Key Points
Cardiomyocyte and cardiac fibroblast PAR1 contribute to doxorubicin-induced cardiac injury.
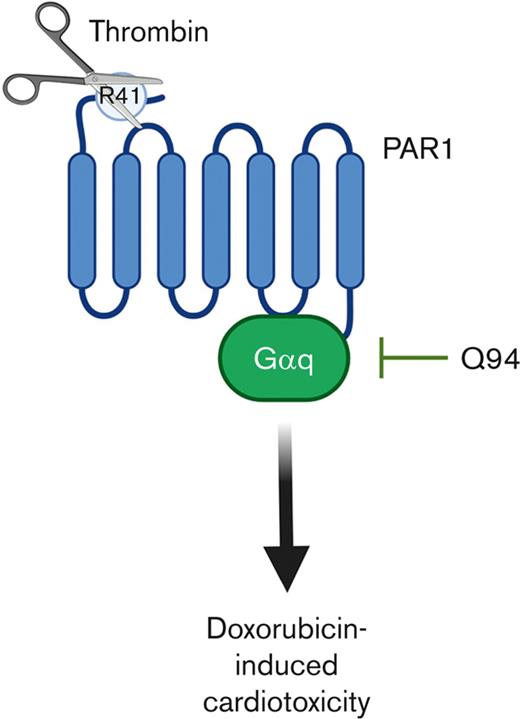
PAR1 cleavage at R41 confers pathologic Galphaq signaling during doxorubicin treatment leading to cardiac injury.
Abstract
The chemotherapeutic drug doxorubicin is cardiotoxic and can cause irreversible heart failure. In addition to being cardiotoxic, doxorubicin also induces the activation of coagulation. We determined the effect of thrombin-mediated activation of protease-activated receptor 1 (PAR1) on doxorubicin-induced cardiac injury. Administration of doxorubicin to mice resulted in a significant increase in plasma prothrombin fragment 1+2, thrombin-antithrombin complexes, and extracellular vesicle tissue factor activity. Doxorubicin-treated mice expressing low levels of tissue factor, but not factor XII-deficient mice, had reduced plasma thrombin-antithrombin complexes compared to controls. To evaluate the role of thrombin-mediated activation of PAR1, transgenic mice insensitive to thrombin (Par1R41Q) or activated protein C (Par1R46Q) were subjected to acute and chronic models of doxorubicin-induced cardiac injury and compared with Par1 wild-type (Par1+/+) and PAR1 deficient (Par1-/-) mice. Par1R41Q and Par1-/- mice, but not Par1R46Q mice, demonstrated similar reductions in the cardiac injury marker cardiac troponin I, preserved cardiac function, and reduced cardiac fibrosis compared to Par1+/+ controls after administration of doxorubicin. Furthermore, inhibition of Gαq signaling downstream of PAR1 with the small molecule inhibitor Q94 significantly preserved cardiac function in Par1+/+ mice, but not in Par1R41Q mice subjected to the acute model of cardiac injury when compared to vehicle controls. In addition, mice with PAR1 deleted in either cardiomyocytes or cardiac fibroblasts demonstrated reduced cardiac injury compared to controls. Taken together, these data suggest that thrombin-mediated activation of PAR1 contributes to doxorubicin-induced cardiac injury.
Introduction
Doxorubicin, an anthracycline antibiotic-based chemotherapeutic agent, is effective in the treatment of a wide range of malignancies, including breast cancer, lymphoma, leukemia, and sarcomas. However, doxorubicin treatment has been associated with a high incidence of dose-limiting cardiovascular toxicities that can manifest acutely or years after treatment.1 Common cardiotoxicities include left ventricular dysfunction, myocardial ischemia, conduction disturbances, and arrhythmias.2,3 These cardiotoxicities lead to irreversible congestive heart failure in roughly a quarter of patients receiving the maximum recommended cumulative dose of doxorubicin.2,3 Despite updated regimens designed to reduce cardiac injury, doxorubicin-induced cardiotoxicity is still prevalent, and its prevention and management remain a major challenge for both cardiologists and oncologists.4
Doxorubicin-induced cardiotoxicity occurs through complex and multifactorial mechanisms.5 Oxidative stress due to mitochondrial dysfunction has been linked to cardiomyocyte injury and death.5,6 Other mechanisms include genomic instability due to topoisomerase inhibition, iron overload, myocardial atrophy, and nonapoptotic cell death.5,7-9
Doxorubicin administration also leads to activation of coagulation.5 Plasma levels of thrombin-antithrombin (TAT) complexes were found to be significantly elevated in patients with breast cancer treated with doxorubicin containing anticancer regimes.10 Similarly, in preclinical studies doxorubicin increased plasma levels of TAT complexes in mice.11,12 Doxorubicin has also been found to increase endothelial cell surface tissue factor (TF) activity in vitro.13 Further, the related anthracycline daunorubicin increased release of the TF-positive extracellular vesicles (EV) from human monocytic cells.14 In addition, doxorubicin has been shown to increase the release of the intrinsic pathway activator cell-free DNA (cfDNA) from neutrophils.11 However, the relative contribution of these different procoagulant pathways to doxorubicin-induced thrombin generation in vivo has not been determined.
