Key Points
We report a simple 4 variables-based laboratory score taken on day +100 to predict transplant outcomes.
The Day100 score stratifies patients into 3 groups for moderate-severe GVHD and TRM.
Abstract
The aim of this study was to develop a predictive score for moderate-severe chronic graft-versus-host disease (cGVHD) on day +100 after allogeneic stem cell transplantation (HSCT). We studied 1292 patients allografted between 1990 and 2016, alive on day +100 after transplant, without cGVHD, and with full biochemistry laboratory values available. Patients were randomly assigned to a training and a validation cohort (ratio 1:1). In the training cohort, a multivariate analysis identified 4 independent predictors of moderate-severe cGVHD: gamma-glutamyl transferase ≥75 UI/l, creatinine ≥1 mg/dl, cholinesterase ≤4576 UI/l, and albumin ≤4 g/dl. A score of 1 was assigned to each variable, producing a low (0 to 1), intermediate (2 to 3), and high (4) score. The cumulative incidence of moderate-severe cGVHD was 12%, 20%, and 52% (P < .0001) in the training cohort, and 13%, 24%, and 33% (P = .002) in the validation cohort, respectively. The 5-year cumulative incidence of transplant-related mortality (TRM) was 5%, 14%, 27% (P < .0001) and 5%, 16%, 31% (P < .0001), respectively. The 5-year survival was 64%, 57%, 54% (P = .009) and 70%, 59%, 42% (P = .0008) in the 2 cohorts, respectively. In conclusion, Day100 score predicts cGVHD, TRM, and survival and, if validated in a separate group of patients, could be considered for trials of preemptive therapy.
Introduction
Chronic graft-versus-host disease (cGVHD) is the most common long-term complication of allogeneic stem cell transplantation (HSCT), developing in over 50% of patients surviving the transplant.1-4 Established risk factors for developing cGVHD are previous acute GVHD (aGVHD), donor-recipient HLA mismatch, no in vivo T depletion, advanced recipient age, and the use of peripheral blood (PB) stem cells.5-8
A registry study on 26 563 patients has clearly shown an increased incidence of cGVHD over the study time period of 1995 to 2007. Reasons for increased incidence were older patient age, increased use of alternative donors, and PB as a stem cell source.9 Mortality related to cGVHD is high due to impaired function of involved organs, infectious complications related to delayed immune recovery, or toxicity caused by prolonged use of immunosuppressive therapy. This is particularly true for severe cGVHD or progressive-onset cGVHD.10,11 In addition, despite the recent interesting results with new agents such as ibrutinib, belumosudil, and ruxolitinib for the treatment of cGVHD, the latter complication remains the most relevant cause of late nonrelapse mortality.6
A predictive score has been proposed at the time when the patient is diagnosed with cGVHD,12 and identifies patients at higher risk of transplant-related mortality (TRM). But if one could predict the occurrence of cGVHD before it develops, this would allow for trials of preemptive treatment to be designed.
The aim of the present study was to assess whether simple laboratory tests, assessed on day+100 after a transplant, would predict moderate-severe cGVHD: a multivariate analysis on laboratory and clinical data has allowed us to identify 4 simple laboratory tests predictive of cGVHD. We have devised a Day100 score and are reporting its predictive value on the occurrence of moderate-severe cGVHD, TRM, and overall survival (OS).
Methods
Patients
This study was approved by the ethics committee of the Policlinico Gemelli Roma, Protocol ID number 4061, and was performed in accordance with the Declaration of Helsinki. Patient data are collected prospectively from all patients undergoing an allogeneic HSCT in the Bone Marrow Transplant Unit of San Martino Hospital in Genoa and Policlinico Gemelli Hospital in Rome and entered into a relational database. The entire studied population consists of 1292 patients with hematological disorders who underwent an HSCT between 1990 and 2016 and fulfilled the following eligibility criteria: (1) alive on day +100 after transplantation, (2) grade 0 to I aGVHD on day +100, (3) no cGVHD on Day100, and (4) valid laboratory (chemistry and blood count) and clinical data for all investigated variables.
The study population was randomly divided into a training (n = 646) and a validation (n = 646) cohort with a ratio of 1:1 and with comparable characteristics in terms of demographic, disease, and transplants features (Table 1). Laboratory values on day +100 after HSCT included in the analysis were: hematocrit, hemoglobin, white blood cells, neutrophils, lymphocytes, monocytes, platelets, bilirubin, aspartate transaminase, alanine transaminase, gamma-glutamyl transferase (γGT), alkaline phosphatase, glucose, urea, creatinine, cholinesterase, total serum protein, albumin, IGG, IgA, IgM, cholesterol, triglycerides, transferrin, iron, and lactate dehydrogenase. Clinical data were retrospectively obtained from an electronic database updated at each visit for every patient and medical charts consultation: Karnofsky score, weight, GVHD assessment (organ involvement, grading, maximum stage reached), immunosuppressive drugs and dose, and relapse occurrence. cGVHD was diagnosed and staged according to National Institutes of Health criteria.10
Characteristics of the training and validation cohorts
| Characteristics . | Training cohort (n = 646) . | Validation cohort (n = 646) . | P . |
|---|---|---|---|
| Karnofsky, median (range) | 90 (30-100) | 90 (30-100) | .95 |
