Key Points
PV is effective in patients with LBCL who relapse or progress after anti-CD19 CAR T-cell therapy.
Duration of response to PV after CAR T-cell therapy is short, except for patients with low tumor burden disease.
Abstract
Polatuzumab vedotin (PV) is an antibody–drug conjugate targeting CD79b that is approved for patients with relapsed/refractory large B-cell lymphoma (LBCL). Patients who relapse after chimeric antigen receptor (CAR) T-cell therapy were not included in the registration study, and reports of PV use after CAR T cells are limited. This multicenter retrospective analysis included patients with LBCL who relapsed or progressed after CAR T-cell therapy and subsequently received PV with or without rituximab and bendamustine between July 2019 and May 2021. Response to treatment and progression were assessed based on the 2014 Lugano criteria. Fifty-seven patients were included in the study: 18 (32%) patients were primary refractory to CAR T-cell therapy, and 34 (60%) patients received PV-based therapy immediately after CAR T-cell therapy. PV was combined with rituximab in 54 (95%) patients and administered with bendamustine in 35 (61%) patients. A response was achieved in 25 (44%) patients, including complete remission in 8 (14%). No significant association between baseline characteristics and response was observed. After a median follow-up of 47 weeks (95% confidence interval [CI], 40-54), 46 (81%) patients had disease progression or died, and the median progression-free survival was 10 weeks (95% CI, 5-15). On a multivariate analysis, bone marrow involvement (hazard ratio, 5.2; 95% CI, 1.8-15; P = .003) and elevated lactate dehydrogenase levels (hazard ratio, 5.0; 95% CI, 1.4-16; P = .01) were associated with shorter progression-free survival. Studies aimed at better characterizing the intrinsic mechanism of resistance and identifying optimal consolidation strategies for these patients are warranted.
Introduction
Autologous chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has represented a paradigm shift in the management of patients with relapsed or refractory large B-cell lymphoma (LBCL), with an increase in cure rate from 10% to 40%.1-7 Outcomes for patients who relapse after CAR T-cell therapy are poor, and use of salvage treatments is frequently limited by persistent severe cytopenia.8,9
CD19 downregulation represents a common mechanism of resistance in these patients. Alternatively spliced exons or CD19 mutations are frequently described in acute lymphoblastic leukemia but not in lymphoma. In the latter, selective survival advantage of tumor cells with low CD19 expression is the most common pattern of immune escape.10-14 Notably, other B-cell lineage markers, including CD79b, are preserved, supporting its targeting as a therapeutic strategy for patients who relapse or progress after anti-CD19 CAR T-cell therapy.12
Polatuzumab vedotin (PV) is an antibody–drug conjugate composed of a monoclonal antibody against CD79b covalently conjugated to the antimitotic cytotoxic agent monomethyl auristatin. In a phase 1 study, 1.8 mg/kg every 3 weeks was identified as the recommended phase 2 dose, and the most common grade 3 to 4 adverse events were neutropenia (40%), anemia (11%), and peripheral sensory neuropathy (9%).15 The efficacy of PV in combination with rituximab has been investigated in a phase 2 study (ROMULUS), including 39 patients with relapsed or refractory LBCL showing an objective response of 54% and a complete response (CR) rate of 21%.16 More recently, the safety and efficacy of PV in combination with bendamustine and rituximab (BR) were investigated in a phase 2 randomized study (GO29365), including 80 patients with relapsed or refractory LBCL. PV plus BR yielded higher CR rates (40% vs 18%) and prolonged median progression-free survival (PFS) (9.5 vs 3.7 months) compared with BR.17 These findings led to accelerated US Food and Drug Administration (FDA) approval of this combination in patients who have received at least 2 previous lines of systemic therapy. However, patients who relapsed or progressed after CAR T-cell therapy were not included in the registration study, and reports of PV use after CAR T cells in the real world are limited.
Methods
Patient selection
This is a multicenter retrospective study of patients with LBCL relapsed or refractory after autologous anti-CD19 CAR T-cell therapy and treated with standard-of-care PV at participating sites between July 2019 and May 2021. Standard of care was defined as administration of a commercial product outside of a clinical trial. Data cutoff was July 31, 2021. Patients received PV 1.8 mg/kg intravenously every 3 weeks, dose-reduced according to toxicity, and variably combined with bendamustine or rituximab, as previously described.17 The study was approved by the institutional review board of all participating sites and was conducted in accordance with all sites' institutional guidelines and the principles of the Declaration of Helsinki.
