Key Points
Oral alendronate is a safe and effective primary prophylaxis against loss in bone mineral density in lymphoma patients.
Abstract
Lymphoma patients often receive high glucocorticoid doses as part of standard therapy. Observational studies have shown a substantial risk of glucocorticoid-induced osteoporosis (GIO) with associated fractures. The aim of the SIESTA trial was to determine if oral alendronate (ALN) is a safe and effective prophylaxis against GIO in lymphoma. SIESTA was a single-center, randomized, double-blinded, phase 2 study of lymphoma patients planned for glucocorticoid-containing chemotherapy. After randomization, patients received weekly ALN 70 mg or placebo for a total of 52 weeks. Bone mineral density (BMD) was assessed at baseline, after completion of chemotherapy (end of treatment [EOT]) (4 to 6 months), and at the end of the study (EOS) (12 months). Vertebral fracture and biomarkers were assessed at baseline and EOS. Patients with baseline BMD assessment and at least 1 follow-up BMD assessment were analyzed for efficacy. The primary endpoint was a change in lumbar spine T-score from baseline to EOS. Of the 59 patients enrolled, 23 of 30 in the ALN arm and 24 of 29 in the placebo arm were analyzed for efficacy. The mean change in T-score from baseline to 12 months at the lumbar spine was +0.15 for ALN and -0.12 for placebo (P = .023). The difference in ΔTEOS between the ALN and placebo groups was larger among females (ALN 0.28; placebo -0.28; P = .01). Biomarker analyses confirmed reduced bone resorption in ALN-treated patients. In conclusion, ALN is a safe and effective primary prophylaxis against loss in BMD following glucocorticoid-containing chemotherapy. Despite reduced BMD loss in the ALN arm, the treatment did not influence fracture risk in this small cohort of patients.
Introduction
A substantial fraction of lymphoma patients become long-term survivors, and the research focus is gradually shifting from being almost exclusively focused on developing more effective therapies to a more holistic view on patient outcomes that also include a focus on survivorship.1 Glucocorticoids are included in many treatment regimens for lymphoma. The typical glucocorticoid-containing treatment schedule for lymphoma uses short-pulse therapy with high doses of prednisone repeated with each chemotherapy cycle. For example, R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone), the internationally accepted first-line therapy for diffuse large B-cell lymphoma (DLBCL), includes oral prednisone 100 mg once daily for 5 consecutive days in each treatment cycle.2 After standard treatment with 6 R-CHOP cycles, patients have received a total cumulative dose of 3000 mg of prednisone over 18 weeks, corresponding to an average of 24 mg/day during that period.
Glucocorticoids inhibit bone formation and vascularity, especially in the initial treatment phase,3 by promoting apoptosis of osteoblasts and osteocytes,4,5 resulting in a decrease in bone mineral density (BMD). Glucocorticoids increase the lifespan of osteoclasts, leading to increased bone resorption, which contributes to further bone loss.6,7 International consensus guidelines recommend the use of primary prophylaxis against glucocorticoid-induced osteoporosis (GIO), provided certain criteria are fulfilled. A working group from the International Osteoporosis Foundation and the European Society of Calcified Tissues recommend GIO risk assessment for patients ≥18 years when oral glucocorticoid therapy is planned for a duration of ≥3 months.8,9 Several recent studies have highlighted the risk of poor bone health, including the risk of fractures, after glucocorticoid-containing chemotherapy for lymphoma. In a study from our institution, vertebral compression fractures were identified on surveillance computed tomography (CT) scans in 14% of patients treated with R-CHOP after 2 years of follow-up, and a significant decrease in CT-assessed BMD was seen.10 In a large observational study of 13 570 elderly non-Hodgkin lymphoma patients with up to 11 years follow-up, those receiving chemotherapy had an increased risk of osteoporosis and fractures compared with patients not receiving chemotherapy (10.1% vs 8.3% and 31.1% vs 18.5%, respectively).11 Population-based data from 2589 DLBCL and follicular lymphoma (FL) patients treated with R-CHOP (-like) or R-CVP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) showed a 10-year cumulative risk of osteoporotic events of 16.3% vs 13.5% in the background population of 12 945 age and sex-matched persons (P < .01).12
The aim of this randomized controlled trial was to investigate the safety and efficacy of alendronate (ALN), an easy to administer, inexpensive oral therapy against osteoporosis, as primary prophylaxis against the observed GIO complications in lymphoma patients.
Methods
Study design
The SIESTA trial (EudraCT number: 2015-005688-18) was a randomized, double-blinded, and placebo-controlled phase 2 study of ALN as primary prophylaxis against GIO in lymphoma patients undergoing treatment with glucocorticoid-containing chemotherapy. The trial was a single-center study performed at the Department of Hematology, Aalborg University Hospital, Denmark. Patients were randomized (1:1 ratio) to receive oral ALN 70 mg or placebo once weekly for 52 weeks. All patients received a daily supplement of calcium (800 to 1200 mg daily) and vitamin D (20 to 40 µg daily). This was given for free to all patients enrolled in the trial.
The primary endpoint of the study was a change in T-score from baseline to EOS after 12 months, ΔTEOS = T1y − Tbaseline, measured by dual-energy X-ray absorptiometry scan (DXA) at lumbar spine L3 level. Key secondary efficacy endpoints were ΔTEOS at total hip and femoral neck, the incidence of new vertebral fractures, safety, and change in T-score at end of treatment (EOT) (4 to 6 months), ΔTEOT = TEOT − Tbaseline for lumbar spine, total hip, and femoral neck.