| Diagnosis | |||
| AA | 36 | 38 | .98 |
| AML | 193 | 201 | |
| ALL | 96 | 99 | |
| MPD | 164 | 153 | |
| LPD | 93 | 94 | |
| MDS | 53 | 53 | |
| Other | 11 | 8 | |
| Disease phase | |||
| CR | 433 | 449 | .34 |
| Non-CR | 213 | 197 | |
| Year | |||
| 1990-1999 | 183 | 189 | .50 |
| 2000-2009 | 255 | 235 | |
| 2010-2016 | 208 | 222 | |
| Median recipient age, median (range) | 40 (9-71) | 41 (14-74) | .53 |
| Median donor age, median (range) | 35 (0-69) | 35 (0-73) | .97 |
| Recipient sex | |||
| M | 365 | 386 | .24 |
| F | 281 | 260 | |
| Donor sex | |||
| M | 368 | 376 | .65 |
| F | 278 | 270 | |
| HLA-Match | |||
| Full match | 426 | 431 | .82 |
| 1 Ag Mismatch | 75 | 68 | |
| Haplo | 145 | 147 | |
| Relation | |||
| Unrelated | 464 | 490 | .10 |
| Related | 182 | 156 | |
| Conditioning | |||
| MAC | 418 | 440 | .19 |
| RIC | 228 | 206 | |
| Stem cell source | |||
| BM | 434 | 475 | .05 |
| PB | 168 | 147 | |
| CB | 44 | 24 | |
| Median dose*108/Kg, median (range) | 4.12 (0.04-25.8) | 4.05 (0.07-26.4) | .57 |
| GVHD prophylaxis | |||
| CSA+MMF | 174 | 157 | .3 |
| CSA+MTX | 468 | 483 | |
| Missing | 4 | 6 | |
| T-cell depletion | |||
| ATG y/n | 220 | 207 | .8 |
| Cy-post SCT | 123 | 128 | |
| None | 298 | 304 | |
| Missing | 5 | 7 | |
| aGVHD grade II-IV, n (%) | 188 (26) | 174 (29) | .4 |
| cGVHD moderate/severe, n (%) | 144 (22) | 148 (23) | .7 |
| Outcomes (%) | |||
| 5-y OS | 59 | 60 | .87 |
| 5-y TRM | 15 | 16 | .30 |
| 5-y DFS | 46 | 47 | .75 |
| Characteristics . | Training cohort (n = 646) . | Validation cohort (n = 646) . | P . |
|---|---|---|---|
| Karnofsky, median (range) | 90 (30-100) | 90 (30-100) | .95 |
| Diagnosis | |||
| AA | 36 | 38 | .98 |
| AML | 193 | 201 | |
| ALL | 96 | 99 | |
| MPD | 164 | 153 | |
| LPD | 93 | 94 | |
| MDS | 53 | 53 | |
| Other | 11 | 8 | |
| Disease phase | |||
| CR | 433 | 449 | .34 |
| Non-CR | 213 | 197 | |
| Year | |||
| 1990-1999 | 183 | 189 | .50 |
| 2000-2009 | 255 | 235 | |
| 2010-2016 | 208 | 222 | |
| Median recipient age, median (range) | 40 (9-71) | 41 (14-74) | .53 |
| Median donor age, median (range) | 35 (0-69) | 35 (0-73) | .97 |
| Recipient sex | |||
| M | 365 | 386 | .24 |
| F | 281 | 260 | |
| Donor sex | |||
| M | 368 | 376 | .65 |
| F | 278 | 270 | |
| HLA-Match | |||
| Full match | 426 | 431 | .82 |
| 1 Ag Mismatch | 75 | 68 | |
| Haplo | 145 | 147 | |
| Relation | |||
| Unrelated | 464 | 490 | .10 |
| Related | 182 | 156 | |
| Conditioning | |||
| MAC | 418 | 440 | .19 |
| RIC | 228 | 206 | |
| Stem cell source | |||
| BM | 434 | 475 | .05 |
| PB | 168 | 147 | |
| CB | 44 | 24 | |
| Median dose*108/Kg, median (range) | 4.12 (0.04-25.8) | 4.05 (0.07-26.4) | .57 |
| GVHD prophylaxis | |||
| CSA+MMF | 174 | 157 | .3 |
| CSA+MTX | 468 | 483 | |
| Missing | 4 | 6 | |
| T-cell depletion | |||
| ATG y/n | 220 | 207 | .8 |
| Cy-post SCT | 123 | 128 | |
| None | 298 | 304 | |
| Missing | 5 | 7 | |
| aGVHD grade II-IV, n (%) | 188 (26) | 174 (29) | .4 |
| cGVHD moderate/severe, n (%) | 144 (22) | 148 (23) | .7 |
| Outcomes (%) | |||
| 5-y OS | 59 | 60 | .87 |
| 5-y TRM | 15 | 16 | .30 |
| 5-y DFS | 46 | 47 | .75 |
AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulins; CB, cord blood; CR, complete response; CSA, cyclosporine A; Cy-post, cychophosphamide post-transplant; F, female; LPD, lymphoproliferative disease other than ALL; M, male; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MMF, mycophenolate; MPD, myeloproliferative disease; MTX, methotrexate; non-CR, other than CR; RIC, reduced intensity conditioning.
Statistical methods
Characteristics of the training and validation cohorts were compared using Pearson’s χ-Square test for categorical variables and Mann-Whitney U test for continuous variables. Kaplan-Meier actuarial survival and disease-free survival (DFS) were compared with the log-rank test. Cumulative incidence curves were used to define the risk of moderate-severe cGVHD after HSCT, with death in the absence of cGVHD or relapse of the original disease as competing factors. Cumulative incidence of TRM was constructed using relapse as a competing factor and the opposite for the cumulative incidence of relapse. The Fine and Gray’s test was used to compare cumulative incidence curves.
Univariate analysis was used to define laboratory tests and clinical variables associated with moderate-severe cGVHD in the training cohort. ROC curve identified cutoff levels for the variables significantly associated with moderate-severe cGVHD. According to these cutoff values, continuous laboratory variables were transformed into dichotomic variables. A multivariate Cox regression model was then built to identify independent predictors for moderate-severe cGVHD among clinical and laboratory variables selected in univariate analysis (P < .05). This model was run first in the training set and then in the validation set. The Day100 score was finally studied for its predictive effect on the occurrence of cGVHD, TRM, and OS, together with transplant and patient variables, first in the training set and then in the validation set.
For the analysis, we used the NCSS 2019 Statistical Software (NCSS, LLC. Kaysville, Utah, ncss.com/software/ncss).
Results
Identifying variables predictive of moderate-severe cGVHD
A univariate analysis for moderate-severe cGVHD occurrence after HSCT showed a significant association with 7 continuous laboratory variables: platelets (PLTs) (P = .002), γGT (P = .002), creatinine (Cr) (P = .0007), cholinesterase (CHE) (P = .03), total protein (Prot) (P = .0001), Alb (P < .0001), and triglycerides (TGR) (P < .0001).