We previously demonstrated that activation of the G-protein coupled receptor protease-activated receptor 1 (PAR1) contributed to doxorubicin-induced cardiac injury in mice.6 Several proteases, including thrombin, activated protein C (APC), matrix metalloproteinase (MMP) 1 and 13, can activate PAR1 through cleavage at the N terminus that generates different tethered ligand agonists.15
Critically, activation of PAR1 can occur in a biased manner with cleavage by thrombin at Arg41 leading to proinflammatory Gαq and Gα12 dependent biased signaling.16,17 In contrast, cleavage by APC at Arg46 leads to cytoprotective β-arrestin-2 dependent biased signaling.18,19 Importantly, the generation of transgenic cleavage site mutant mice has allowed the relative contribution of thrombin and APC mediated activation of PAR1 to be evaluated in vivo.20
Here, we performed a detailed characterization of the cellular sources of PAR1 that contribute to doxorubicin induced cardiac injury, the mechanisms of doxorubicin-induced thrombin generation and the consequence of thrombin mediated activation of PAR1 on doxorubicin-induced cardiac injury. We first evaluated the cellular source of PAR1 that contributes to doxorubicin-induced cardiac injury using cell type–specific PAR1 knockout mice. Next, we explored the mechanism by which doxorubicin induces thrombin generation in vivo. The relative contribution of thrombin and APC-mediated activation of PAR1 to doxorubicin-induced cardiac injury was then evaluated using transgenic cleavage site mutant PAR1 mice. Finally, we explored the effect of inhibiting cytotoxic PAR1 mediated Gαq signaling on doxorubicin-induced cardiac injury.
Methods
Mice
All procedures were approved by the University of North Carolina (UNC) at the Chapel Hill Institutional Animal Care and Use Committee and complied with the National Institutes of Health (NIH) guidelines. Mice deficient in murine TF expressing low levels of human TF under the control of the human TF promoter (mTF-/-;hTF+/+ [TFlow]) and wild-type (wt) controls (mTF+/+;hTF+/+ [TFwt]) maintained on a C57Bl6J background were used.21F12-/- and wild-type F12+/+ mice maintained on a C57Bl6J background were obtained from David Gailani (Vanderbilt University, Nashville, TN). Several PAR1 transgenic mice with manipulations of the factor 2 receptor (F2r) gene (referred to as Par1) were used. Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice were maintained on a C57BL/6J background.20,22Par1fl/fl mice were generated as previously described and crossed with mice expressing cre recombinase under the control of the cardiomyocyte specific myosin light chain 2v promoter (Mlc2vcre).23,24 Cardiomyocyte specific PAR1 deficient mice (Par1fl/fl;Mlc2vcre) and littermate controls (Par1fl/fl) were generated. In addition, Par1fl/fl mice were crossed with mice expressing cre recombinase under the control of the cardiac fibroblast specific tamoxifen inducible transcription factor 21 promoter (Tcf21creERT2).24 Cardiac fibroblast PAR1deficient mice (Par1fl/fl;Tcf21creERT2) and littermate controls (Par1fl/fl) were generated. To induce cre recombinase expression in an appropriately controlled manner Par1fl/fl;Tcf21creERT2 and Par1fl/fl mice were gavaged with tamoxifen (2 mg/mouse, Sigma-Aldrich, St. Louis, MO) for 5 days. The genotypes of the experimental mice were determined by standard polymerase chain reaction analysis.
Doxorubicin-induced cardiac injury models
Male and female mice (12-16 week of age) were subjected to acute or chronic models of doxorubicin-induced cardiac injury. In the acute model, mice were administered a single dose of doxorubicin (Fresenius Kabi, Bad Homburg, Germany) by intraperitoneal injection at a dose of 20 mg/kg, and cardiac function was assessed at day 5 after administration. In the chronic model, mice were administered doxorubicin at a dose of 5 mg/kg weekly for 5 weeks (25 mg/kg cumulative dose) and cardiac function was assessed on day 25. Cohorts of mice subject to the acute model were administered the selective PAR1 Gαq antagonist Q94 (Axon Medchem, Reston, VA) by twice daily intraperitoneal injection at a dose of 5 μg/g starting at the time of doxorubicin administration.