The study was conducted in accordance with the Helsinki Declaration, and all patients provided written consent. The study was approved by the regional ethics committee of the North Region Denmark (N-20160004), the Danish Medicines Agency (No. 2016040045), and the Danish Data protection agency (2008-58-0028). External trial monitoring was performed by the Danish Good Clinical Practice Units to ensure good clinical practice compliance for study procedures.
Randomization in blocks of 2 to 8 patients was performed by the hospital. Only the pharmacy had access to the randomization key. Unblinding was performed after the last patient had the last study visit and all DXA scan results had been reported. All analyses were prespecified in the statistical analysis plan, with the final version signed prior to study unblinding.
Patients
Patients were eligible if they fulfilled the following inclusion criteria: 1) newly diagnosed or relapsed malignant lymphoma; 2) age ≥18; 3) planned for treatment with a glucocorticoid-containing chemotherapy regimen (such as R-CVP and all variants of R-CHOP), and; 4) life expectancy of ≥2 years judged by the treating physician. Patients who received central nervous system prophylaxis or radiation therapy were also eligible. Additional glucocorticoid treatment of a maximum of 4 weeks at the time of screening was allowed. Local estrogen therapy (eg, estradiol vaginal inserts) was permitted regardless of duration. All patients had to be able to stand or sit upright for at least 30 minutes.
Patients were excluded if they fulfilled any of the following criteria: 1) contraindications to ALN; 2) treatment with any antiresorptive or anabolic medications including hormone replacement therapy (such as bisphosphonates, denosumab, strontium ranelate, selective estrogen receptor modulators, and estrogen used to treat symptoms associated with female menopause, teriparatide); 3) ongoing lithium or anticonvulsants treatment; 4) abnormalities of the esophagus or other conditions delaying esophageal emptying; 5) Glomerular filtration rate <35 ml/min; 6) planned for upfront autologous stem cell transplantation consolidation; and 7) pregnancy or lactating.
Study assessments
BMD (g/cm2) was measured at the lumbar spine (L1-L4), proximal femur, and femoral neck using DXA (Hologic Discovery, Marlborough, MA). Vertebral fracture assessment (VFA) was used to detect vertebral fractures (Hologic Discovery).
BMD was measured at baseline (within 2 weeks), EOT (typically 4 to 6 months), and EOS (12 months ± 4 weeks). VFA was performed at baseline and EOS. Routine quality control was performed for our site in 2017, and the coefficients of variation in vivo were 1.091% at the lumbar spine, 1.15% at the total hip, and 1.77% at the femoral neck.13
Hemoglobin, white blood cells, calcium, creatinine, N-terminal propeptides of collagen type 1 (P1NP), and C-terminal telopeptide crosslinks (CTX) were measured after overnight fast and were obtained at baseline, EOT, and EOS. Follicle-stimulating hormone, luteinizing hormone, testosterone/estrogen, parathyroid hormone, and 25-hydroxy vitamin D were measured at baseline and EOS. CTX and P1NP were measured in EDTA-plasma on an automated Cobas analyzer from Roche Diagnostics (Mannheim, Germany). The interassay analytical variation coefficients, according to the manufacturers, were CTX <6% and P1NP <4%.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03. Grade 1 to 4 AEs were registered for the gastrointestinal (GI) canal, as these were of special interest, and registrations for other AEs were limited to grades 3 and 4. A potential relationship of AEs to study medication was assessed by the attending physician.
Statistical analysis
Prespecified analyses were performed according to statistical analyses plan version 1.2 (supplemental Material).
Differences in ΔTEOS and ΔTEOT between treatment groups were tested using a 2-sided Student t test assuming equal variance. If the assumption of equal variance was not fulfilled (based on Bartlett’s test), a Student t test without the assumption of equal variance was used. Normally distribution of changes in T-scores was tested using the Shapiro-Wilk test, and in cases where ΔT was judged to be nonnormally distributed, the Wilcoxon rank-sum test was used to test for ΔT differences between the groups. In exploratory analyses, the effect of ALN was investigated within specific patient subgroups stratified by age (<60 and ≥60), sex, body mass index (BMI) (<25 and ≥25), chemotherapy regiment (R-CHOP and other), and Eastern Cooperative Oncology Group performance status (0 and >0). Differences in clinicopathologic factors and AEs were tested using the Wilcoxon rank-sum test (continuous variables) and Fishers’ exact test (discrete variables). Interaction tests were used to confirm the differential effects of ALN between patient subgroups in linear regression models. The efficacy population for primary and secondary endpoints was patients with baseline BMD assessment and at least 1 follow-up BMD assessment. The safety population was all patients who received at least 1 dose of study medication. All P values ≤5% were considered statistically significant.
Results
Patient characteristics
In total, 59 (30 in the ALN arm and 29 in the placebo arm) patients were enrolled in the study during the preplanned recruitment period (December 2016 until February 2020) (Table 1). The median age was 66 years in the ALN arm and 65 years in the placebo arm, with the majority being males (75%). In total, 36 patients were diagnosed with DLBCL, 15 patients diagnosed with FL, and 8 patients with other lymphoma diagnoses (Table 1). Bone marrow involvement was found for 14 patients at baseline. Fifty-five patients (93%) received first-line treatment, whereas 4 patients (7%) received later-lines treatment. Baseline characteristics were generally well balanced between the ALN arm and the placebo arm, except for advanced-stage disease, which was observed more frequently in the ALN arm (70% vs 41%), and lower baseline T-score at the lumbar spine level in the ALN arm (median T-score of −0.6 vs 1.0). Importantly, the cumulative doses of corticosteroids are similar between the 2 treatment arms. One patient was diagnosed with a pathological fracture at baseline.