ROC curves were used to assess a cutoff value for each of these laboratory variables according to moderate-severe cGVHD occurrence.
The following cutoff values were identified: PLTs counts ≤165 × 109/L (Area under the curve (AUC) 0.577, 95% Confidence interval (CI) 0.523-0.626, sensitivity 85%, specificity 30%, P = .003); γGT ≥75 UI/l (AUC 0.588, 95% CI 0.532-0.638, sensitivity 48.2%, specificity 68.7%, P = .001); creatinine ≥1 mg/dl (AUC 0.611, 95% CI 0.558-0.659, sensitivity 63.9%, specificity 51.2%, P < .0001); CHE ≤4576 UI/l (AUC 0.551, 95% CI 0.496-0.600, sensitivity 78.9%, specificity 33.3%, P = .04); serum total proteins <6.5 g/dl (AUC 0.587, 95% CI 0.532-0.636, sensitivity 60.1%, specificity 51.4%, P = .001); albumin ≤4 g/dl (AUC 0.576, 95% CI 0.521-0.626, sensitivity 72.3%, specificity 44.6%, P = .005); and TGR ≥295 mg/dl (AUC 0.581, 95% CI 0.524-0.633, sensitivity 38.4%, specificity 75.3%, P = .003). Laboratory variables were transformed from continuous to dichotomized variables according to these cutoff levels.
A multivariate COX regression was built including clinical and laboratory variables significantly associated with moderate-severe cGVHD in univariate analysis (Table 2): γGT ≥75 UI/l (Hazard ratio (HR) 1.93, 95% CI 1.35-2.76, P = .0003); Creatinine (Cr) ≥1 mg/dl (HR 1.49, 95% CI 1.03-2.14, P = .03); Cholinesterase (CHE) ≤4576 UI/l (HR 1.57, 95% CI 1.01-2.43, P = .04), and Alb ≤4 g/dl (HR 1.63, 95% CI 1.05-2.52, P = .03) were confirmed as independent predictors for moderate-severe cGVHD, together with a previous diagnosis of aGVHD (HR 2.11, 95% CI 1.19-3.74, P = .01); a more recent transplant era (HR 0.39, 95% CI 0.25-0.61, P < .0001; for years 2000-2009; HR 0.56, 95% CI 0.36-0.87, P = .01 for years 2010-2016, compared with years <2000); and a female donor to a male recipient (HR 1.61, 95% CI 1.12-2.34, P = .01).
Multivariate Cox regression for moderate-severe cGVHD in the training and validation set
| . | Training set . | Validation set . | ||||
|---|---|---|---|---|---|---|
| Variable . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Karnofsky | 1.00 | 0.99-1.02 | .06 | 0.98 | 0.97-0.99 | .001 |
| aGVHD: II-IV vs 0-I | 2.11 | 1.19-3.74 | .01 | 2.1 | 1.51-3.01 | .001 |
| Year | ||||||
| 1990-1999 | 1.00 | 1.00 | ||||
| 2000-2009 | 0.39 | 0.25-0.61 | <.0001 | 0.75 | 0.50-0.99 | .01 |
| 2010-2016 | 0.56 | 0.36-0.87 | .01 | 0.69 | 0.38-0.87 | .01 |
| Donor sex: F→M vs others | 1.61 | 1.12-2.34 | .01 | — | — | — |
| PLTs: ≤ vs >165*109/L vs | — | — | — | — | — | — |
| γGT: ≥ vs <75UI/l vs others | 1.93 | 1.35-2.76 | .0003 | 1.59 | 1.02-1.96 | .01 |
| Cr: ≥ vs <1mg/dl | 1.49 | 1.03-2.14 | .03 | 1.59 | 1.12-2.25 | .009 |
| CHE: ≤ vs >4576UI/l vs others | 1.57 | 1.01-2.43 | .04 | 1.05 | 1.00-1.58 | .04 |
| Alb: ≤ vs >4g/dl vs others | 1.63 | 1.05-2.52 | .03 | 1.54 | 1.07-2.08 | .02 |
| Donor age (continuous) | — | — | — | 1.01 | 1.00-1.03 | .04 |
| ATG: yes vs no | — | — | — | 0.57 | 0.36-0.89 | .01 |
| Cy-post: yes vs no | — | — | — | 0.19 | 0.05-0.78 | .02 |
| . | Training set . | Validation set . | ||||
|---|---|---|---|---|---|---|
| Variable . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Karnofsky | 1.00 | 0.99-1.02 | .06 | 0.98 | 0.97-0.99 | .001 |
| aGVHD: II-IV vs 0-I | 2.11 | 1.19-3.74 | .01 | 2.1 | 1.51-3.01 | .001 |
| Year | ||||||
| 1990-1999 | 1.00 | 1.00 | ||||
| 2000-2009 | 0.39 | 0.25-0.61 | <.0001 | 0.75 | 0.50-0.99 | .01 |
| 2010-2016 | 0.56 | 0.36-0.87 | .01 | 0.69 | 0.38-0.87 | .01 |
| Donor sex: F→M vs others | 1.61 | 1.12-2.34 | .01 | — | — | — |
| PLTs: ≤ vs >165*109/L vs | — | — | — | — | — | — |
| γGT: ≥ vs <75UI/l vs others | 1.93 | 1.35-2.76 | .0003 | 1.59 | 1.02-1.96 | .01 |
| Cr: ≥ vs <1mg/dl | 1.49 | 1.03-2.14 | .03 | 1.59 | 1.12-2.25 | .009 |
| CHE: ≤ vs >4576UI/l vs others | 1.57 | 1.01-2.43 | .04 | 1.05 | 1.00-1.58 | .04 |
| Alb: ≤ vs >4g/dl vs others | 1.63 | 1.05-2.52 | .03 | 1.54 | 1.07-2.08 | .02 |
| Donor age (continuous) | — | — | — | 1.01 | 1.00-1.03 | .04 |
| ATG: yes vs no | — | — | — | 0.57 | 0.36-0.89 | .01 |
| Cy-post: yes vs no | — | — | — | 0.19 | 0.05-0.78 | .02 |
AL, acute leukemia; Alb, albumin; ATG, anti-thymocyte globulins; CB, cord blood; CHE, cholinesterase; Cr, creatinine; CR, complete remission; Cy-post, cyclophosphamide post-transplant; F, female; Haplo, haploidentical donor; M, male; MAC, myeloablative conditioning; MMF, micophenolic acid; MTX, methotrexate; MUD, matched unrelated donor; PLTs, platelets; Prot, protein totali; RIC, reduced intensity conditioning; SIB, relative fully-matched donor; TGR, triglycerides; γGT, gamma-glutamyl transferase.