Echocardiography
Cardiac function was assessed by echocardiography of conscious mice using a Vevo 2100 ultrahigh frequency ultrasound system (VisualSonics, Ontario, Canada). Left ventricle function (LV) was assessed by obtaining M-mode echocardiographic traces in the short-axis view at the midventricular level. LV fractional shortening (FS) was determined by measuring the percentage reduction in ventricular lumen diameter between end-diastole and end-systole ([end-diastolic diameter – end-systolic diameter]/end-diastolic diameter × 100 [%]). Echocardiographic recordings and analyses were performed in a blinded manner.
Blood and tissue collection
In the acute model, blood was collected on days 1, 2, and 5 after doxorubicin administration. In the chronic model, blood and heart samples were collected on day 35 after the initiation of doxorubicin administration. To collect blood, 200 μL of sodium citrate (3.8%, Ricca, Arlington, TX) was injected into the inferior vena cava to prevent activation of coagulation, and 500 μL of anticoagulated blood was collected. Plasma was generated by centrifugation of whole blood at 4500 g for 15 min at room temperature. Plasma was aliquoted and stored at −80°C. Hearts were removed and fixed in formalin (10%, VWR, Radnor, PA) overnight and transferred to 70% ethanol for histological processing.
Plasma assays
Plasma levels of Prothrombin F1+2 (Biomatik, Ontario, Canada), TAT complexes (Enzygnost, Siemens Healthcare, Marburg, Germany), and cardiac troponin I (cTnI, Life Diagnostics, West Chester, PA) were determined using commercial assays according to the manufacturers’ instructions. Plasma levels of EV-TF were determined using an in-house assay as previously described.25 Plasma levels of cfDNA were measured using a Quant-iT Picrogreen double stranded DNA assay kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions.
Histology analysis
Formalin fixed paraffin embedded heart sections were stained with picrosirius red. Slides were imaged using a ScanScope AT2 (Leica, Wetzlar, Germany). The collagen positive percentage area was determined using the Color Pixel Counter plugin in Image J analysis software (v2.3.0; NIH, Bethesda, MD).
Statistical analysis
The normality of the data was assessed by Shapiro-Wilk tests, and parametric or nonparametric tests were selected as appropriate. For pairwise comparisons parametric Student’s t tests or nonparametric Mann-Whitney U tests were used. For multiple group tests, parametric two-way analysis of variance tests with a post hoc Holm-Sidak test were used. Data were analyzed using the Prism software (v9.3.1; GraphPad, San Diego, CA).
Results
Cardiomyocyte and cardiac fibroblast PAR1 both contribute to doxorubicin-induced cardiac injury
To evaluate the relative contribution of PAR1 expressed by either cardiomyocytes or cardiac fibroblasts to doxorubicin-induced cardiac injury cell type–specific PAR1 knockout mice were used. To selectively delete PAR1 from cardiomyocytes and fibroblasts, Par1fl/fl mice were crossed with Mlc2vcre or Tcf21creERT2 expressing mice. These cell type–specific PAR1 knockout mice were evaluated in an acute doxorubicin-induced cardiac injury model. In the acute model, mice were treated with a single bolus of doxorubicin (20 mg/kg), and cardiac injury was assessed using conscious echocardiography-based measurements of FS at day 5 after treatment. At baseline, no significant difference in FS was observed between Par1fl/fl;Mlc2vcre or Par1fl/fl control mice (Figure 1A). On day 5, however, Par1fl/fl;Mlc2vcre demonstrated significantly preserved FS compared to Par1fl/fl control mice (Figure 1A). At baseline, no significant difference in FS was observed between Par1fl/fl;Tcf21creERT2 and Par1fl/fl control mice (Figure 1B). At day 5, Par1fl/fl;Tcf21creERT2 had significantly preserved FS compared with Par1fl/fl control mice (Figure 1B). The observed protection against doxorubicin-induced cardiac injury was greater in cardiomyocyte specific PAR1 knockouts (78% protection in FS) than in cardiac fibroblast PAR1 knockouts (53% protection in FS). These results indicate that PAR1 expression in both cardiomyocytes and cardiac fibroblasts contributes to doxorubicin-induced cardiac injury.
Cardiomyocyte and cardiac fibroblast cell types specific knockout mice demonstrate reduced doxorubicin-induced cardiac injury. LV FS was assessed in (A) Par1fl/fl;Mlc2vcre+ cardiomyocyte-specific Par1 knockout mice and Par1fl/fl wild-type controls (n = 7-10/group per timepoint) or (B) Par1fl/fl;Tcf21creERT2+ cardiac fibroblast specific Par1 knockout mice and Par1fl/fl wild-type controls (n = 5-7/group per timepoint) at baseline and on day 5 in the acute model of doxorubicin-induced cardiac injury by conscious echocardiography. #P < .05 vs baseline of the respective genotype; ∗∗P < .01; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with the mean and SEM.