Baseline characteristics of 59 adult lymphoma patients enrolled in the Siesta trial
| . | ALN (n =30), n (%) . | Placebo (n = 29), n (%) . | P . |
|---|---|---|---|
| Age, mean (SD) | 66 (9.2) | 65 (11.2) | 1.000 |
| Sex | 1.000 | ||
| Male | 22 (73.3) | 22 (75.9) | |
| Female | 8 (26.7) | 7 (24.1) | |
| Postmenopausal | 8 (100.0) | 7 (100.0) | 1.000 |
| Performance status | .212 | ||
| 0 | 21 (70.0) | 24 (85.7) | |
| >0 | 9 (30.0) | 4 (14.3) | |
| BMI, mean (range) | 29 (19-43) | 27 (22-37) | .088 |
| Subtype | .301 | ||
| DLBCL | 18 (60.0) | 18 (62.1) | |
| FL | 6 (20.0) | 9 (31.0) | |
| Other (marginal zone lymphoma, Hodgkin lymphoma, T-cell lymphoma, unspecified low grade lymphoma) | 6 (20.0) | 2 (6.9) | |
| Bone marrow involvement | 9 (30.0) | 5 (17.2) | .360 |
| Ann Arbor stage | .037 | ||
| 1 to 2 | 9 (30.0) | 17 (58.6) | |
| 3 to 4 | 21 (70.0) | 12 (41.4) | |
| LDH | .438 | ||
| Normal | 18 (60.0) | 14 (48.3) | |
| Elevated | 12 (40.0) | 15 (51.7) | |
| Treatment line | 1.000 | ||
| First-line | 28 (93.3) | 27 (93.1) | |
| Second- or later-lines | 2 (6.7) | 2 (6.9) | |
| Chemotherapy | 1.000 | ||
| R-CHOP | 15 (50.0) | 14 (48.3) | |
| R-CVP | 10 (33.3) | 10 (34.5) | |
| Other | 5 (16.6) | 5 (17.2) | |
| CNS prophylaxis with high doses MTX | .472 | ||
| No | 24 (80.0) | 26 (89.7) | |
| Yes | 6 (20.0) | 3 (10.3) | |
| Total prednisolone doses, mean (range) | 3291 (2400-4400) | 3398 (2000-4000) | .400 |
| T-score (lumbar spine) | |||
| Available | 25 (83.3) | 27 (93.1) | .424 |
| Lumbar spine, median (range) | −0.6 (−3.1 to 3.8) | 1.0 (−3.2 to 2.7) | .073 |
| T-score (hip/femoral neck) | |||
| Available | 26 (86.7) | 25 (86.2) | 1.000 |
| Total hip, median (range) | −0.2(−2.5 to 1.5) | 0.0 (−3.5 to 1.6) | .784 |
| Femoral neck, median (range) | −0.8(−3.0 to 1.5) | −0.6(−3.2 to 1.0) | .734 |
| Completion status | .289 | ||
| Completed | 22 (73.3) | 23 (79.3) | |
| Dead | 0 (0.0) | 1 (3.4) | |
| Withdrawal | 5 (16.7) | 1 (3.4) | |
| Drop-out | 3 (10.0) | 4 (13.8) | |
| . | ALN (n =30), n (%) . | Placebo (n = 29), n (%) . | P . |
|---|---|---|---|
| Age, mean (SD) | 66 (9.2) | 65 (11.2) | 1.000 |
| Sex | 1.000 | ||
| Male | 22 (73.3) | 22 (75.9) | |
| Female | 8 (26.7) | 7 (24.1) | |
| Postmenopausal | 8 (100.0) | 7 (100.0) | 1.000 |
| Performance status | .212 | ||
| 0 | 21 (70.0) | 24 (85.7) | |
| >0 | 9 (30.0) | 4 (14.3) | |
| BMI, mean (range) | 29 (19-43) | 27 (22-37) | .088 |
| Subtype | .301 | ||
| DLBCL | 18 (60.0) | 18 (62.1) | |
| FL | 6 (20.0) | 9 (31.0) | |
| Other (marginal zone lymphoma, Hodgkin lymphoma, T-cell lymphoma, unspecified low grade lymphoma) | 6 (20.0) | 2 (6.9) | |
| Bone marrow involvement | 9 (30.0) | 5 (17.2) | .360 |
| Ann Arbor stage | .037 | ||
| 1 to 2 | 9 (30.0) | 17 (58.6) | |
| 3 to 4 | 21 (70.0) | 12 (41.4) | |
| LDH | .438 | ||
| Normal | 18 (60.0) | 14 (48.3) | |
| Elevated | 12 (40.0) | 15 (51.7) | |
| Treatment line | 1.000 | ||
| First-line | 28 (93.3) | 27 (93.1) | |
| Second- or later-lines | 2 (6.7) | 2 (6.9) | |
| Chemotherapy | 1.000 | ||
| R-CHOP | 15 (50.0) | 14 (48.3) | |
| R-CVP | 10 (33.3) | 10 (34.5) | |
| Other | 5 (16.6) | 5 (17.2) | |
| CNS prophylaxis with high doses MTX | .472 | ||
| No | 24 (80.0) | 26 (89.7) | |
| Yes | 6 (20.0) | 3 (10.3) | |
| Total prednisolone doses, mean (range) | 3291 (2400-4400) | 3398 (2000-4000) | .400 |
| T-score (lumbar spine) | |||
| Available | 25 (83.3) | 27 (93.1) | .424 |
| Lumbar spine, median (range) | −0.6 (−3.1 to 3.8) | 1.0 (−3.2 to 2.7) | .073 |
| T-score (hip/femoral neck) | |||
| Available | 26 (86.7) | 25 (86.2) | 1.000 |
| Total hip, median (range) | −0.2(−2.5 to 1.5) | 0.0 (−3.5 to 1.6) | .784 |
| Femoral neck, median (range) | −0.8(−3.0 to 1.5) | −0.6(−3.2 to 1.0) | .734 |
| Completion status | .289 | ||
| Completed | 22 (73.3) | 23 (79.3) | |
| Dead | 0 (0.0) | 1 (3.4) | |
| Withdrawal | 5 (16.7) | 1 (3.4) | |
| Drop-out | 3 (10.0) | 4 (13.8) | |
Performance status, CNS, central nervous system; Eastern Cooperative Oncology Group performance status (ECOG); LDH, lactate dehydrogenase; MTX, high-dose Methotrexate.