Table 2 outlines the same multivariate Cox analysis on moderate-severe cGVHD in the validation cohort, with the 4 laboratory tests being confirmed as predictors: γGT ≥75 UI/l (HR 1.59, 95% CI 1.02-1.96, P = .01); Cr ≥1 mg/dl (HR 1.59, 95% CI 1.12-2.25, P = .009); CHE ≤4576 UI/l (HR 1.05, 95% CI 1.00-1.58, P = .04); and Alb ≤4 g/dl (HR 1.54, 95% CI 1.07-2.08, P = .02). Other predictors in the validation cohort were Karnofsky’s performance, increasing donor age, a previous diagnosis of aGVHD, year of transplant, the use of ATG, and the use of cyclophosphamide posttransplant (Table 2).
Day100 score in the training cohort
cGVHD. A score of 1 was assigned for each of the following variables: γGT ≥75 UI/l, Cr ≥1 mg/dl, CHE ≤4576 UI/l, and Alb ≤4 g/dl. The global Day100 score was calculated for every patient on day +100 after HSCT: score 0 (n = 42), score 1 (n = 137), score 2 (n = 208), score 3 (n = 198), and score 4 (n = 61). A statistical difference in the incidence of moderate-severe cGVHD was observed for scores 2 to 3 (HR 2.02, 95% CI 1.26-3.24, P = .003) and score 4 (HR 6.43, 95% CI 3.58-11.58, P < .0001), when compared with scores 0 to 1. Therefore, we decided to divide patients into 3 risk groups: low risk (score 0 to 1, n = 179) intermediate risk (score 2 to 3, n = 406), and high risk (score 4, n = 61). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value for each risk group were as follows: 100%, 0%, 22%, and 100% for the low-risk group; 84%, 31%, 25%, and 87% for the intermediate-risk group; and 22%, 94%, 52%, and 80% for the high-risk group, respectively.
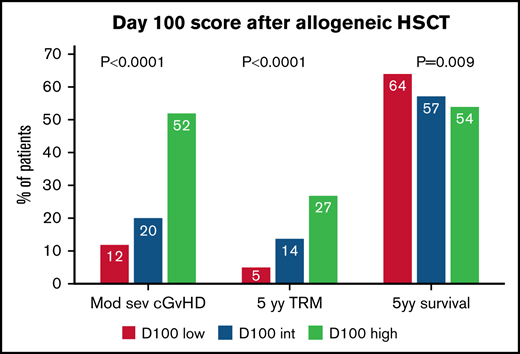
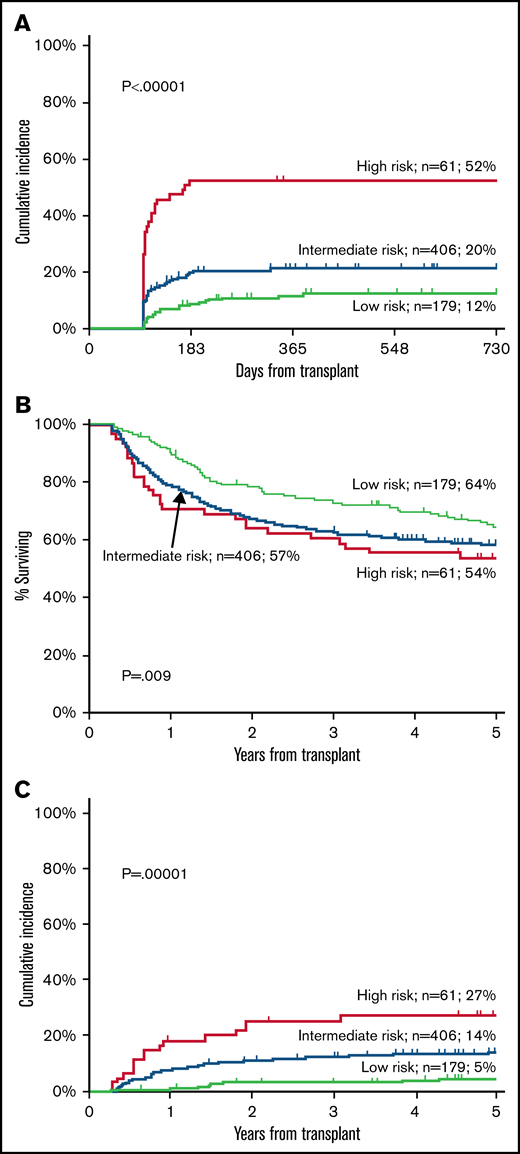
The 2-year cumulative incidence of moderate-severe cGVHD was 12% (95% CI 8-18) in the low-risk group, 20% (95% CI 16-24) in the intermediate-risk group, and 52% (95% CI 40-65) in the high-risk group (P < .00001) (Figure 1A).
Training cohort. Cumulative incidence of moderate-severe cGVHD (A), OS (B), and TRM (C) in the training cohort according to Day100 score.
Training cohort. Cumulative incidence of moderate-severe cGVHD (A), OS (B), and TRM (C) in the training cohort according to Day100 score.