Baseline characteristics and response assessment
The clinical characteristics and laboratory features before initiation of PV were confirmed by review of medical records. Performance status was defined according to the Eastern Cooperative Oncology Group.18 The International Prognostic Index was calculated as previously described.19 Best response was determined according to the 2014 Lugano classification and collected per clinical records.20 Either immunohistochemistry or flow cytometry was used for assessment of CD19 and CD79b status on available tissue biopsy specimens collected before initiation of PV.
Statistical methods
Association between categorical variables was evaluated by using χ2 tests or Fisher’s exact tests. The difference in a continuous variable between patient groups was evaluated by using the Mann-Whitney U test. PFS was defined as the time from the start of PV to progression of disease, death, or last follow-up (whichever occurred first). Overall survival (OS) was defined as the time from the start of PV to death from any cause or last follow-up. PFS and OS were calculated for all patients in the study and for subgroups of patients according to Kaplan-Meier estimates, and they were compared between subgroups by using the log-rank test. Multivariable Cox regression analysis was performed to assess the associations between patient characteristics and PFS or OS. A P value ≤ .05 (two-tailed) was considered statistically significant. Statistical analyses were completed by using SPSS version 24 (IBM SPSS Statistics, IBM Corporation) and GraphPad Prism 8 (GraphPad Software).
Results
Baseline characteristics and treatment
Across all sites, 348 patients with relapsed or refractory LBCL received standard-of-care CAR T-cell therapy over the course of 2 years. Of these, 186 (53%) relapsed or progressed, and 57 (30%) of 186 received a polatuzumab-based regimen outside of a clinical trial. These 57 patients were included in the study, and baseline characteristics at time of PV initiation are shown in Table 1. No patients had received PV before CAR T-cell therapy. Their median age was 60 years (range, 22-79 years), and 33 patients (58%) had an International Prognostic Index score ≥3. Eastern Cooperative Oncology Group performance status was 0 in 4 (8%), 1 in 37 (70%), 2 in 10 (18%), and 3 to 4 in 2 (4%) of 53 patients. Seventeen (30%) patients previously had autologous stem cell transplant (SCT) and 2 (4%) had allogeneic SCT. Eighteen (32%) patients were primary refractory to CAR T-cell therapy (defined as relapse or progression within 90 days), and median time from CAR T-cell therapy to PV was 5 months (range, 1-40 months). CD19 status at the time of relapse after CAR T-cell therapy relapse was assessed in 43 patients and was positive in 36 (84%); CD79b status was assessed in 14 patients and was positive in all cases (100%).
Baseline characteristics (before initiation of PV)
| Total (N = 57) . | Value . |
|---|---|
| Age, y | 60 [22-79] |
| Age >60 y | 28 (49) |
| Male | 40 (70) |
| ECOG performance status 3-4 | 2/53 (4) |
| DLBCL/HGBCL* | 44 (77) |
| Ann Arbor stage III-IV | 53 (93) |
| International Prognostic Index score 3-5 | 33/48 (69) |
| Bone marrow involvement | 6/46 (13) |
| Prior CNS lymphoma | 3/57 (5) |
| Previous treatment | |
| Systemic therapies before CAR T-cell therapy | 2 [2-6] |
| >2 Lines before CAR T-cell therapy | 25 (44) |
| Previous autologous SCT | 17 (30) |
| Previous allogeneic SCT | 2 (4) |
| Systemic therapies after CAR T-cell therapy | 0 [0-5] |
| ≥1 Line after CAR T-cell therapy | 23 (40) |
| Primary refractory to CAR T-cell therapy | 18 (32) |