Eight patients in the ALN arm and 6 patients in the placebo arm discontinued study treatment before final study assessments (Consort diagram in supplemental Material). For 2 patients discontinuing study treatment, DXA was performed.
Efficacy
In total, 47 patients (23 in the ALN arm and 24 in the placebo arm) were included in the primary efficacy analysis of BMD after 12 months (Table 2). Two patients were not included in selected BMD analyses; one patient had previous lower back surgery (only femur BMD was performed), and the other had prior hip surgery (only spine BMD was performed).
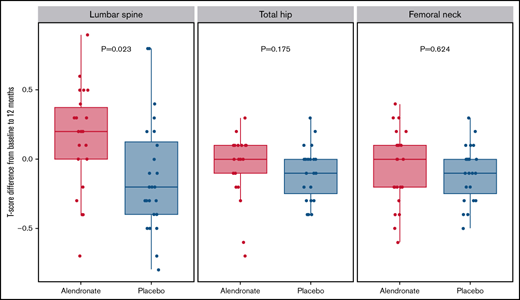
The mean change in lumbar spine T-scores from baseline to 12 months (ΔTEOS) was +0.15 for patients randomized to ALN and −0.12 for patients randomized to placebo (P = .02). For total hip, mean ΔTEOS was −0.05 and −0.10 for ALN and placebo, respectively (P = .18), whereas, for femoral neck, the median ΔTEOS was −0.07 and −0.10 for ALN and placebo, respectively (P = .62) (Table 3).
Baseline characteristics efficacy group (47 patients)
| . | ALN (n = 23), n (%) . | Placebo (n = 24), n (%) . | P . |
|---|---|---|---|
| Age, mean (SD) | 66 (7.8) | 64 (10.3) | .617 |
| Sex | 1.000 | ||
| Male | 17 (73.9) | 18 (75.0) | |
| Female | 6 (26.1) | 6 (25.0) | |
| Postmenopausal | 6 (100.0) | 6 (100.0) | 1.000 |
| Performance status | .188 | ||
| 0 | 19 (82.6) | 23 (95.8) | |
| >0 | 4 (17.4) | 1 (4.2) | |
| BMI, mean (range) | 28 (19-38) | 27 (22-37) | .441 |
| Subtype | .311 | ||
| DLBCL | 13 (56.5) | 13 (54.2) | |
| FL | 5 (21.7) | 9 (37.5) | |
| Other (marginal zone lymphoma, Hodgkin lymphoma, T-cell lymphoma) | 5 (21.7) | 2 (8.3) | |
| Bone marrow involvement | 7 (30.4) | 5 (20.8) | .517 |
| Ann Arbor stage | .041 | ||
| 1 to 2 | 7 (30.4) | 15 (62.5) | |
| 3 to 4 | 16 (69.6) | 9 (37.5) | |
| LDH | .147 | ||
| Normal | 15 (65.2) | 10 (41.7) | |
| Elevated | 8 (34.8) | 14 (58.3) | |
| Treatment line | 1.000 | ||
| First-line | 21 (91.3) | 22 (91.7) | |
| Second- or later-lines | 2 (8.7) | 2 (8.3) | |
| Chemotherapy | 1.000 | ||
| R-CHOP | 11 (47.8) | 10 (41.7) | |
| R-CVP | 9 (39.1) | 10 (41.7) | |
| Other | 3 (13.1) | 4 (16.6) | |
| CNS prophylaxis with high-dose MTX | .287 | ||
| No | 17 (73.9) | 21 (87.5) | |
| Yes | 6 (26.1) | 3 (12.5) | |
| Total prednisolone doses, mean (range) | 3291 (2400-4400) | 3398 (2000-4000) | .400 |
| T-score (lumbar spine) | |||
| Available | 22 (95.7) | 24 (100.0) | .489 |
| Lumbar spine, median (range) | −0.8 (−3.1 to 3.8) | 1.0 (−3.2 to 2.7) | .097 |
| T-score (hip/femoral neck) | |||
| Available | 23 (100.0) | 23 (95.8) | 1.000 |
| Total hip, median (range) | −0.2 (−2.5 to 1.5) | 0.1 (−2.3 to 1.6) | .322 |
| Femoral neck, median (range) | −0.9 (−3.0 to 1.5) | −0.4 (−3.1 to 1.0) | .475 |
| Completion status | 1.000 | ||
| Completed | 22 (95.7) | 23 (95.8) | |
| Dead | 0 (0.0) | 0 (0.0) | |
| Withdrawal | 0 (0.0) | 0 (0.0) | |
| Drop-out | 1 (4.3) | 1 (4.2) | |
| . | ALN (n = 23), n (%) . | Placebo (n = 24), n (%) . | P . |
|---|---|---|---|
| Age, mean (SD) | 66 (7.8) | 64 (10.3) | .617 |
| Sex | 1.000 | ||
| Male | 17 (73.9) | 18 (75.0) | |
| Female | 6 (26.1) | 6 (25.0) | |