The score maintained its prognostic power on cGVHD regardless of donor type and year of transplant. In particular, among patients receiving a sibling donor, the 2-year cumulative incidence of moderate-severe cGVHD was 14%, 22%, and 55% in the low-, intermediate-, and high-risk groups, respectively (P < .0001). Similarly, among patients receiving a matched unrelated donor, the 2-year cumulative incidence of moderate-severe cGVHD was 7%, 18%, and 45% in the 3 groups, respectively (P = .003). Finally, among patients receiving haploidentical graft, 2-year cumulative incidence of moderate-severe cGVHD was 10%, 14%, and 60%, respectively, in the 3 groups (P < .0001). For patients receiving BM grafts the figures were 13%, 17%, and 56%, respectively (P < .0001), and for PB they were 5%, 26%, and 46%, respectively (P = .002). As to the year of transplant, the 2-year cumulative incidence of moderate-severe cGVHD was 9%, 22%, and 50%, respectively, for patients transplanted before 2010 (P < .0001), and 14%, 17%, 60%, respectively, for patients transplanted after 2010 (P < .0001). In a multivariate analysis, the Day100 score was an independent predictor of moderate-severe cGVHD, together with previous aGVHD grade II to IV, year of transplant, and female donor to male recipient (Table 3). The use of PB cells had a borderline negative effect (P = .06). Finally, when applying a bootstrapping analysis (500 simulations), the 95% CI for intermediate- vs low-risk Day100 score is 1.34-2.52, and for high- vs low-risk Day100 score, it is 2.79-6.20 in a Cox model.
Multivariate Cox analysis: predictive effect of Day100 score on cGVHD, TRM, and survival in the training set
| . | cGVHD . | TRM . | OS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Day100 score | |||||||||
| Int. vs low | 2.02 | 1.26-3.24 | .003 | 2.05 | 1.18-3.50 | .009 | 1.24 | 0.93-1.64 | .13 |
| High vs low | 6.43 | 3.58-11.58 | <.0001 | 3.57 | 1.80-7.03 | .0003 | 1.49 | 0.96-2.30 | .07 |
| Karnofsky <90% | — | — | — | — | — | — | 1.50 | 1.15-1.94 | .002 |
| Diagnosis AL vs other | — | — | — | — | — | — | 1.60 | 1.26-2.03 | .0001 |
| Year of transplant | |||||||||
| 2000-2009 vs <2000 | 0.39 | 0.26-0.59 | <.0001 | 0.53 | 0.34-0.84 | .007 | 0.72 | 0.54-0.95 | .02 |
| 2010-2016 vs <2000 | 0.54 | 0.36-0.81 | .003 | 0.46 | 0.27-0.79 | .005 | 0.60 | 0.44-0.83 | .002 |
| no CR vs CR | — | — | — | — | — | — | 2.44 | 1.89-3.15 | <.0001 |
| Donor sex F→M vs other | 1.55 | 1.08-2.21 | .04 | 1.71 | 1.11-2.65 | .01 | — | — | — |
| aGVHD II-IV | 1.66 | 1.13-2.43 | .008 | — | — | — | — | — | — |
| Cy post: yes vs no | — | — | — | — | — | — | |||
| ATG: yes vs no | — | — | — | — | — | — | |||
| Stem cell source | |||||||||
| PB vs BM | 1.58 | 0.94-2.42 | .06 | — | — | — | |||
| CB vs BM | — | — | — | — | — | — | |||
| Recipient age yy (cont) | — | — | — | 1.02 | 1.00-1.03 | .01 | 1.01 | 1.00-1.02 | .0001 |
| Donor age yy (cont) | — | — | — | ||||||
| . | cGVHD . | TRM . | OS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Day100 score | |||||||||
| Int. vs low | 2.02 | 1.26-3.24 | .003 | 2.05 | 1.18-3.50 | .009 | 1.24 | 0.93-1.64 | .13 |
| High vs low | 6.43 | 3.58-11.58 | <.0001 | 3.57 | 1.80-7.03 | .0003 | 1.49 | 0.96-2.30 | .07 |
| Karnofsky <90% | — | — | — | — | — | — | 1.50 | 1.15-1.94 | .002 |
| Diagnosis AL vs other | — | — | — | — | — | — | 1.60 | 1.26-2.03 | .0001 |
| Year of transplant | |||||||||
| 2000-2009 vs <2000 | 0.39 | 0.26-0.59 | <.0001 | 0.53 | 0.34-0.84 | .007 | 0.72 | 0.54-0.95 | .02 |
| 2010-2016 vs <2000 | 0.54 | 0.36-0.81 | .003 | 0.46 | 0.27-0.79 | .005 | 0.60 | 0.44-0.83 | .002 |
| no CR vs CR | — | — | — | — | — | — | 2.44 | 1.89-3.15 | <.0001 |
| Donor sex F→M vs other | 1.55 | 1.08-2.21 | .04 | 1.71 | 1.11-2.65 | .01 | — | — | — |
| aGVHD II-IV | 1.66 | 1.13-2.43 | .008 | — | — | — | — | — | — |
| Cy post: yes vs no | — | — | — | — | — | — | |||
| ATG: yes vs no | — | — | — | — | — | — | |||
| Stem cell source | |||||||||
| PB vs BM | 1.58 | 0.94-2.42 | .06 | — | — | — | |||
| CB vs BM | — | — | — | — | — | — | |||
| Recipient age yy (cont) | — | — | — | 1.02 | 1.00-1.03 | .01 | 1.01 | 1.00-1.02 | .0001 |
| Donor age yy (cont) | — | — | — | ||||||
AL, acute leukemia; ATG, anti-thymocyte globulins; CB, cord blood; (cont), continuous variable; CR, complete remission; Cy-post, cyclophosphamide post-transplant; F, female; Haplo, haploidentical donor; M, male; MAC, myeloablative conditioning; MMF, micophenolic acid; MTX, methotrexate; MUD, matched unrelated donor; RIC, reduced intensity conditioning; SIB, relative fully-matched donor.
OS and TRM.
The 5-year OS is shown in Figure 1B: 64% (95% CI 57-71) for the low-risk group, 57% (95% CI 53-62) for the intermediate-risk, and 54% (95% CI 41%-66%) for the high-risk group (P = .009) (Figure 1B). In a Cox multivariate analysis, survival was predicted by Karnofsky’s performance, recipient age, year of transplant, a diagnosis of acute leukemia, and remission status at transplant. Day100 score had a borderline effect (Table 3).
