Key Points
Adults with SCD and HP 1 allele have lower cell-free hemoglobin-to-HP ratios at steady state and during a vaso-occlusive episode.
The HP 1 allele is associated with a twofold lower risk of multiorgan failure on longitudinal follow-up.
Abstract
Haptoglobin (HP) is an acute-phase protein and the main scavenger of cell-free hemoglobin. When HP is depleted, as observed in hemolytic conditions such as sickle cell disease (SCD), cell-free hemoglobin can lead to acute organ damage. The impact of the HP 1-1, 2-1, and 2-2 isoforms on HP and cell-free hemoglobin concentrations and SCD-related complications is unclear. In a longitudinal cohort of patients with SCD, the HP 1 allele was associated with higher HP and lower cell-free hemoglobin concentrations at a routine clinic visit as well as during hospitalization for a vaso-occlusive episode or acute chest syndrome. With a median follow-up of 6.8 years, acute chest syndrome occurred in 42% (n = 163) and multiorgan failure in 14% (n = 53) of 391 patients with SCD with a minimum follow-up of 6 months. The HP 1 allele was independently associated with lower risk of developing multiorgan failure during acute chest syndrome (additive model hazard ratio, 0.5; P < .001). Future studies assessing the regulation of HP concentrations and ability to bind cell-free hemoglobin according to the HP genotype may help to identify patients with SCD at high risk for multiorgan failure and to guide interventions, such as rapid initiation of exchange transfusion or HP replacement therapy.
Introduction
Approximately 30% of the hemolysis in sickle cell disease (SCD) is intravascular, resulting in median plasma cell–free hemoglobin concentrations of 3.6 μM (interquartile range [IQR], 2.5-4.7 μM) at steady state increasing to 9.2 μM (IQR, 7.3-13.5 μM) during a vaso-occlusive pain episode (VOE).1-3 Cell-free hemoglobin contributes to organ damage through direct oxidative injury, consumption of nitric oxide, and upregulation of inflammatory pathways.4
The acute-phase protein haptoglobin (HP) is the main scavenger of cell-free hemoglobin in circulation. HP binds cell-free hemoglobin with high affinity, attenuating the toxic effects of cell-free hemoglobin and facilitating its rapid removal from the blood by binding of the HP-hemoglobin complex to CD163 on monocytes and macrophages.5 A genetic polymorphism in HP results in 3 major genotypes: HP 1-1, HP 2-1, and HP 2-2.6 These isoforms are characterized by changes in the α-chain of HP, resulting in differences in molecular weight and polymer formation. There have been conflicting reports on whether the HP isoforms affect the binding efficiency of cell-free hemoglobin to HP7-9 or of the HP-hemoglobin complex to CD163.10,11 Furthermore, some non-SCD studies have demonstrated that the HP 1-1 genotype is protective from kidney injury and cardiovascular disease,7,12 whereas others indicate that this genotype is a risk factor for neurologic or cardiovascular complications compared with the HP 2-2 genotype.13,14 Studies in patients with SCD have also shown mixed results, with some demonstrating that the HP 1 allele is associated with fewer SCD-related complications15,16 and others not reporting differences by the HP genotype.17,18
We conducted a longitudinal study to determine whether the (1) HP genotype is associated with HP and cell-free hemoglobin concentrations at steady state, VOE, and acute chest syndrome; and (2) HP 1 allele is protective against developing multiorgan failure during an episode of acute chest syndrome.
Methods
Institutional review board approval and written informed consent were obtained before recruitment and biosample collection. Between October 2009 and June 2018, 431 patients with SCD were recruited into a longitudinal registry at our institution during a routine clinic visit, which we defined as steady state. Baseline clinical and laboratory data were collected at enrollment. Between September 2020 and November 2021, we collected blood from 51 patients with SCD within 24 hours of hospital admission for VOE or acute chest syndrome from all patients who provided consent. HP isoform genotyping was performed by polymerase chain reaction, as previously described.19 Blood was collected using larger (smaller gauge) needles to minimize hemolysis, when possible. Serum concentrations of HP (R&D Systems; Minneapolis, MN) and cell-free hemoglobin (Bethyl Laboratories Inc; Montgomery, TX) were measured by enzyme-linked immunosorbent assay in all available samples (steady state, n = 243; VOE, n = 42; acute chest syndrome n = 9).