| Postmenopausal | 6 (100.0) | 6 (100.0) | 1.000 |
| Performance status | .188 | ||
| 0 | 19 (82.6) | 23 (95.8) | |
| >0 | 4 (17.4) | 1 (4.2) | |
| BMI, mean (range) | 28 (19-38) | 27 (22-37) | .441 |
| Subtype | .311 | ||
| DLBCL | 13 (56.5) | 13 (54.2) | |
| FL | 5 (21.7) | 9 (37.5) | |
| Other (marginal zone lymphoma, Hodgkin lymphoma, T-cell lymphoma) | 5 (21.7) | 2 (8.3) | |
| Bone marrow involvement | 7 (30.4) | 5 (20.8) | .517 |
| Ann Arbor stage | .041 | ||
| 1 to 2 | 7 (30.4) | 15 (62.5) | |
| 3 to 4 | 16 (69.6) | 9 (37.5) | |
| LDH | .147 | ||
| Normal | 15 (65.2) | 10 (41.7) | |
| Elevated | 8 (34.8) | 14 (58.3) | |
| Treatment line | 1.000 | ||
| First-line | 21 (91.3) | 22 (91.7) | |
| Second- or later-lines | 2 (8.7) | 2 (8.3) | |
| Chemotherapy | 1.000 | ||
| R-CHOP | 11 (47.8) | 10 (41.7) | |
| R-CVP | 9 (39.1) | 10 (41.7) | |
| Other | 3 (13.1) | 4 (16.6) | |
| CNS prophylaxis with high-dose MTX | .287 | ||
| No | 17 (73.9) | 21 (87.5) | |
| Yes | 6 (26.1) | 3 (12.5) | |
| Total prednisolone doses, mean (range) | 3291 (2400-4400) | 3398 (2000-4000) | .400 |
| T-score (lumbar spine) | |||
| Available | 22 (95.7) | 24 (100.0) | .489 |
| Lumbar spine, median (range) | −0.8 (−3.1 to 3.8) | 1.0 (−3.2 to 2.7) | .097 |
| T-score (hip/femoral neck) | |||
| Available | 23 (100.0) | 23 (95.8) | 1.000 |
| Total hip, median (range) | −0.2 (−2.5 to 1.5) | 0.1 (−2.3 to 1.6) | .322 |
| Femoral neck, median (range) | −0.9 (−3.0 to 1.5) | −0.4 (−3.1 to 1.0) | .475 |
| Completion status | 1.000 | ||
| Completed | 22 (95.7) | 23 (95.8) | |
| Dead | 0 (0.0) | 0 (0.0) | |
| Withdrawal | 0 (0.0) | 0 (0.0) | |
| Drop-out | 1 (4.3) | 1 (4.2) | |
See Table 1 for definitions.
Mean (range) T-score difference from baseline to EOS and baseline to EOT at lumbar spine, total hip, and femoral neck, respectively (efficacy group; 47 patients)
| . | ΔTEOS (ALN) . | ΔTEOS (placebo) . | Difference . | P . |
|---|---|---|---|---|
| EOS (12 mo) | ||||
| Lumbar spine* | 0.15 (−0.70 to 0.90) | −0.12 (−0.80 to 0.80) | 0.28 (0.04−0.51) | .023 |
| Total hip† | −0.05 (−0.70 to 0.30) | −0.10 (−0.40 to 0.30) | 0.05 | .175 |
| Femoral neck* | −0.07 (−0.60 to 0.40) | −0.10 (−0.50 to 0.30) | 0.03 (−0.11 to 0.18) | .624 |
| EOT (4 to 6 mo) | ||||
| Lumbar spine* | 0.01 (−0.60 to 0.70) | −0.00 (−0.60 to 1.00) | 0.01 (−0.19 to 0.22) | .896 |
| Total hip‡ | −0.05 (−0.70 to 0.50) | −0.05 (−0.30 to 0.20) | 0.00 (−0.12 to 0.12) | 1.000 |
| Femoral neck† | −0.11 (−0.40 to 0.40) | −0.02 (−0.40 to 0.30) | −0.10 | .175 |
| . | ΔTEOS (ALN) . | ΔTEOS (placebo) . | Difference . | P . |
|---|---|---|---|---|
| EOS (12 mo) | ||||
| Lumbar spine* | 0.15 (−0.70 to 0.90) | −0.12 (−0.80 to 0.80) | 0.28 (0.04−0.51) | .023 |
| Total hip† | −0.05 (−0.70 to 0.30) | −0.10 (−0.40 to 0.30) | 0.05 | .175 |
| Femoral neck* | −0.07 (−0.60 to 0.40) | −0.10 (−0.50 to 0.30) | 0.03 (−0.11 to 0.18) | .624 |
| EOT (4 to 6 mo) | ||||
| Lumbar spine* | 0.01 (−0.60 to 0.70) | −0.00 (−0.60 to 1.00) | 0.01 (−0.19 to 0.22) | .896 |
| Total hip‡ | −0.05 (−0.70 to 0.50) | −0.05 (−0.30 to 0.20) | 0.00 (−0.12 to 0.12) | 1.000 |
| Femoral neck† | −0.11 (−0.40 to 0.40) | −0.02 (−0.40 to 0.30) | −0.10 | .175 |
Student t test with equal variance.