Cardiomyocyte and cardiac fibroblast cell types specific knockout mice demonstrate reduced doxorubicin-induced cardiac injury. LV FS was assessed in (A) Par1fl/fl;Mlc2vcre+ cardiomyocyte-specific Par1 knockout mice and Par1fl/fl wild-type controls (n = 7-10/group per timepoint) or (B) Par1fl/fl;Tcf21creERT2+ cardiac fibroblast specific Par1 knockout mice and Par1fl/fl wild-type controls (n = 5-7/group per timepoint) at baseline and on day 5 in the acute model of doxorubicin-induced cardiac injury by conscious echocardiography. #P < .05 vs baseline of the respective genotype; ∗∗P < .01; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with the mean and SEM.
Doxorubicin treatment induces TF-dependent thrombin generation in vivo
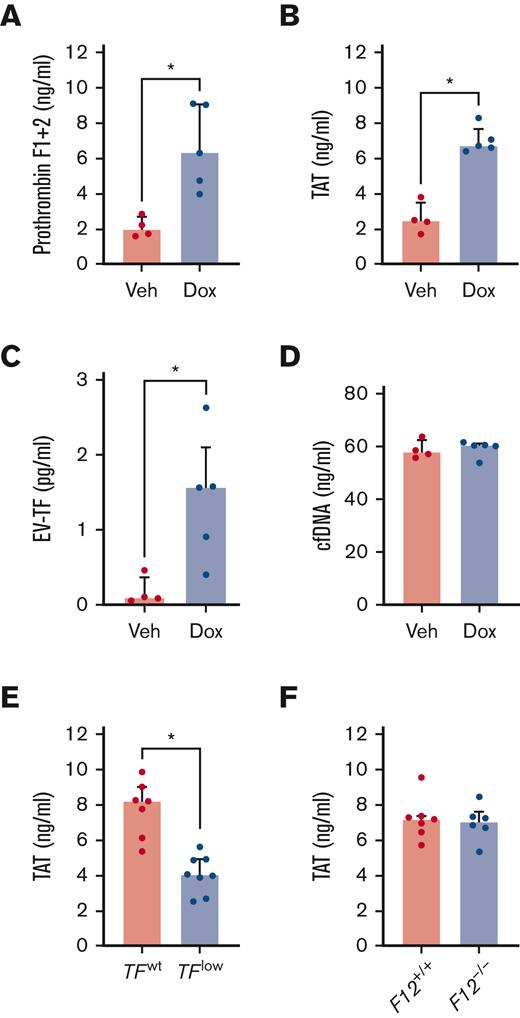
Mice treated with doxorubicin demonstrated an acute increase in plasma levels of prothrombin fragment 1+2 and TAT complexes compared with vehicle treated controls, which indicated systemic activation of coagulation (Figure 2A-B). Plasma levels of activators of the extrinsic and intrinsic pathways of coagulation were measured in mice treated with doxorubicin. Plasma EV-TF levels were significantly increased in mice treated with doxorubicin compared to those in the vehicle controls (Figure 2C). By contrast, no significant differences were observed in the plasma levels of cfDNA (Figure 2D). To determine the contribution of TF to the observed increases in doxorubicin-induced activation of coagulation, TAT complexes were measured in the plasma of low TF mice or controls treated with doxorubicin. Low TF mice treated with doxorubicin demonstrated significantly lower plasma levels of TAT complexes compared to wild-type controls (Figure 2E). Consistent with the lack of an effect of doxorubicin on cfDNA, no significant difference in plasma TAT complexes was observed between doxorubicin-treated F12-/- mice and wild-type controls (Figure 2F).
Doxorubicin-induced activation of coagulation in a tissue factor- dependent manner. Levels of markers of activation of coagulation (A) Prothrombin fragment 1+2 and (B) TAT complexes were measured in the plasma of wild-type mice (n = 4-5/group) 24 hours after administration of doxorubicin (Dox) or vehicle control (Veh). Levels of procoagulants, (C) extracellular vesicle tissue factor (EV-TF), and (D) cell-free DNA (cfDNA) were measured in the plasma of wild-type mice (n = 4-5/group) 24 hours after administration of doxorubicin or vehicle control. Levels of TAT were measured in the plasma of (E) TFlow and TFwt or (F) F12+/+ and F12-/- mice (n = 6-8/group) 24 hours after administration of doxorubicin (20 mg/kg). ∗P < .05 Mann-Whitney tests. Data are represented as individual values with the median and interquartile ranges.
