in the R-CHOP arm of the LNH-98.5 trial (10-year PFS and OS: 36.5% and 43.5%, respectively), which involved a similar population. Therefore, we observed a modest improvement in long-term outcomes after R-CHOP in the last decade in a particularly challenging group (60 to 80 years) to manage and treat. To our knowledge, no other long-term outcome prospective reports after R-CHOP treatment in this population of older patients are available in the literature. Here, we confirm with a longer follow-up that dose-dense R-CHOP14 does not provide a longer PFS or OS in this population. The addition of darbepoetin alfa did not affect any survival endpoints. No plateau was reached for any of the survival endpoints, as shown in Figures 2 and 3. This finding is explained by deaths from other causes (either related or unrelated to the lymphoma or its treatment) in this older group of patients. Indeed, in our study, only 3.7% of patients had a relapse after 5 years, whereas the probability of death continued to increase beyond 5 years. In the study by Wang and colleagues, 48 patients were >60 years, and the cumulative incidence of late relapses that occurred after achieving event-free survival at 24 months was 9.3% at 5 years and 10.3% at 8 years.17 This result is consistent with our observations indicating the probability of progression or relapse plateaus from approximately 5 years after diagnosis, whereas the probability of death constantly increases. Of note, we did not have information on biopsy at relapse or on the percentage of patients who relapsed with indolent disease.
As usually seen in DLBCL, patient outcomes after disease progression were poor, with 61% of the patients dying from lymphoma within the first 2 years after progression. An initial progression-free period of <1 year was strongly associated with poor outcomes at relapse (median OS2: 6 months). This finding is consistent with the results of the SCHOLAR-1 study.6 This long-term study also revealed that 99 (51%) relapsed or refractory patients were eligible for intensive chemotherapy, and yet only 20% of them (or 10.3% of the total 194 patients) then went onto autologous transplantation procedures. In our study, we do not have sufficient data to comment on the reasons why nearly 80% of older patients who received intensive chemotherapy ultimately did not receive an ASCT, but our findings are consistent with those previously reported in this age group in a large population-based study.18 In the present work, the benefit of intensive chemotherapy over nonintensive treatment in this age group was found in terms of PFS but was not clear in OS. On the other hand, even if ASCT is usually associated with higher toxicity and lower efficacy in this older population, the very rare and highly selective patients who may be chemosensitive to relapse treatment and can receive ASCT have longer survival, close to that of young patients in this situation.19 Simple “chronological” age is not sufficient to determine patient eligibility for ASCT. Other criteria should be considered in patients aged >60 years to assess ASCT eligibility: performance status, comorbidities, general condition, and “functional age.”20
We would like to highlight several limitations of our study. First, the population of patients described in the long-term follow-up program (n = 256) is not comparable with the whole population (n = 600). We observed some differences, with fewer high-risk baseline characteristics in the long-term follow-up program population. When isolating this population of 256 patients, we induced a selection bias because those patients were selected on the fact that they were alive at the previous analysis. In addition, data from 128 of the 384 patients who were still alive at the end of the LNH03-6b trial were not updated in this study (patients outside France, centers not volunteering for the long-term follow-up program, patient opposition, and other reasons), which may represent a selection bias.
Second, long-term outcome data collection was difficult and not exhaustive. Indeed, histology and CD20 expression at relapse, relapse site, IPI score at relapse, some causes of death (especially for patients who did not experience lymphoma relapse), late adverse events such as cardiovascular and infectious events, dementia, or other aging-specific adverse events were missing.21,22 These data were rarely collected in the centers, which seem to comprehensively collect the status of the disease at each visit, but not systematically the data regarding long-term toxicity.
To overcome the problem of missing (or potentially miscoded) causes of death, an interesting study would be to estimate the long-term excess mortality hazard in this population compared with the general population to see how it changes with time and according to prognosis factors. Indeed, in considering the treatment outcomes for a population of patients with a number of competing risks for death and only 3.7% of relapses beyond 5 years, it would be valuable to investigate long-term DLBCL-specific mortality hazard compared with the expected mortality hazard in the general population.