Wilcoxon rank-sum test (no confidence intervals are provided from this test).
Student t test with unequal variance.
The mean ΔTEOT at the lumbar spine was 0.01 for the ALN group and 0.00 for the placebo group (P = .90). For total hip, mean ΔTEOT was −0.05 and −0.05 for ALN and placebo, respectively (P = 1.00), whereas, for femoral neck, the mean ΔTEOT was −0.11 and −0.02 for ALN and placebo, respectively (P = .18) (Table 3).
In the ALN arm, 2 patients with baseline osteopenia (T-score of −1.0 to −2.5) had normal BMD at the end of the study, and 1 patient with normal BMD at baseline developed osteopenia at the end of the study. In the placebo arm, 1 patient with baseline osteopenia developed osteoporosis (T-score ≤−2.5), and 1 patient went from having osteopenia to normal BMD. One new fracture was observed in the placebo group, no additional major osteoporotic fractures were identified.
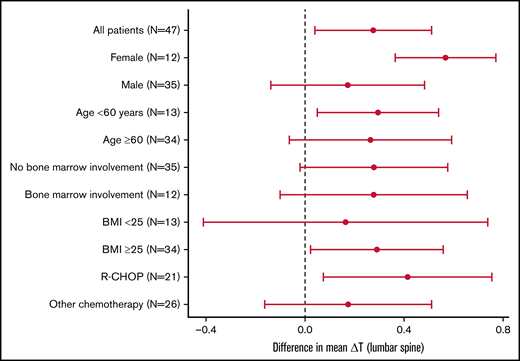
The difference in ΔTEOS between the ALN and placebo groups was larger among females (ALN, 0.28; placebo, −0.28; P = .01) compared with males (ALN, 0.10; placebo, −0.07; P = .27) (Figure 1).
The difference in mean ΔT at the lumbar spine (L1-L4) between patients in the efficacy group (n = 47) allocated to ALN and placebo groups. The displayed 95% confidence intervals indicate whether ALN has a significant effect in each subgroup. Test for differential effect of ALN between clinical subgroups did not reveal any significant differences: female vs male (P = .145), age <60 years vs age ≥60 years (P = .913), bone marrow involvement vs no involvement (P = .995), BMI <25 vs BMI ≥25 (P = .655), and R-CHOP vs other chemotherapy (P = .572).
The difference in mean ΔT at the lumbar spine (L1-L4) between patients in the efficacy group (n = 47) allocated to ALN and placebo groups. The displayed 95% confidence intervals indicate whether ALN has a significant effect in each subgroup. Test for differential effect of ALN between clinical subgroups did not reveal any significant differences: female vs male (P = .145), age <60 years vs age ≥60 years (P = .913), bone marrow involvement vs no involvement (P = .995), BMI <25 vs BMI ≥25 (P = .655), and R-CHOP vs other chemotherapy (P = .572).
Safety
Serious adverse events (SAEs) were balanced in the 2 treatment arms, with 15 (50%) patients experiencing SAEs in the ALN arm and 14 (48%) experiencing SAEs in the placebo arm (Table 4). Nine patients experienced AEs related to the upper GI system (7 grade 1 to 2; 2 grade 3 to 4), with 5 AEs assessed as related to the study treatment (3 in the ALN group and 2 in the placebo group). One patient (placebo) discontinued study treatment due to upper GI bleeding. Six patients experienced AE related to the lower GI system, all assessed unrelated to study medication.