The 5-year cumulative incidence of TRM was 5% (95% CI 9-16) for the low-risk group, 14% (95% CI 11-17) in the intermediate-risk group, and 27% (95% CI 17-41) in the high-risk group (P < .0001) (Figure 1C). In a multivariate Cox analysis, the Day100 score was an independent risk factor for TRM: HR 2.05 for intermediate-risk patients (95% CI 1.18-3.50, P = .009) and HR 3.57 for high-risk patients (95% CI 1.80-7.03, P = .0003) as compared with low-risk patients. Other predictors were increasing recipient age, a female donor to a male recipient, and year of transplant (Table 3).
Day100 score in the validation cohort
Patients in the validation cohort were scored by day +100 as follows: low risk (n = 197), intermediate risk (n = 391), and high risk (n = 58).
cGVHD.
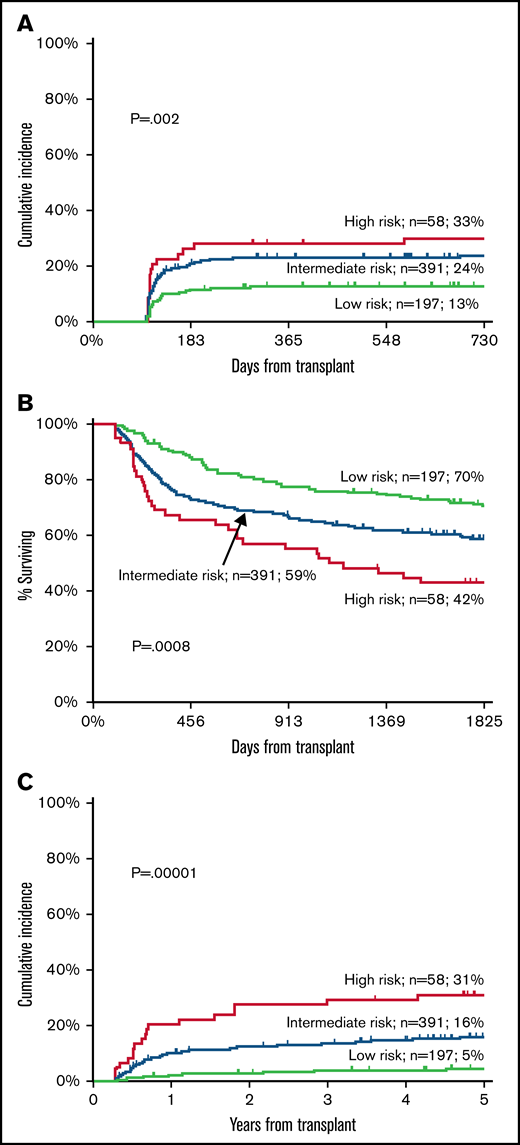
The cumulative incidence of moderate-severe cGVHD was 13% (95% CI 8-18) in the low-risk group (n = 197), 24% (95% CI 19-28) in the intermediate-risk group (n = 391), and 33% (95% CI 20-44) in the high-risk group (n = 58) (P = .002) (Figure 2A).
Validation cohort. Cumulative incidence of moderate-severe cGVHD (A), OS (B), and TRM (C) according to Day100 score.
Validation cohort. Cumulative incidence of moderate-severe cGVHD (A), OS (B), and TRM (C) according to Day100 score.
Table 4 outlines independent predictors of moderate-severe cGVHD when the Day100 score was added in the model: these were the Day100 score, the year of transplant, aGVHD, ATG, and the use of PB as a stem cell source (Table 4).
Multivariate Cox analysis: predictive effect of Day100 score on cGVHD, TRM, and survival in the validation set
| . | cGVHD . | TRM . | OS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Day100 score | |||||||||
| Int. vs low | 1.91 | 1.26-2.89 | .002 | 2.01 | 1.24-3.25 | .007 | 1.30 | 0.99-1.70 | .05 |
| High vs low | 2.31 | 1.21-4.42 | .01 | 2.45 | 1.29-4.67 | .002 | 1.55 | 1.02-2.36 | .03 |
| Karnofsky <90% | — | — | — | 2.29 | 1.60-3.27 | <.0001 | 1.86 | 1.45-2.04 | <.0001 |
| Diagnosis AL vs other | — | — | — | — | — | — | 1.71 | 1.34-2.19 | <.0001 |
| Year of transplant | |||||||||
| 2000-2009 vs <2000 | 0.75 | 0.50-1.12 | .07 | 0.41 | 0.26-0.65 | .002 | 0.79 | 0.59-1.06 | .11 |
| 2010-2016 vs <2000 | 0.69 | 0.38-1.24 | .06 | 0.41 | 0.24-0.69 | .007 | 0.57 | 0.40-0.80 | .001 |
| no CR vs CR | — | — | — | — | — | — | 2.22 | 1.70-2.90 | <.0001 |
| Donor sex F→M vs other | — | — | — | 1.60 | 1.09-2.33 | .008 | — | — | — |
| aGVHD II-IV | 2.09 | 1.46-2.98 | .001 | — | — | — | — | — | — |
| Cy post: yes vs no | — | — | — | — | — | — | — | — | — |
| ATG: yes vs no | 0.57 | 0.36-0.89 | .01 | — | — | — | — | — | — |
| Stem cell source | |||||||||
| PB vs BM | 2.06 | 135-3.20 | .0009 | — | — | — | — | — | — |
| CB vs BM | — | — | — | — | — | — | — | — | — |
| Recipient age (cont) | — | — | — | 1.03 | 1.02-1.05 | .0001 | 1.02 | 1.01-1.03 | <.0001 |
| Donor age (cont) | — | — | — | — | — | — | — | — | — |
| . | cGVHD . | TRM . | OS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| Day100 score | |||||||||
| Int. vs low | 1.91 | 1.26-2.89 | .002 | 2.01 | 1.24-3.25 | .007 | 1.30 | 0.99-1.70 | .05 |
| High vs low | 2.31 | 1.21-4.42 | .01 | 2.45 | 1.29-4.67 | .002 | 1.55 | 1.02-2.36 | .03 |
| Karnofsky <90% | — | — | — | 2.29 | 1.60-3.27 | <.0001 | 1.86 | 1.45-2.04 | <.0001 |
| Diagnosis AL vs other | — | — | — | — | — | — | 1.71 | 1.34-2.19 | <.0001 |
| Year of transplant | |||||||||
| 2000-2009 vs <2000 | 0.75 | 0.50-1.12 | .07 | 0.41 | 0.26-0.65 | .002 | 0.79 | 0.59-1.06 | .11 |
| 2010-2016 vs <2000 | 0.69 | 0.38-1.24 | .06 | 0.41 | 0.24-0.69 | .007 | 0.57 | 0.40-0.80 | .001 |
| no CR vs CR | — | — | — | — | — | — | 2.22 | 1.70-2.90 | <.0001 |
| Donor sex F→M vs other | — | — | — | 1.60 | 1.09-2.33 | .008 | — | — | — |
| aGVHD II-IV | 2.09 | 1.46-2.98 | .001 | — | — | — | — | — | — |
| Cy post: yes vs no | — | — | — | — | — | — | — | — | — |
| ATG: yes vs no | 0.57 | 0.36-0.89 | .01 | — | — | — | — | — | — |
| Stem cell source | |||||||||
| PB vs BM | 2.06 | 135-3.20 | .0009 | — | — | — | — | — | — |
| CB vs BM | — | — | — | — | — | — | — | — | — |
| Recipient age (cont) | — | — | — | 1.03 | 1.02-1.05 | .0001 | 1.02 | 1.01-1.03 | <.0001 |
| Donor age (cont) | — | — | — | — | — | — | — | — | — |
See Table 3 for term definitions.