For the longitudinal analysis, we focused on 391 patients with SCD with >6 months of follow-up at our institution. A VOE was defined as a hospitalization for SCD-associated pain (excluding headaches) and requiring opioid treatment for management.15 Acute chest syndrome was defined as a new pulmonary infiltrate on chest imaging accompanied by fever or respiratory symptoms (chest pain, cough, wheezing, tachypnea, or a reduction in oxygen saturation of >2% from baseline).15 An acute chest syndrome event was first identified based on the inpatient physician’s discharge summary diagnosis and the definition confirmed by an investigator blinded to the patient’s HP genotype. Of 438 acute chest syndrome events listed as a diagnosis in 178 patients, 423 events (96%) in 163 patients (92%) were confirmed based on the definition provided above; 15 events were excluded based on the absence of a new pulmonary infiltrate in the radiologist’s report. Rapidly progressive acute chest syndrome was defined as acute chest syndrome requiring ≥3 L of oxygen to maintain oxygen hemoglobin saturation ≥90% or mechanical ventilation within 24 hours of onset of respiratory symptoms.20 Multiorgan failure was defined during an acute chest syndrome episode as having injury to ≥2 of the following organs: lungs (new oxygen requirement >3 L or ventilatory support), kidneys (defined according to the Kidney Disease Improving Global Outcomes guidelines),21 central nervous system (encephalopathy or stroke), liver (acute elevation of alanine aminotransferase >3× upper limit of normal or intrahepatic cholestasis [total bilirubin >15 mg/dL and ≥50% direct bilirubin]), and/or cardiovascular system (vasopressor support or myocardial infarction).22
We examined the association of the HP genotype with laboratory and clinical variables using an additive allelic model based on previous studies.23,24 Baseline variables were compared by the HP genotype (1-1, 2-1, or 2-2) using the Cochran-Armitage trend test for categorical variables and the test for linear trend for linear variables. The following variables were log transformed to approximate normal distribution for the analyses: systolic blood pressure, body mass index, white blood cell count, hemoglobin F%, absolute reticulocyte count, lactate dehydrogenase (LDH), total bilirubin, aspartate aminotransferase, urine albumin-to-creatinine ratio, serum HP, cell-free hemoglobin, cell-free hemoglobin-to-HP ratio, and VOE and acute chest syndrome incidence. Serum HP, cell-free hemoglobin, and cell-free hemoglobin-to-HP ratio were compared by the HP genotype at steady state by linear regression analysis, adjusting for age, sex, SCD genotype, hydroxyurea use, LDH concentration, and time intervals between sample collection and preceding VOE and prior red blood cell transfusion; comparisons during a VOE or an acute chest syndrome event were performed using the test for linear trend. A time duration of >4 months for preceding VOE or red blood cell transfusion was assigned a value of 120 days. The associations of the HP genotype with VOE incidence, acute chest syndrome, rapidly progressive acute chest syndrome, and multiorgan failure were performed using linear or logistic regression analysis, adjusting for age, sex, SCD genotype, hydroxyurea use, asthma, and tobacco history. The association of the HP genotype with multiorgan failure was also assessed using the log-rank method to compare Kaplan-Meier survival curves and Cox proportional hazards models, with similar adjustments. Analyses were performed using Systat 13 (Systat Software Corporation; Chicago, IL). Median and IQR or mean ± standard error of mean values are provided.
Results and discussion
The median age of the cohort was 32 years (IQR, 24-43 years), 57% were female, 76% were SS/Sβ0-thalassemia genotype, and 46% were on hydroxyurea at enrollment. The prevalence of the HP genotypes was HP 1-1, 30% (129 of 431); HP 2-1, 47% (203 of 431); and HP 2-2, 23% (99 of 431). The distribution of these genotypes was similar to what has been reported in the general African population (HP 1-1, 29%; HP 2-1, 40%; and HP 2-2, 22%).8 There were no statistically significant clinical differences at baseline by the HP genotype (Table 1). The mean time interval between biosample collection and the preceding VOE (HP 1-1, 103 ±3 days; HP 2-1, 107 ±2 days; and HP 2-2, 112 ±3 days) or red blood cell transfusion (HP 1-1, 113 ±2 days; HP 2-1, 111 ±2 days; and HP 2-2, 115 ±2 days) were similar between the HP genotypes (P = .3).