Number of patients with adverse events among 59 lymphoma patients stratified by randomization
| . | ALN (n = 30), n (%) . | Placebo (n = 29), n (%) . |
|---|---|---|
| Any AEs | 19 (63.3) | 15 (51.7) |
| Any serious AE | 15 (50.0) | 14 (48.3) |
| AE related to infection | 6 (20.0) | 9 (31.0) |
| AE related to upper GI tract | 7 (23.3) | 2 (6.9) |
| AE related to lower GI tract | 3 (10.0) | 3 (10.3) |
| AE related to renal toxicity | 1 (3.3) | 2 (6.9) |
| Other AEs | 12 (40.0) | 8 (27.6) |
| AE grade 1 to 2 related to study drug | 3 (10.0) | 1 (3.4) |
| AE grade 3 to 4 related to study drug | 0 (0.0) | 1 (3.4) |
| AE resulting in termination of study drug | 1 (3.3) | 2 (6.9) |
| . | ALN (n = 30), n (%) . | Placebo (n = 29), n (%) . |
|---|---|---|
| Any AEs | 19 (63.3) | 15 (51.7) |
| Any serious AE | 15 (50.0) | 14 (48.3) |
| AE related to infection | 6 (20.0) | 9 (31.0) |
| AE related to upper GI tract | 7 (23.3) | 2 (6.9) |
| AE related to lower GI tract | 3 (10.0) | 3 (10.3) |
| AE related to renal toxicity | 1 (3.3) | 2 (6.9) |
| Other AEs | 12 (40.0) | 8 (27.6) |
| AE grade 1 to 2 related to study drug | 3 (10.0) | 1 (3.4) |
| AE grade 3 to 4 related to study drug | 0 (0.0) | 1 (3.4) |
| AE resulting in termination of study drug | 1 (3.3) | 2 (6.9) |
Biomarker analyses
Dynamics over time for blood biomarkers of bone turnover (CTX as a marker of bone resorption and P1NP as a marker for bone formation)14 are illustrated in Figure 2. Median levels of CTX and P1NP were similar between treatment arms at baseline (supplemental Material). From baseline to EOT, the mean change in CTX was −0.17 in the ALN group and 0.10 in the placebo group, respectively (P < .001). From baseline to EOS, the mean change in CTX was −0.19 in the ALN group and 0.00 in the placebo group, respectively (P = .002). For P1NP, EOT mean changes were 3.93 in the ALN group and 40.45 in the placebo group (P < .001), and EOS mean changes were 7.76 in the ALN group and 30.52 in the placebo group (P = .045).
Boxplot of CTX and P1NP at baseline, EOT, and 12 months stratified by randomization arm.
Boxplot of CTX and P1NP at baseline, EOT, and 12 months stratified by randomization arm.
Discussion
The present study of ALN as primary prophylaxis against GIO in lymphoma patients treated with glucocorticoid-containing chemotherapy regimens met its primary endpoint as ALN prevented a reduction in BMD at lumbar spine level from baseline to the 12-month assessment. In contrast, patients in the placebo arm experienced a reduction in BMD during the same period. Biomarker analyses supported a favorable effect of ALN on bone health, as CTX decreased over time among patients in the ALN arm and remained unchanged for patients in the placebo arm. However, ALN had no protective effects on BMD at the total hip and left femoral neck level at any time point. One year should be sufficient to appreciate changes in T-scores as glucocorticoids seem to cause marked vertebral bone loss in the initial months of therapy.15 Consistently, Svendsen et al showed that the BMD loss primarily occurred in the first year following treatment.10 One new fracture occurred during follow-up in the placebo group, therefore no firm conclusions can be made on whether the reduced BMD loss at lumbar spine level eventually translates into a clinically relevant reduction in fractures.
Only 2 previous randomized clinical trials have addressed the question of prophylaxis for lymphoma patients receiving glucocorticoid-containing chemotherapy. A trial by Kim et al from 2004 randomized between IV pamidronate (30 mg) every 3 months for 1 year vs placebo. Among the 50 patients randomized, patients assigned to pamidronate had a reduction in both bone loss and vertebral fractures risk.16 New vertebral fractures occurred in 6 of 20 patients in the placebo group vs 1 of 25 in the pamidronate group (P = .01).16 Very large doses of glucocorticoids were used in this trial, as many patients received older chemotherapy regiments (ProMACE/CytaBOM). Patients in this trial received a mean of 7573 mg prednisone in the pamidronate group and 7527 mg prednisone in the placebo group. Prednisone doses are not directly comparable to prednisone doses in R-CHOP-like regiments. Westin et al conducted a single-center study of 74 newly diagnosed lymphoma patients who, on top of oral calcium and vitamin D, received IV zoledronic acid (ZOL) or placebo. The study showed that ZOL could stabilize BMD at the lumbar spine and femoral neck level after 12 months.17 However, 21 of 74 patients were not evaluable at 1-year follow-up due to study dropout for financial or personal reasons.
Our results are consistent with these observations as the present study showed a clinically meaningful effect of oral ALN. The explanation for the lack of consistent results between BMD at the lumbar spine level and the total hip or femoral neck level is likely that bone loss occurs more rapidly in trabecular bone such as the lumbar spine compared with the cortical bone of the hip and femoral neck.18
Kim et al identified 6 new fractures,16 but no fractures were identified in the trial by Westin et al17 where fractures were detected clinically and not by imaging. As prednisone doses in the trial by Kim et al are very high, the risk of fractures in the placebo group is not directly comparable with the fracture risk for patients receiving R-CHOP/R-CVP. Increased risk of fractures has been found in observational studies of GIO in lymphoma patients,10,12,19,20 but causality cannot be established in this setting since cancer patients may have increased fragility and treatment-related factors such as loss of muscle mass, peripheral neuropathy, and general weakness, which may lead to fractures independent of bone health. Possible explanations for the low fracture rate in our study are the short study duration of only 12 months and that patients enrolled in clinical trials are typically healthier and often have better performance; in addition, more fractures are expected for the elderly and women.21 A British study by Booth et al based on 877 patients age ≥70 years with a follow-up of 18 months showed a fracture rate of 11.4% in patients receiving R-CHOP.20 In the present study, the median age was 67 years, and only 25.4% were women (median age for women in the study was 67 years). These demographic differences may at least partially explain the differences in fracture rate observed across studies. This is supported by the fact that the analyses of BMD by sex, although not significant, show that the difference in ΔTEOS between the ALN and placebo groups was larger among females, despite the fact that only 15 women, all postmenopausal, were recruited to the present study. Efficacy results by gender were not provided in the previously mentioned randomized trials by Kim et al or Westin et al.16,17
The 2 treatment arms were generally well balanced with respect to baseline clinical characteristics, including sex, although patients in the ALN arm had a lower baseline T-score at lumbar spine level (median T-score, −0.6 vs 1.0 for placebo arm). However, the effect of ALN remained significant after adjusting for baseline T-scores (difference in δ T-score, −0.26; P = .036) in multivariable linear regression.