Doxorubicin-induced activation of coagulation in a tissue factor- dependent manner. Levels of markers of activation of coagulation (A) Prothrombin fragment 1+2 and (B) TAT complexes were measured in the plasma of wild-type mice (n = 4-5/group) 24 hours after administration of doxorubicin (Dox) or vehicle control (Veh). Levels of procoagulants, (C) extracellular vesicle tissue factor (EV-TF), and (D) cell-free DNA (cfDNA) were measured in the plasma of wild-type mice (n = 4-5/group) 24 hours after administration of doxorubicin or vehicle control. Levels of TAT were measured in the plasma of (E) TFlow and TFwt or (F) F12+/+ and F12-/- mice (n = 6-8/group) 24 hours after administration of doxorubicin (20 mg/kg). ∗P < .05 Mann-Whitney tests. Data are represented as individual values with the median and interquartile ranges.
Activation of PAR1 at R41, but not R46, contributes to doxorubicin-induced cardiac injury
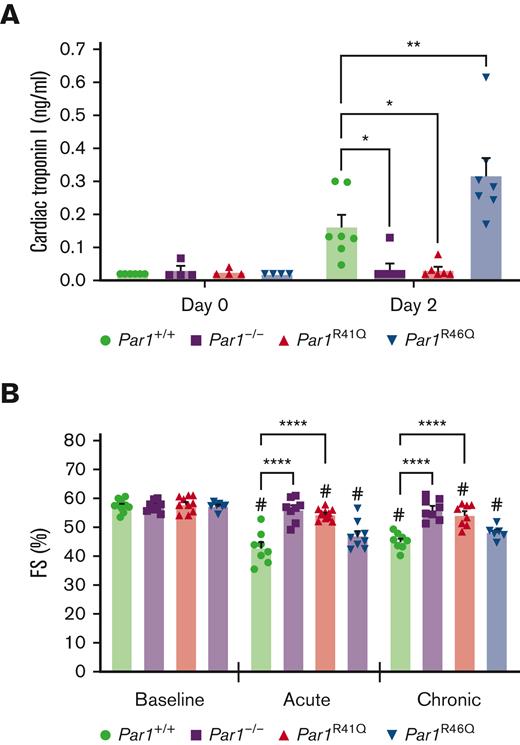
To determine the effect of loss of either thrombin or APC-mediated activation of PAR1 activation on doxorubicin-induced cardiac injury, Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice were subjected to acute and chronic models. In the acute model, mice were treated with a single bolus of doxorubicin (20 mg/kg) with cardiac injury assessed by measurements of plasma cTnI on day 2 post-treatment, and cardiac function assessed by conscious echocardiography-based measurements of FS on day 5 posttreatment. No significant difference in plasma levels of cTnI was observed between Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline (Figure 3A). At day 2 after treatment, Par1-/- and Par1R41Q mice had significantly reduced plasma levels of cTnI, whereas Par1R46Q demonstrated significantly increased plasma levels of cTnI compared with Par1+/+ controls (Figure 3A). No significant difference in FS was observed in Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline (Figure 3B-C). However, at day 5 after treatment, Par1-/- and Par1R41Q mice had significantly preserved FS compared with Par1+/+ controls (Figure 3B-C). No significant difference in FS was observed in PAR1R46Q mice when compared to Par1+/+ controls (Figure 3B-C).
Thrombin insensitive PAR1 transgenic mice demonstrate reduced doxorubicin-induced cardiac injury. (A) Plasma levels of cardiac troponin I were measured in Par1+/+, Par1-/-, Par1R41Q and Par1R46Q mice (n = 4-7/group) at baseline and 48 hours after administration of doxorubicin. (B) LV FS was assessed in Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline and on day 5 in acute and day 35 in the chronic models of doxorubicin-induced cardiac injury by conscious echocardiography (n = 6-11/group). #P < .05 vs baseline of the respective genotype; ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with mean and SEM.
Thrombin insensitive PAR1 transgenic mice demonstrate reduced doxorubicin-induced cardiac injury. (A) Plasma levels of cardiac troponin I were measured in Par1+/+, Par1-/-, Par1R41Q and Par1R46Q mice (n = 4-7/group) at baseline and 48 hours after administration of doxorubicin. (B) LV FS was assessed in Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline and on day 5 in acute and day 35 in the chronic models of doxorubicin-induced cardiac injury by conscious echocardiography (n = 6-11/group). #P < .05 vs baseline of the respective genotype; ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with mean and SEM.
In the chronic model, mice were treated once weekly with doxorubicin (5 mg/kg; 25 mg/kg, total), and cardiac function was assessed using conscious echocardiography-based measurements of FS at week 5 after treatment initiation. As observed in the acute model, Par1-/- and Par1R41Q mice had significantly preserved FS compared to Par1+/+ controls in the chronic model (Figure 3B-C). Again, no significant difference in FS was observed in PAR1R46Q mice when compared to Par1+/+ controls in the chronic model (Figure 3B-C).