Patient characteristics at the time of enrollment
| . | HP 1-1 (n = 129) . | HP 2-1 (n = 203) . | HP 2-2 (n = 99) . | P value . |
|---|---|---|---|---|
| Age (y) | 34 (26-44) | 32 (24-43) | 30 (23-41) | .07 |
| Female | 78 (60%) | 118 (58%) | 51 (52%) | .2 |
| Sickle cell genotype | .3 | |||
| Hb SS or Sβ0-thalassemia | 93 (72%) | 157 (77%) | 76 (77%) | |
| Hb SC | 22 (17%) | 37 (18%) | 15 (15%) | |
| Hb Sβ+-thalassemia | 14 (11%) | 9 (4%) | 8 (8%) | |
| Asthma, n (%) | 24 (19%) | 48 (24%) | 20 (20%) | .6 |
| Hydroxyurea, n (%) | 60 (47%) | 92 (45%) | 46 (46%) | 1.0 |
| Tobacco use, n (%) | 37 (29%) | 47 (23%) | 22 (22%) | .2 |
| Systolic blood pressure (mm Hg) | 120 (111-129) | 119 (110-128) | 120 (110-130) | .9 |
| Body mass index (kg/m2) | 25 (22-28) | 23 (21-27) | 24 (21-28) | .1 |
| WBC count (× 103/μL) | 9.2 (6.9-11.8) | 9.8 (7.9-11.9) | 9.8 (7.4-11.8) | .1 |
| Hemoglobin (g/dL) | 9.2 (8.2-10.7) | 9.3 (8.1-10.4) | 9.3 (8.3-10.4) | .9 |
| Hemoglobin F (%) | 5.1 (1.7-9.7) | 4.2 (1.9-8.4) | 4.3 (2.5-9.0) | .9 |
| Platelet count (× 103/μL) | 343 (253-460) | 392 (291-493) | 372 (281-487) | .04 |
| Absolute reticulocyte count (× 103/μL) | 245 (164-372) | 284 (186-393) | 272 (168-411) | .6 |
| LDH (U/L) | 309 (238-436) | 304 (231-425) | 295 (235-386) | .4 |
| Ferritin (ng/mL) | 271 (74-1056) | 393 (128-1264) | 473 (114-1202) | .5 |
| Total bilirubin (mg/dL) | 2.3 (1.3-3.9) | 2.3 (1.5-3.5) | 2.1 (1.4-3.4) | .1 |
| AST (U/L) | 35 (26-52) | 36 (27-49) | 35 (27-42) | .3 |
| ALT (U/L) | 22 (16-31) | 22 (16-31) | 23 (16-30) | .6 |
| Urine albumin concentration (mg/g creatinine) | 31 (12-163) | 36 (12-198) | 19 (8-79) | .2 |
| eGFR (mL/min/1.73 m2) | 115 (87-127) | 117 (94-129) | 123 (98-132) | .06 |
| Vaso-occlusive episodes (preceding year)∗ | 2 (1-5) | 2 (0-4) | 2 (0-4) | .2 |
| Acute chest syndrome history, n (%) | 63 (50%) | 112 (55%) | 58 (59%) | .1 |
| Stroke history, n (%) | 22 (17%) | 41 (20%) | 17 (17%) | .9 |
| Priapism history, n (%) | 11/51 (22%) | 16/85 (19%) | 11/48 (23%) | .9 |
| . | HP 1-1 (n = 129) . | HP 2-1 (n = 203) . | HP 2-2 (n = 99) . | P value . |
|---|---|---|---|---|
| Age (y) | 34 (26-44) | 32 (24-43) | 30 (23-41) | .07 |
| Female | 78 (60%) | 118 (58%) | 51 (52%) | .2 |
| Sickle cell genotype | .3 | |||
| Hb SS or Sβ0-thalassemia | 93 (72%) | 157 (77%) | 76 (77%) | |
| Hb SC | 22 (17%) | 37 (18%) | 15 (15%) | |
| Hb Sβ+-thalassemia | 14 (11%) | 9 (4%) | 8 (8%) | |
| Asthma, n (%) | 24 (19%) | 48 (24%) | 20 (20%) | .6 |