| Time from CAR T-cell therapy to PV, mo | 5 [1-40] |
| Laboratory values | |
| Absolute neutrophil count, 109/L | 2.9 [0.5-19] |
| Hemoglobin, g/dL | 10.2 [6.5-16.3] |
| Platelet count, 109/L | 102 [15-437] |
| Grade 3-4 cytopenia | 20/57 (35%) |
| LDH above normal limit | 46/55 (84) |
| Bilirubin total, mg/dL | 0.5 [0.2-1.8] |
| Creatinine, mg/dL | 1 [0.4-2.2] |
| Biological markers | |
| CD19 positive | 36/43 (84) |
| CD79 positive | 14/14 (100) |
| Total (N = 57) . | Value . |
|---|---|
| Age, y | 60 [22-79] |
| Age >60 y | 28 (49) |
| Male | 40 (70) |
| ECOG performance status 3-4 | 2/53 (4) |
| DLBCL/HGBCL* | 44 (77) |
| Ann Arbor stage III-IV | 53 (93) |
| International Prognostic Index score 3-5 | 33/48 (69) |
| Bone marrow involvement | 6/46 (13) |
| Prior CNS lymphoma | 3/57 (5) |
| Previous treatment | |
| Systemic therapies before CAR T-cell therapy | 2 [2-6] |
| >2 Lines before CAR T-cell therapy | 25 (44) |
| Previous autologous SCT | 17 (30) |
| Previous allogeneic SCT | 2 (4) |
| Systemic therapies after CAR T-cell therapy | 0 [0-5] |
| ≥1 Line after CAR T-cell therapy | 23 (40) |
| Primary refractory to CAR T-cell therapy | 18 (32) |
| Time from CAR T-cell therapy to PV, mo | 5 [1-40] |
| Laboratory values | |
| Absolute neutrophil count, 109/L | 2.9 [0.5-19] |
| Hemoglobin, g/dL | 10.2 [6.5-16.3] |
| Platelet count, 109/L | 102 [15-437] |
| Grade 3-4 cytopenia | 20/57 (35%) |
| LDH above normal limit | 46/55 (84) |
| Bilirubin total, mg/dL | 0.5 [0.2-1.8] |
| Creatinine, mg/dL | 1 [0.4-2.2] |
| Biological markers | |
| CD19 positive | 36/43 (84) |
| CD79 positive | 14/14 (100) |
Data are presented as median [range], No. (%), or n/N (%).
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HGBCL, high-grade B-cell lymphoma; LDH, lactate dehydrogenase.
DLBCL/HGBCL vs primary mediastinal B-cell lymphoma and/or transformed follicular lymphoma.
Thirty-four (60%) patients received PV-based therapy immediately after CAR T-cell therapy, whereas 23 (40%) had intervening treatments (median, 1; range, 1-5); these included immunotherapy in 16 patients, chemotherapy in 7, radiotherapy in 5, anti-CD3/CD20 bispecific antibody in 4, cellular therapy in 4, tafasitamab in 2, and oral biological therapy in 2 patients. PV was given at the standard dose of 1.8 mg/kg intravenously every 3 weeks in all patients, except for 1 patient, who received 1.4 mg/kg. The median number of PV cycles was 2 (range, 1-16). Rituximab was omitted in 3 (5%) patients (owing to CD20 negativity in 2 patients and rituximab refractoriness in 1 patient); bendamustine was omitted in 22 (39%) patients (owing to cytopenia in 15 patients, chemo-refractoriness in 4, indolent clinical course in 1, wish to preserve CAR T-cell function in 1, and previous fungal infection in 1).
Response to PV and treatment discontinuation
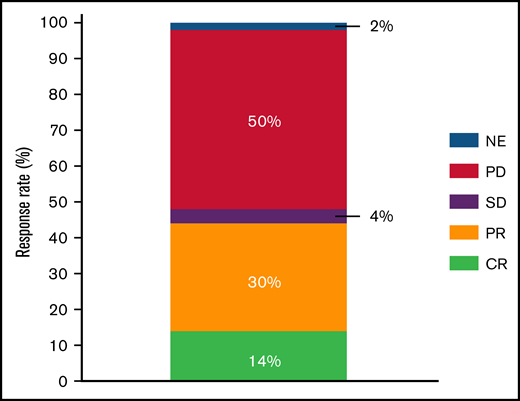
A response to PV-based therapy was achieved in 25 (44%) patients, including CR in 8 (14%) and partial response in 17 (30%) (Figure 1); median duration of response was 11 weeks (95% confidence interval [CI], 5-17) and was not reached for patients in CR. No significant association between the baseline characteristics shown in Table 1 and response was observed. There was also no significant association between use of bendamustine in combination with PV and response (38% vs 55%; P = .28). To date, 52 (91%) patients stopped PV: 40 (70%) due to disease progression, 7 (13%) because of CR/patient decision, 3 (6%) to proceed to allogeneic SCT, 1 (2%) to proceed to an immunotherapy clinical trial (despite absence of progression), and 1 (2%) because of toxicity (peripheral neuropathy).