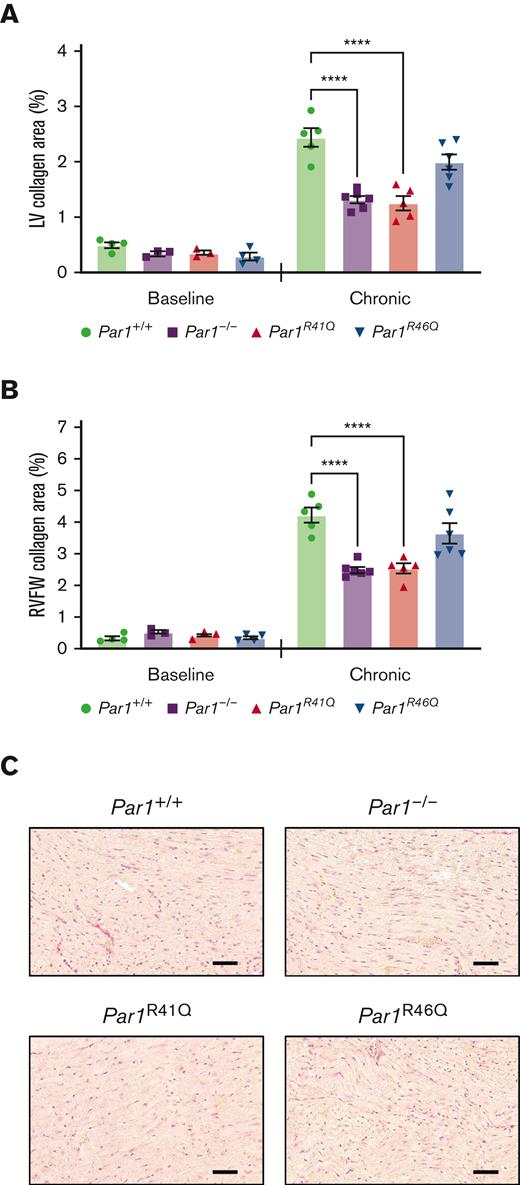
Cardiac fibrosis was evaluated histologically in the hearts of Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline or in mice subjected to a chronic model of doxorubicin-induced cardiac injury on day 35. Par1-/- and Par1R41Q mice had significantly reduced LV and right ventricle free wall (RVFW) collagen content compared with Par1+/+ controls (Figure 4A-C). No significant difference in LV or RVFW collagen content was observed between Par1R46Q and Par1+/+ controls (Figure 4A-C).
Thrombin insensitive PAR1 transgenic mice demonstrate reduced doxorubicin-induced cardiac fibrosis. Quantification of (A) LV and (B) RVFW, and collagen content from picrosirius red-stained heart sections from Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline and on day 35 in the chronic model of doxorubicin-induced cardiac injury (n = 3-6/group). (C) Representative micrographs of picrosirius red-stained heart sections from Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice on day 35 in the chronic model of doxorubicin-induced cardiac injury (Scale bars, 50μm). ∗∗∗∗P < .0001; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with the mean and SEM.
Thrombin insensitive PAR1 transgenic mice demonstrate reduced doxorubicin-induced cardiac fibrosis. Quantification of (A) LV and (B) RVFW, and collagen content from picrosirius red-stained heart sections from Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice at baseline and on day 35 in the chronic model of doxorubicin-induced cardiac injury (n = 3-6/group). (C) Representative micrographs of picrosirius red-stained heart sections from Par1+/+, Par1-/-, Par1R41Q, and Par1R46Q mice on day 35 in the chronic model of doxorubicin-induced cardiac injury (Scale bars, 50μm). ∗∗∗∗P < .0001; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with the mean and SEM.