Most publications showing increased risk of fractures for lymphoma patients have been performed for lymphoma patients treated with standard lymphoma treatment.12,19,20 Although detailed information on prednisone dosing, including actual dose, was not available in these retrospective evaluations, it is likely that the patients in those studies received approximately the same dose of prednisone as in the present study. The fact that only 1 vertebral compression fracture was detected in the present study of 59 patients with only 12 months follow-up does not necessarily provide evidence of clinically limited/irrelevant fracture risk. The low fracture rate may be a type 2 error, as the baseline fracture risk in this relatively young, predominantly male study population may have been minimal. The aspect of prevention is a relevant issue for further exploration in this setting. However, with an observed difference in T-score of the lumbar spine of 0.27 between ALN and placebo, the expected effect on relative fracture risk compared with placebo would be 0.80 for spine fractures and 0.90 for any fracture according to a previous meta-analysis.22 Given that ALN is a simple, inexpensive, and well-tolerated treatment, we believe that fracture risk reduction expected from this effect on BMD is clinically meaningful.
ALN is a widely used drug with a well-established safety profile. AEs reported in the present study are likely more reflective of the side effects related to (immuno)-chemotherapy. Only a few AEs were assessed as being related to the study medicine, but there were no differences for these AEs between the ALN arm and the placebo arm.
High doses of glucocorticoids undoubtedly lead to GIO.23 The isolated effect of chemotherapy on the risk of developing osteoporosis is generally considered limited.24,25 In the context of lymphoma treatment, where glucocorticoid-containing chemotherapy regimens are often used, the question of prevention of GIO is relevant, but for many other diseases where glucocorticoid pulse-treatments or short-term high-dose glucocorticoid treatments are given, the aspect of preventing GIO is also a relevant issue. BMD measurements are rarely performed as a part of standard lymphoma assessment before starting treatment, but probably should be.
The study was designed as an intention-to-treat with an expected balanced dropout rate of 30% in each treatment arm. The observed dropout rates were 27% in the ALN and 24% in the placebo arm. The patients discontinuing study treatment were encouraged to accept the performance of remaining DXA scans, which was done for 2 of 14 patients. As missing data are considered unrelated to the treatment effects and as the number of patients discontinuing study treatment are equally distributed in the 2 treatment groups, the risk of bias due to missing data are considered low.
The number of patients in the study was relatively low, making the trial underpowered for secondary analysis. The primary endpoint was reached, and despite nonsignificant results for the secondary endpoints, the positive effect on T-score for the lumbar spine at 12 months is supported by the biomarker analysis indicating reduced bone resorption.
In conclusion, oral weekly ALN appears to be an effective and safe prophylaxis against loss in BMD for lymphoma patients treated with glucocorticoid-containing chemotherapy regimens. The beneficial effect appears to be stronger in female patients. Biomarker analyses supported a biological effect of ALN in this patient setting. Larger trials with longer follow-up are needed to determine if ALN improve patient outcome by lowering the risk of fractures.
Acknowledgments
The authors would like to thank Liza Kristensen, Rikke Bûlow Eschen at the Clinical Trial Unit, Department of Hematology, Aalborg University Hospital.
Financial support for this study was provided by The Obel Family Foundation (https://obel.com/home/) (to P.J.). The study was an investigator-initiated study, and the institution funding had no direct role in study design, patient recruitment, data collection, analysis, interpretation, or writing.
Authorship
Contribution: P.J., T.C.E.-G., M.B., M.T.S., and P.V. contributed to conception and design of the study; P.J. performed the management and coordination responsibilities; L.H.J., M.B., T.C.E.-G., and P.J. performed analysis of data; P.J. drafted the manuscript; and all authors contributed to acquisition of data and interpretation of the results, revised the manuscript, and read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests in relation to the present study. T.C.E.-G. was previously employed by Roche Ltd (Basel) (2019-2021) and has received a speakers fee from Abbvie (2021) and received research funding from the Danish Cancer Society (not related to this study). L.H.J. received honoraria from Takeda (2019) and Roche (2021). P.V. has received speakers fees from MSD, Amgen, Servier, and Novartis and is an investigator for Kyowa-Kirin.
Correspondence: Paw Jensen, Clinical Cancer Research Center, Aalborg University Hospital, Medicinerhuset, Mølleparkvej 4, Aalborg, 9000 Denmark; e-mail: paje@rn.dk.
References
Author notes
Protocol and data will be shared with qualified researchers, provided that relevant permissions can be obtained from the Danish authorities. Requests for data sharing may be submitted to Paw Jensen (paje@rn.dk).
The full-text version of this article contains a data supplement.