Response to PV after CAR T-cell therapy in LBCL. NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Response to PV after CAR T-cell therapy in LBCL. NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Survival after PV and associated factors
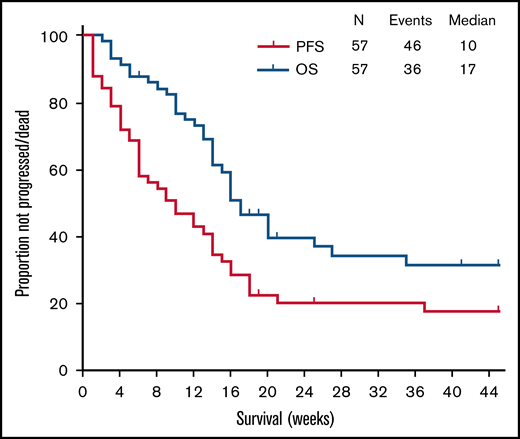
After a median follow-up of 47 weeks (95% CI, 40-54), 46 (81%) patients had disease progression or died, and the median PFS was 10 weeks (95% CI, 5-15). To date, 36 (63%) died, and median OS was 17 weeks (95% CI, 14-21) (Figure 2). Causes of death included progression in 33 patients and transplant-related complications in 3. The latter included 1 patient who underwent allogeneic SCT received as consolidation of response to PV, and 2 patients who received it as consolidation of responses achieved with lines of therapy subsequent to PV. Of the remaining 2 patients who received allogeneic SCT immediately after PV, one died of PD and one is still alive. On univariate analysis including all categorical variables from Table 1, a shorter median PFS was observed for patients with bone marrow (BM) involvement (3 vs 12 weeks; P = .001), prior central nervous system involvement (4 vs 10 weeks; P = .01), and elevated lactate dehydrogenase (LDH) levels (6 weeks vs not reached; P = .001). On multivariate analysis, the association was maintained only for BM involvement (hazard ratio, 5.2; 95% CI, 1.8-15; P = .003) and elevated LDH levels (hazard ratio, 5.0; 1.4-16; P = .01) (Table 2; Figure 3).
Multivariate analysis of factors associated with PFS
| Patients (N = 54) . | Median PFS (wk) . | P, univariate . | HR . | 95% CI . | P, multivariate . |
|---|---|---|---|---|---|
| BM involvement | 3 | .001 | 5.2 | 1.8-15 | .003 |
| No BM involvement | 12 | ||||
| Prior CNS involvement | 4 | .01 | – | – | .23 |
| No prior CNS involvement | 10 | ||||
| Elevated LDH | 6 | .001 | 5 | 1.4-16 | .01 |
| No elevated LDH | NR |
| Patients (N = 54) . | Median PFS (wk) . | P, univariate . | HR . | 95% CI . | P, multivariate . |
|---|---|---|---|---|---|
| BM involvement | 3 | .001 | 5.2 | 1.8-15 | .003 |
| No BM involvement | 12 | ||||
| Prior CNS involvement | 4 | .01 | – | – | .23 |
| No prior CNS involvement | 10 | ||||
| Elevated LDH | 6 | .001 | 5 | 1.4-16 | .01 |
| No elevated LDH | NR |
CNS, central nervous system; HR, hazard ratio; LDH, lactate dehydrogenase.
Factors associated with PFS. LDH, lactate dehydrogenase; NR, not reached.