| Hydroxyurea, n (%) | 60 (47%) | 92 (45%) | 46 (46%) | 1.0 |
| Tobacco use, n (%) | 37 (29%) | 47 (23%) | 22 (22%) | .2 |
| Systolic blood pressure (mm Hg) | 120 (111-129) | 119 (110-128) | 120 (110-130) | .9 |
| Body mass index (kg/m2) | 25 (22-28) | 23 (21-27) | 24 (21-28) | .1 |
| WBC count (× 103/μL) | 9.2 (6.9-11.8) | 9.8 (7.9-11.9) | 9.8 (7.4-11.8) | .1 |
| Hemoglobin (g/dL) | 9.2 (8.2-10.7) | 9.3 (8.1-10.4) | 9.3 (8.3-10.4) | .9 |
| Hemoglobin F (%) | 5.1 (1.7-9.7) | 4.2 (1.9-8.4) | 4.3 (2.5-9.0) | .9 |
| Platelet count (× 103/μL) | 343 (253-460) | 392 (291-493) | 372 (281-487) | .04 |
| Absolute reticulocyte count (× 103/μL) | 245 (164-372) | 284 (186-393) | 272 (168-411) | .6 |
| LDH (U/L) | 309 (238-436) | 304 (231-425) | 295 (235-386) | .4 |
| Ferritin (ng/mL) | 271 (74-1056) | 393 (128-1264) | 473 (114-1202) | .5 |
| Total bilirubin (mg/dL) | 2.3 (1.3-3.9) | 2.3 (1.5-3.5) | 2.1 (1.4-3.4) | .1 |
| AST (U/L) | 35 (26-52) | 36 (27-49) | 35 (27-42) | .3 |
| ALT (U/L) | 22 (16-31) | 22 (16-31) | 23 (16-30) | .6 |
| Urine albumin concentration (mg/g creatinine) | 31 (12-163) | 36 (12-198) | 19 (8-79) | .2 |
| eGFR (mL/min/1.73 m2) | 115 (87-127) | 117 (94-129) | 123 (98-132) | .06 |
| Vaso-occlusive episodes (preceding year)∗ | 2 (1-5) | 2 (0-4) | 2 (0-4) | .2 |
| Acute chest syndrome history, n (%) | 63 (50%) | 112 (55%) | 58 (59%) | .1 |
| Stroke history, n (%) | 22 (17%) | 41 (20%) | 17 (17%) | .9 |
| Priapism history, n (%) | 11/51 (22%) | 16/85 (19%) | 11/48 (23%) | .9 |
P values < .003 are significant after the Bonferroni correction.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; WBC, white blood cell count.
Vaso-occlusive episodes were defined as acute SCD-associated pain requiring hospital admission.
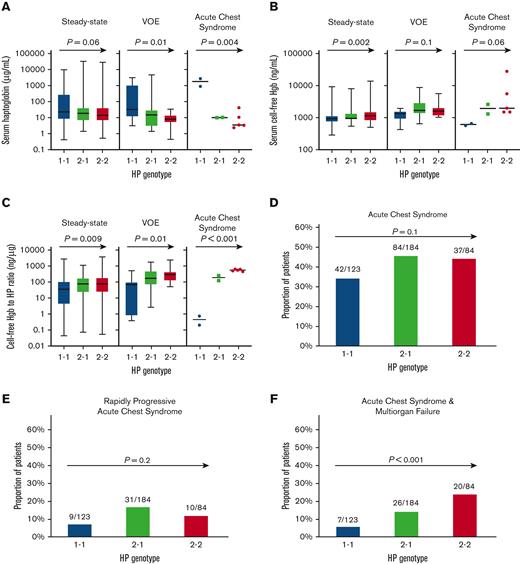
In univariate analysis, steady-state serum HP concentrations trended higher in those with the HP 1 allele (Figure 1A). Independent variables associated with HP levels at steady state included hemoglobin SS/Sβ0-thalassemia genotype (β −1.8 ± 0.4; P < .001), preceding VOE time interval (β −0.012 ± 0.004; P = .001), LDH (natural log β −0.84 ± 0.37; P = .03), hydroxyurea use (β 0.51 ± 0.26; P = .05), and a trend for the HP 1 allele (β 0.25 ± 0.17; P = .1), adjusting for age, sex, and preceding red blood cell transfusion time interval. The HP 1 allele was associated with higher serum HP concentrations during a VOE and during an acute chest syndrome episode (Figure 1A).