OS and TRM.
The 5-year OS was 70% (95% CI 64-77) in the low-risk group, 59% (95% CI 53-63) in the intermediate-risk group, and 42% (95% CI 30-55) in the high-risk group (P = .0008) (Figure 2B). In a Cox analysis, other predictors of survival were Karnofsky’s performance, remission status at transplant, increasing recipient age, year of transplant, and a diagnosis of acute leukemia (Table 4).
The 5-year cumulative incidence of TRM was 5% in the low-risk group (95% CI 2-8), 16% in the intermediate-risk group (95% CI 12-20), and 31% in the high-risk group (95% CI 21-45) (P < .0001) (Figure 2C). In multivariate analysis, other independent predictors were Karnofsky’s performance, year of transplant, female donor to male recipient, and increasing recipient age (Table 4).
Discontinuing immunosuppressive therapy.
The proportion of patients off immunosuppressive therapy at 1 year after HSCT changed during different transplant eras as a consequence of different protocols: it was 27% before 2000, 52% in years 2001 through 2010, and 66% after 2010. Looking only at the most recent period (>2010), the proportion of patients off immunosuppression was 77%, 60%, and 44%, respectively, for Day100 score for low-, intermediate-, and high-risk groups, respectively (P = .005). Similarly, 75%, 56%, and 50% in the 3 score groups were also off oral steroids at 1-year posttransplant (P = .009).
Causes of death.
cGVHD with or without infections was recorded in the training cohort as the cause of death in 2%, 9%, and 16% of patients in the low-, intermediate-, and high-risk Day100 scores (P < .001); these figures were 2%, 10%, and 19% in the validation cohort (P < .001). Patients dying as a consequence of relapse were 33%, 30%, and 22% (P = .3) in the training cohort and 29%, 27%, and 24% (P = .8) in the validation cohort.
Discussion
In this study, we have identified a predictive score for cGVHD based on 4 simple laboratory tests (γGT, creatinine, cholinesterase, and albumin) taken on day +100 after transplantation. The combination of these 4 variables identifies, overall, a group of patients with a low risk of moderate-severe cGVHD (12%), a group with an intermediate risk (21%), and a group with a high risk (41%). In a multivariate Cox analysis, the risk of moderate-severe cGVHD in the high-risk Day100 group is 2- to 6-fold compared with low-risk Day100 patients. In addition, Day100 score also predicts TRM, with a hazard ratio of 2- to 3-fold for high-risk patients. The question then would be the following: Why should these 4 laboratory tests predict the occurrence of significant cGVHD and associated complications? Are they a marker of organ toxicity or indirect evidence of immune reactivity?
γ-glutamyl transferase is a liver enzyme that is commonly increased in patients with GVHD; the revised NIH staging of cGVHD includes liver enzymes as evidence of increasing severity of liver involvement.13 We expected ALT AST or alkaline phosphatase to be predictive of cGVHD, as they are included in the NIH staging of cGVHD, but found γGT instead to be highly predictive. An independent study has shown that patients with an increase of at least 2-fold the normal value of γGT, bilirubin, and alkaline phosphatase have a consistent (32%) risk of severe cGVHD, which reaches 50% when transaminases are also at least 2-fold higher than normal value.14 In addition, high pretransplant levels of γGT are associated with a 3.6-fold increased risk of developing liver disease at 100 days after HSCT.15,16 Barba and colleagues have shown that increasing pretransplant bilirubin and γGT have an adverse effect on the outcome of patients receiving reduced-intensity conditioning transplants. High pretransplant γGT predict worse survival but showed only a trend in multivariate analysis for worsening TRM.17 Despite not being included in the NIH staging of cGVHD, γ-glutamyl transferase remains a sensitive marker for ductal damage and has been strictly linked to toxic and oxidative stress in the liver.18,19
Creatinine is a marker of renal injury, and higher creatinine levels may suggest subclinical endothelial toxicity, as seen in thrombotic microangiopathy.20,21 In addition, increased creatinine may be the result of nephrotoxic drug administration. It may well be that patients with a high creatinine level on day+100 have been exposed to larger doses of cyclosporine or nephrotoxic antivirals, thus suggesting a higher incidence of both aGVHD and viral infections before day +100. Indeed, aGVHD is a strong predictor of cGVHD. In addition, higher creatinine levels call for a faster taper of cyclosporin, which may prompt the development of cGVHD. Serum cholinesterase, another liver enzyme, is a significant indicator of hepatic protein synthetic ability and a predictor of outcome in chronic liver disease, malnutrition, or infections.22,23 Cholinesterase has been used to stage liver cirrhosis,24 and it is no surprise that it would be reduced in patients developing cGVHD. We included cholinesterase in our standard laboratory test a long time ago as a marker of liver function, and we reported an early and significant reduction of cholinesterase in patients who were developing GVHD.25 In our original study, the difference in serum CHE levels according to aGVHD became more prominent with time after transplant, and it was also able to predict TRM.25 We believe CHE may be a marker for a subclinical response of the liver to alloreactivity. Finally, albumin is an important biochemical marker for several acute and chronic disorders, particularly in disorders with high levels of proinflammatory cytokines, like neoplasm, gastrointestinal diseases with mucosa damages, multiple myeloma, or in patients after surgery.26,27 The serum level of albumin is regulated by a complex interaction of synthesis, distribution, and catabolism. Hypoalbuminemia at the onset of aGVHD has been reported to be a significant independent marker of TRM and survival in addition to aGVHD response.28 Pretransplant hypoalbuminemia is a predictor of survival after HSCT,29,30 and a low albumin level on day +90 is significantly associated with TRM and OS posttransplant.31 Finally, enteropathies are often protein-losing disorders, and the gut is a primary target of immune reactivity after an allogeneic HSCT.