Selective inhibition of PAR1 mediated Gαq signaling abrogates doxorubicin-induced cardiac injury
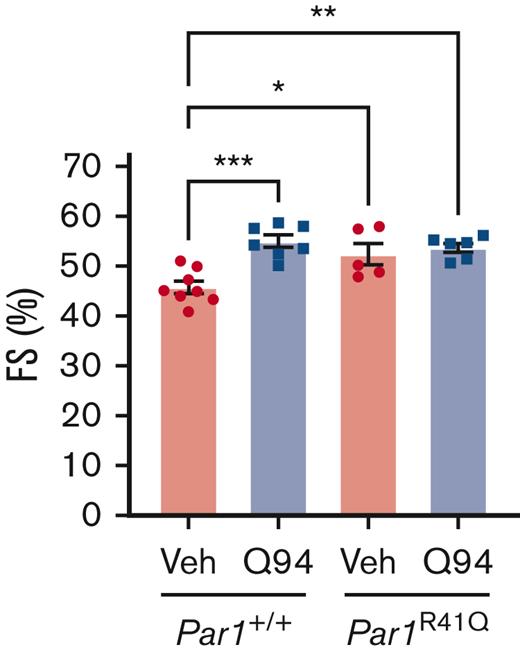
To determine the effect of intracellular signaling downstream of PAR1 activation on doxorubicin-induced cardiac injury, mice were treated with the selective PAR1 Gαq inhibitor Q94. Par1+/+ mice subjected to the acute model of doxorubicin-induced cardiac injury and treated with Q94 had significantly preserved FS on day 5 compared to vehicle treated controls (Figure 5). To evaluate the specificity of Q94 for PAR1, Par1-/- mice, subjected to an acute model of doxorubicin-induced cardiac injury, were treated with Q94. Surprisingly, all Par1-/- mice treated with Q94 (5/5), but none of the Par1-/- mice treated with vehicle (0/4), died or reached the humane end point criteria before day 5. As an alternative approach, the effects of Q94 on Par1R41Q mice were evaluated. Cardiac function was not significantly different in Par1R41Q mice treated with Q94 in the acute doxorubicin-induced cardiac injury model compared when compared to vehicle treated controls (Figure 5).
Inhibition of PAR1-mediated Gαq signaling reduces doxorubicin-induced cardiac injury. LV FS was assessed on day 5 in Par1+/+ mice and Par1R41Q mice treated with Q94 or Veh in an acute model of doxorubicin-induced cardiac injury by conscious echocardiography (n = 5-8/group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with mean and SEM. Veh, vehicle control.
Inhibition of PAR1-mediated Gαq signaling reduces doxorubicin-induced cardiac injury. LV FS was assessed on day 5 in Par1+/+ mice and Par1R41Q mice treated with Q94 or Veh in an acute model of doxorubicin-induced cardiac injury by conscious echocardiography (n = 5-8/group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; 2-way ANOVA with post hoc Holm-Sidak tests. Data are represented as individual values with mean and SEM. Veh, vehicle control.
Discussion
We have previously demonstrated that global PAR1 deficiency reduces doxorubicin-induced cardiac injury in vivo in mice.6 In addition, doxorubicin-induced decreases in cell viability and mitochondrial dysfunction were abrogated in cultured PAR1 deficient cardiomyocytes and cardiac fibroblasts.6 Although cardiomyocytes provide the heart with critical contractile function, evidence supports an important role for cardiac fibroblasts in cardiac physiology and pathology.26 We, therefore, sought to determine the effect of cardiomyocyte and cardiac fibroblast PAR1 on doxorubicin-induced cardiotoxicity in mice. Interestingly, both cardiomyocyte and cardiac fibroblast specific PAR1 deficient mice demonstrated preserved cardiac function in an acute model of doxorubicin-induced cardiac injury. The phenotype observed in cardiomyocyte specific PAR1 deficient mice was expected based on the observed phenotype in global PAR1 deficient mice and the key role of cardiomyocytes in cardiac function. The phenotype observed in cardiac fibroblast specific PAR1 deficient mice was somewhat unexpected but reinforces the important role of cardiac fibroblasts as modulators of cardiac function.26
To further investigate the contribution of PAR1 activation to doxorubicin-induced cardiac injury, we considered the ability of doxorubicin to generate the PAR1 activating protease thrombin. Mice administered doxorubicin demonstrated an acute increase in plasma levels of prothrombin fragment 1+2 and TAT complexes, indicative of enhanced in vivo thrombin generation. This finding is consistent with previous reports of elevated plasma TAT complexes in both acute and chronic models of doxorubicin.11,12
The extrinsic coagulation pathway initiator TF and intrinsic pathway activator cfDNA have been identified as 2 potential mediators of doxorubicin-induced thrombin generation.11,13,14 However, the relative contribution of these triggers to doxorubicin-induced thrombin generation remains unclear. In the present study, doxorubicin administration significantly increased plasma levels of EV-TF procoagulant activity but not plasma levels of cfDNA. The lack of increased cfDNA contrasts to findings of a previous report that demonstrated significantly increased plasma levels of cfDNA in mice treated with either doxorubicin or epirubicin.11 However, larger cfDNA complexes might adhere to the vasculature or be cleared out of the plasma during plasma preparation.27,28
To investigate the mechanism of doxorubicin-induced activation of coagulation more directly, the effects of TF or FXII deficiency on doxorubicin-induced thrombin generation were evaluated. Importantly, mice expressing low levels of TF, but not those with a complete deficiency of FXII, had reduced plasma TAT complexes. These phenotypes are consistent with the observed effects of doxorubicin on EV-TF and cfDNA in the present study. Furthermore, the data suggest that doxorubicin-induced release of EV-TF, but not cfDNA, is the major initiator of thrombin generation in vivo.