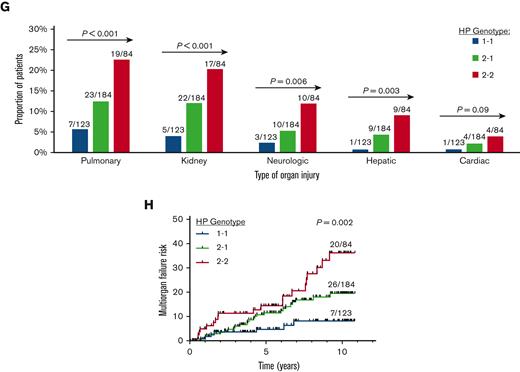
Association of HP 1 allele with lower cell-free hemoglobin-to-HP concentration and multiorgan failure risk in SCD. (A-C) Serum concentrations of (A) HP, (B) cell-free hemoglobin (hgb), and (C) cell-free hgb-to-HP ratio by the HP genotype in patients with SCD at steady state (n = 243), VOE (n = 42), and acute chest syndrome (n = 9). Test for linear trend P values are provided. (D-F) Proportion of patients who developed (D) acute chest syndrome, (E) rapidly progressive acute chest syndrome, and (F) acute chest syndrome complicated by multiorgan failure by the HP genotype on longitudinal follow-up. (G) The proportion of patients with acute organ injury by the HP genotype. (H) The risk for developing acute chest syndrome complicated by multiorgan failure was lower in patients with the HP 1 allele. Log-rank P value provided.
Association of HP 1 allele with lower cell-free hemoglobin-to-HP concentration and multiorgan failure risk in SCD. (A-C) Serum concentrations of (A) HP, (B) cell-free hemoglobin (hgb), and (C) cell-free hgb-to-HP ratio by the HP genotype in patients with SCD at steady state (n = 243), VOE (n = 42), and acute chest syndrome (n = 9). Test for linear trend P values are provided. (D-F) Proportion of patients who developed (D) acute chest syndrome, (E) rapidly progressive acute chest syndrome, and (F) acute chest syndrome complicated by multiorgan failure by the HP genotype on longitudinal follow-up. (G) The proportion of patients with acute organ injury by the HP genotype. (H) The risk for developing acute chest syndrome complicated by multiorgan failure was lower in patients with the HP 1 allele. Log-rank P value provided.
Cell-free hemoglobin concentrations were lower in patients with SCD with the HP 1 allele at steady state (Figure 1B). The HP 1 allele was an independent predictor for baseline cell-free hemoglobin (β −0.14 ± 0.05; P = .007) in the fully adjusted model. Cell-free hemoglobin concentrations trended lower in patients with SCD with the HP 1 allele during a VOE and during an acute chest syndrome episode (Figure 1B). The ratio of cell-free hemoglobin-to-HP was lower in patients with SCD with the HP 1 allele at steady state (Figure 1C). In the fully adjusted model, independent variables associated with the cell-free hemoglobin-to-HP ratio included the hemoglobin SS/Sβ0-thalassemia genotype (β 1.8 ± 0.4; P < .001), preceding VOE time interval (β 0.011 ± 0.004; P = .007), hydroxyurea use (β −0.65 ± 0.29; P = .03), HP 1 allele (β −0.40 ± 0.19; P = .04), and LDH (natural log β 0.69 ± 0.41; P = .09). The cell-free hemoglobin-to-HP ratio was also significantly lower during VOE or acute chest syndrome episodes in those with the HP 1 allele (P ≤ .01) (Figure 1C).
With a median follow-up of 6.8 years (IQR, 3.5-8.9 years), the mean incidence rates of VOE and acute chest syndrome episodes were 2.6 ± 0.2 and 0.2 ± 0.02 per patient per year, respectively. A distribution of the VOE, acute chest syndrome, and multiorgan failure events by age, sex, and SCD genotype are provided in supplemental Figure 1. We observed a trend for the HP 1 allele being associated with a lower incidence of VOE (supplemental Figure 2A), but this was not statistically significant on multivariate analysis (β 0.09 ± 0.1; P = .4). Four hundred twenty-three acute chest syndrome events occurred in 42% (163 of 391) of patients with SCD. Four of the acute chest syndrome events occurred in the pregnancy setting and 7 in the postoperative period. A trend for the HP allele being associated with acute chest syndrome was observed in univariate analysis (Figure 1D, supplemental Figure 2B). In multivariate analysis, the hemoglobin SS/Sβ0-thalassemia genotype (odds ratio [OR], 3.1; 95% confidence interval [CI], 1.8-5.4; P < .001) and asthma (OR, 1.6; 95% CI, 1.0-2.6; P = .07), but not HP 1 allele (OR, 0.8; 95% CI, 0.6-1.1; P = .2), were independently associated with risk of acute chest syndrome after adjusting for age, sex, hydroxyurea, and tobacco use. The proportion of patients treated for acute chest syndrome with exchange transfusion was not significantly different by the HP genotype (HP 1-1, 10% [12 of 123]; HP 2-1, 14% [25 of 184]; HP 2-2, 12% [10 of 84]; P = .6). Rapidly progressive acute chest syndrome occurred in 13% (50 of 391) of patients with SCD. We did not observe an association between the HP genotype and rapidly progressive acute chest syndrome in univariate (Figure 1E) or multivariate (OR, 0.8; 95% CI, 0.5-1.3; P = .4) analysis, whereas asthma (OR, 2.0; 95% CI, 1.0-3.8; P = .04) was an independent risk factor in the multivariate model.