Therefore, it comes as no surprise that these 4 biochemical markers would be associated with cGVHD. The point is that slight abnormalities in these 4 markers are detected on day +100 in patients who are otherwise clinically free of acute and cGVHD but possibly have subclinical evidence of an ongoing or imminent immune reaction. In our study, the 4 markers are all independent predictors of moderate-severe cGVHD: when combined in a Day100 score, they can identify patients with low, intermediate, or high risk of developing extensive cGVHD. In the training set, the risk of moderate-severe cGVHD was 2-fold for the intermediate-risk group and 6-fold for the high-risk group compared with low-risk patients. The predictive value of our Day100 score was confirmed in the validation set of patients and was independent of donor type, stem cell source, patients’ age, GVHD prophylaxis (ATG or PTCY), and year of transplant. This last observation is interesting because GVHD prophylaxis has been changing over the years, and we are now using much more posttransplant high-dose cyclophosphamide. With these changes, the overall rate of cGVHD has been reduced, at least in our transplant units, but the Day100 score maintained its predictive value in the most recent transplant era.
In addition, and perhaps more importantly, the Day100 score had a strong impact on TRM both in univariate and multivariate analysis, independent of recipient’s age, year of transplant, donor-recipient gender mismatch, and phase of the disease at transplant: TRM was 2- to 3-fold greater in the high- as compared with the low-risk group, both in the training and validation cohort. The effect on survival was less pronounced, possibly because of the protective effect of cGVHD on relapse. Indeed, there was a trend for patients in the high-risk Day100 group to have a lower proportion of relapse-related deaths.
This is not the first predictive score in patients with GVHD, although most studies assess the risk of TRM and survival at the onset of the disease. The Endothelial Activation and Stress Index is calculated at the onset of aGVHD.20 Similarly, the CIBMTR has developed a score based on 10 items assessed at the onset of cGVHD,12 which is highly predictive of mortality. The CIBMTR risk score has been revised with the addition of 2 additional variables, eosinophils and lymphocyte count, again at the onset of GVHD.32 Another risk score has been proposed by Vaughn and colleagues, who implemented the hematopoietic cell transplant comorbidity index (HCT-CI),33 including laboratory variables assessed before transplantation. In particular, low albumin and platelet count and high ferritin levels predicted TRM and survival after HSCT. This augmented HCT-CI score identifies patients with a 2-fold risk of TRM.30
The Day100 score we have described in this study is independent of clinical and transplant data, including patients’ age. Older patients are known to be at higher risk of complications and mortality, particularly if they develop GVHD. When applied to patients >50 years, the Day100 score identifies patients with a low risk of cGVHD (16%), intermediate risk (32%), and high risk (57%) (P = .004), with associated significantly different TRM (2% vs 17% vs 42%, P < .0001). The Day100 score also identifies patients who are more likely to come off immunosuppressive therapy at 1 year: 77% in the low-risk vs 44% in the high-risk group.
The PPV for moderate-severe cGVHD of 52% in the high-risk group may be sufficient to justify a trial of preemptive therapy, for example, JAk2 or ROC inhibitors and extracorporeal-photopheresis,34-36 because the option of reducing a 50% risk of severe cGVHD may be attractive.
We recognize 3 main limitations of our study. First, only 2 centers were involved, and for this reason, we are activating a multicenter prospective study. Second, the analysis is retrospective, although data were collected prospectively at the time of the outpatient visit on day +100. Third, the patient population is heterogeneous, although this has allowed us to test the Day100 score in different settings, such as marrow or PB grafts or different donor types, and have found the Day100 score to be always predictive.
In conclusion, if the Day100 score will be confirmed prospectively in a different set of patients, possibly different institutions, we could then assess an individualized transplant-related risk on day +100: patients with low risk could be eligible for early discontinuation of immunosuppressive therapy, whereas high-risk patients could enter a trial of preemptive treatment with currently available agents approved for the treatment of cGVHD.
Acknowledgments
This study was supported by Associazione Italiana Ricerca contro il Cancro (AIRC), Milano, grant to A.B., and Centro di Ricerca sulle Cellule Staminali Emopoietiche e le Terapie Cellulari, Linea D1, Università Cattolica del Sacro Cuore in Rome, Italy.
Authorship
Contribution: E.M. and A.B. designed the study, performed statistical analysis, and wrote the paper; I.M.C., T.L., A.M.R., A.G., F. Galaverna, F. Gualandi, C.D.G., A.D., R.V., S.S., P.C., F.S., S.G., L.L., and E.A. collected patients’ data, contributed in writing the paper, and critically revised the final version of the manuscript; and A.S. participated in the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea Bacigalupo, Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario Agostino Gemelli IRCCS. Largo Agostino gemelli n.8, 00168, Rome, Italy; email: andrea.bacigalupo@unicatt.it.
References
Author notes
Requests for data sharing may be submitted to Andrea Bacigalupo (andrea.bacigalupo@unicatt.it).