Discussion
We report the largest real-world experience of PV-based therapy for the treatment of patients with LBCL who relapse or progress after autologous anti-CD19 CAR T-cell therapy. A German multicenter retrospective study previously reported the outcomes of 12 patients treated with standard-of-care PV after autologous anti-CD19 CAR T-cell therapy.21
In the current study, the use of PV after CAR T-cell therapy was safe, with only 1 patient discontinuing therapy due to peripheral sensory neuropathy. The latter has been reported as one of the most common severe adverse events in previous phase 1 and 2 studies investigating the safety and efficacy of PV, either as a single agent or in combination with rituximab or BR.15-17,21 Of interest, cytopenias, the most common severe toxicity associated with the use of PV, did not result in treatment discontinuation, despite severe and persistent myelosuppression being observed in about one-third of patients treated with CAR T-cell therapy.9
In this analysis, 44% of patients responded to PV after CAR T-cell failure, similar to what was reported in the registration study, despite exclusion from the latter study of patients who previously received CAR T-cell therapy.17 No efficacy data for the treatment of patients with LBCL who relapse after CAR T-cell therapy are currently available for tafasitamab or selinexor, also approved by the FDA for patients with LBCL who relapse or progress after 2 lines of systemic therapy.22,23 Limited data are available in this setting for loncastuximab tesirine, with a response rate of 46% in 13 patients who experienced relapse or progression after CAR T-cell therapy, similar to that reported for PV in our analysis.24
Despite a high response rate, the duration of response to PV was very limited, likely due to a low CR rate, comparing unfavorably to what was reported in patients not previously exposed to CAR T-cell therapy. In fact, in the registration study, 64% of patients with an initial response had an ongoing response at 6 months and 48% at 12 months.17,25 Although real-world data have shown less durable remissions for these patients, our data suggest that patients who achieve a response with PV after CAR T-cell therapy may be considered for consolidation with SCT; safety for the latter in this setting needs to be further investigated, however, and PV cost taken into consideration.26-28
Finally, in our study, patients with high tumor burden before PV initiation, indicated by elevated serum LDH levels and BM involvement, experienced the shortest duration of response, as already described for patients with LBCL treated with chemoimmunotherapy or CAR T-cell therapy.29-31 Although CD79b expression levels according to chromogenic immunohistochemistry did not correlate with quality of response to PV in the registration study,17 preclinical data have shown that upregulation of BCL-2 alternative proteins may be responsible for primary or acquired resistance to this agent.32 Further investigation of this pathway may result in novel combinations and improved efficacy for PV-based regimens in patients with LBCL who relapse or progress after CAR T-cell therapy.
We acknowledge the multiple limitations of the current study, including its retrospective nature, its relatively small sample size, and the lack of consistent collection of tissue biopsy specimens before PV initiation to characterize mechanism of refractoriness to PV. It is also important to acknowledge that, although it is not possible to retrospectively determine which factors favored PV selection for the treatment of patients included in this study, it is possible to speculate that patients not eligible for clinical trials (which is the preferred treatment for patients with LBCL who relapse after CAR T-cell therapy) and those with CD19 downregulation were more likely to be treated with a commercial product targeting CD79b.
In summary, PV is effective but has a short duration of response for patients with low tumor burden LBCL who relapse or progress after CAR T-cell therapy. Patients not eligible for clinical trials and those with CD19 downregulation may benefit from its selection over other FDA-approved agents with a similar indication. Although its incorporation into frontline regimens may limit its use in the future as salvage therapy,33 further investigation of intrinsic mechanisms of resistance and of the safety and efficacy of potential consolidation strategies, including SCT, for patients who achieve a response is warranted.
Acknowledgments
This research was supported in part by The University of Texas Anderson Cancer Center Support Grant from the National Institutes of Health, National Cancer Institute (P30 CA016672), and by the Lymphoma Research Foundation Clinical Research Mentoring Program. The salary of P.S. is supported by the Lymphoma Research Foundation Career Development Award and by the R21 NIH grant.
Authorship
Contribution: S.G. analyzed data and wrote the paper; A.C.R., J.L.C., A.I., M.K.K., B.H., S.S.N., A.K., Y.L., M.I., and R.W.M. provided clinical care to patients and coauthored the paper; G.W. and A.A. collected clinical data and coauthored the paper; L.F. provided statistical support and coauthored the paper; and P.S. designed the study, analyzed the data, provided clinical care to patients, and wrote the paper.
Conflict-of-interest disclosure: P.S. is a consultant for Roche-Genentech, Hutchison MediPharma, TG Therapeutics, and ADC Therapeutics; and received research funds from AstraZeneca-Acerta and ALX Oncology. A.I. is a consultant for AstraZeneca and TG Therapeutics. R.W.M. is a consultant for Genmab and Adaptive Biotechnologies; and received research funds from Bristol Myers Squibb, Merck, and Genentech/Roche. J.L.C. is a consultant for Incyte and Karyopharm; and received research funding from Bayer, AbbVie, Merck, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Paolo Strati, Department of Lymphoma and Myeloma, Department of Translational Molecular Pathology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: pstrati@mdanderson.org.
References
Author notes
S.G. and A.C.R. contributed equally to this study.
R.W.M. and P.S. contributed equally to this study.
Requests for data sharing may be submitted to Paolo Strati (pstrati@mdanderson.org).