Acute chest syndrome complicated by multiorgan failure occurred in 14% (53 of 391) of patients with SCD. The distribution and type of organ failure are provided in supplemental Figure 3. A significantly lower proportion of patients with SCD with the HP 1 allele developed multiorgan failure in univariate analysis (Figure 1F) and in the fully adjusted regression model (OR, 0.4; 95% CI, 0.3-0.7; P < .001). Pulmonary, kidney, neurologic, and hepatic injury occurred in a lower proportion of patients with SCD with the HP 1 allele (Figure 1G). In the time-to-event analysis, the HP 1 allele was independently associated with a lower risk of developing multiorgan failure (hazard ratio, 0.5; 95% CI, 0.4-0.6; P < .001) (Figure 1H).
Lower circulating HP concentrations are associated with kidney injury in the setting of acute hemolysis after cardiac surgery or severe burns.7,25 Some studies have reported that the HP 2-2 and 2-1 genotypes are associated with lower circulating HP concentrations compared with the HP 1-1 genotype in European ancestry and African populations.8,26 The association of the HP genotypes with circulating HP concentrations in patients with SCD is less clear. One SCD cohort demonstrated that the HP 1-1 genotype was associated with higher HP concentrations than HP 2-1 or 2-2,16 whereas another study did not find any difference.27
We observed that the HP 1 allele is associated with higher HP and lower cell-free hemoglobin concentrations at steady state as well as during VOE. Although the number of patients is small, we found a similar trend during acute chest syndrome. Furthermore, patients with SCD with the HP 1 allele were at a twofold lower risk of developing multiorgan failure on longitudinal follow-up. Consistent with a previous pediatric SCD study,15 we observed a trend for a lower incidence of VOE with the HP 1 allele, although this was not statistically significant after adjusting for covariates. Our study was focused on adults with SCD, but admissions for SCD-related pain, such as acute on chronic pain or pain related to avascular necrosis, may have affected our findings.
Limitations of our study include only capturing VOE and acute chest syndrome events at our institution and the relatively small sample size. The small sample size limits our ability to perform subgroup analyses, such as the effects of the HP genotype on clinical outcomes in the different SCD genotypes. We expanded our protocol to collect biosamples in hospitalized patients with SCD after September 2020, resulting in a smaller number of biosamples collected during an acute chest syndrome event. Another limitation of our study is that circulating concentrations of HP and cell-free hemoglobin are dynamic, and we used single time points for steady-state, VOE, and acute chest syndrome episodes. Although our findings demonstrate that the HP genotype is associated with concentrations of HP in circulation at steady state and during hospitalization, functional differences in cell-free hemoglobin scavenging and HP-hemoglobin complex clearance by the HP genotype are also plausible. Future studies investigating the regulation of HP concentrations and scavenging function based on the HP genotype and serially measuring HP concentrations during hospitalization for VOE or acute chest syndrome may contribute to identifying patients with SCD for aggressive measures, such as rapid initiation of red blood cell exchange transfusions or exogenous administration of HP.
Acknowledgments
The project described was financially supported by the National Institutes of Health through grants R03-HL146788, R01-HL153161 (S.L.S.), and R01-HL111656 (R.F.M.).
Authorship
Contribution: M.A.R., B.N.S., G.R., and S.L.S. designed and performed the research, analyzed the data, and wrote the paper; F.H., F.N., R.F.M., and V.R.G. designed and performed the research and wrote the paper.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Santosh L. Saraf, Division of Hematology & Oncology, Department of Medicine, University of Illinois at Chicago, 820 South Wood Street, MC 712, Chicago, IL 60612; e-mail: ssaraf@uic.edu.
References
Author notes
Data are available on request from the corresponding author, Santosh L. Saraf (ssaraf@uic.edu).
The full-text version of this article contains a data supplement.