In addition, the data we collected on the treatments given after the first relapse show the extreme heterogeneity of the indications proposed within a group of investigators used to work together. Of note, no data regarding well-known prognostic biomarkers (cell of origin, MYCandBCL-2and/orBCL-6 rearrangements, and total metabolic tumor volume) were available.23
Finally, the very poor prognosis of 60- to 80-year-old patients with DLBCL who relapse emphasizes the important need to improve first-line treatment in this age group. The recently reported results of the POLARIX trial, in which 69.2% of patients were >60 years, are hence of strong interest.24 Indeed, treatment with polatuzumab–vedotin, rituximab, doxorubicin, and prednisone (pola-R-CHP) resulted in a risk of disease progression, relapse, or death that was 27% lower (stratified HR, 0.73; 95% CI, 0.57 to 0.95; P = .02) than that with R-CHOP. On the other hand, to improve the prognosis of older patients who relapse, it seems necessary to improve salvage treatments. In particular, the use of CAR T cells, whose feasibility and target population are wider than those of ASCT, appears promising in the management of relapsed, older patients with DLBCL.9,10 With this finding in mind, the LYSA is currently studying axicabtagene ciloleucel as a second-line therapy in patients with relapsed or refractory DLBCL who are ineligible for ASCT (ALYCANTE trial, NCT04531046).
Conclusions
Ten years after randomization, in 60- to 80-year-old patients with newly diagnosed DLBCL, outcomes were similar between the R-CHOP21 and R-CHOP14 treatment groups. Our results confirm that the beneficial effects of R-CHOP are sustained over a 10-year follow-up period. Relapse or progression led to an adverse prognosis, except for 10.3% of thoroughly selected patients who received ASCT. New combinations are expected to improve frontline therapy results and spare retreatment in this population of patients aged 60 to 80 years. Other alternatives, including CAR T-cell therapy, need to be investigated as a second-line treatment in this hard-to-treat older population.
Acknowledgments
The authors thank the patients and their families, LYSARC, and all of the investigators in the Lymphoma Study Association (LYSA) centers; Christophe Bonnet, a reviewer at LYSA; and Fabrice Inglès, clinical project coordinator at LYSARC, Lyon, France.
This study was sponsored by Amgen and LYSARC.
Authorship
Contribution: V.C. analyzed and interpreted the data and wrote the manuscript; L.O., D.S., H.G., C.T., C.F., O.C., J.-M.M., T.J.M., A. Bosly, C.H., E.N.-V, P.F., O.F., and R.D. collected the data; A. Belot and C.J. performed the statistical analysis; and H.T. designed and supervised the study, analyzed and interpreted the data, and edited the paper.
Conflict-of-interest disclosure: V.C. received honoraria from Roche, Incyte, Gilead-Kite, Bristol-Myers Squibb, Sanofi, and Novartis; travel grants from Pfizer, Roche, Gilead-Kite, and Novartis; and research grants from Iqone Health Care. H.T. received honoraria from Celgene, Roche, Karyopharm, AstraZeneca, and Bristol-Myers Squibb; and grants from Celgene. R.D. has employment with BeiGene Switzerland GmbH and owns stock in BeiGene and Bristol-Myers Squibb.
Correspondence: Vincent Camus, Centre Henri Becquerel and INSERM U1245,1 rue d'amiens, 76038 Rouen Cedex, Rouen, France; e-mail: vincent.camus@chb.unicancer.fr.
References
Author notes
The sponsor (LYSARC) can provide the full study protocol upon request. Deidentified participant data can be provided by (and after approval from) LYSARC. Requests should be sent to the corresponding author, Vincent Camus (vincent.camus@chb.unicancer.fr). Data are available immediately following publication.
The full-text version of this article contains a data supplement.