Several physiological activators of PAR1 have been identified, including thrombin, APC, and MMPs.15 These proteases activate PAR1 via proteolytic cleavage at distinct sites. Thrombin cleaves PAR1 at Arg41, APC cleaves at Arg46, MMP1 cleaves at Asp39 and MMP13 cleaves at Ser42.29 Signaling downstream of PAR1 has been found to vary depending on the activating protease leading to biased signaling.18 To study this biased agonism transgenic mice have been developed with mutated PAR1 cleavage sites for thrombin (Par1R41Q) and APC (Par1R46Q). Par1R41Q mice are considered to be insensitive to thrombin-mediated activation whereas Par1R46Q mice are considered to be insensitive to APC-mediated activation.20
In the acute model, both Par1R41Q and Par1-/- mice were protected to a similar extent from doxorubicin-induced increases in the plasma marker of cardiomyocyte injury cTnI compared with Par1+/+ controls. Moreover, in both acute and chronic models of doxorubicin-induced cardiac injury, Par1R41Q mice demonstrated an equivalent level of protection in cardiac function as Par1-/- mice, when compared to Par1+/+ controls. Therefore, loss of thrombin-mediated activation of PAR1 was protective against doxorubicin-induced cardiac injury in vivo. This further suggests that the activation of PAR1 by thrombin potentiates cardiac injury induced by doxorubicin.
Thrombin-mediated activation of PAR1 on fibroblasts has been found to induce release of inflammatory cytokines, including Gαq mediated induction of CCL2.30 CCL2 in addition to functioning as an important chemoattractant also induces expression of transforming growth factor β.31 Transforming growth factor β is an important inducer of collagen expression in cardiac fibroblasts.32,33 Moreover, cardiac PAR1 activation has been linked to cardiac fibroblasts activation and cardiac fibrosis in different heart failure models.34-37 Histological analysis revealed that hearts from Par1-/- and Par1R41Q mice had reduced fibrosis in the chronic model of doxorubicin-induced cardiac injury. This provides further evidence for the role of PAR1 in cardiac fibroblasts as a mediator of the pathological response to doxorubicin. Further, the reduced cardiac fibrosis observed in PAR1R41Q mouse hearts is consistent with the established proinflammatory effect of thrombin-mediated activation of PAR1 on cardiac fibroblasts.38
Beyond providing insights into the relative contribution of thrombin-mediated activation of PAR1 in the Par1-/- and Par1R41Q phenotypes, our results may provide further insights into PAR-mediated signaling. A complete loss of PAR1 disrupts the formation of a heterodimer with PAR2. It is not clear whether PAR1-PAR2 heterodimers are preassembled or formed after receptor cleavage in vivo. Importantly, proteolytic cleavage of PAR1 by thrombin was shown to be required for heterodimer formation of PAR1 with other PARs such as PAR4 in vitro.39 In Par1R41Q mice, PAR1 is still present but would not be able to initiate heterodimer formation with PAR2 due to its thrombin insensitivity. Currently, it is not clear whether the heterodimer formation of PAR1-PAR2 is thrombin-cleavage dependent in vivo. Moreover, thrombin-mediated activation of PAR1-PAR2 heterodimers has been shown to induce cytoprotective β-arrestin mediated signaling.40 In addition, PAR2 activation seems to facilitate a prosurvival signal during certain chemotherapeutic regimes.41 The equivalence in phenotype of Par1-/- and Par1R41Q mice in doxorubicin-induced cardiac injury models suggests that in this setting PAR1 may primarily act as a single receptor or homodimer mediating deleterious Gαq signaling. Broadly speaking, the single point mutation of R41Q in the extracellular domain of PAR1 may alter general receptor interactions involving the cell surface and intracellular binding partners of PAR1. This possible change in receptor interactions should be the aim of future studies.
Interestingly, in the acute model, Par1R46Q mice demonstrated an increased release of cTI into the plasma, indicating increased cardiomyocyte injury compared to Par1+/+ controls. This suggests that APC cleavage of PAR1 may provide protection against doxorubicin-induced cardiac injury. However, in both acute and chronic models of doxorubicin-induced cardiac injury, no significant difference in cardiac function was observed between Par1R46Q and Par1+/+ mice. This indicates that although the loss of APC-mediated activation of PAR1 may lead to enhanced cardiomyocyte injury, it does not result in further reduction in cardiac function in models evaluated here. This finding does not preclude the potential protective effects of exogenous APC. Indeed, given the observed protective effects of cytoprotective APC variants, such as 3K3A-APC, in other pathologies this warrants further investigation.20,42-44
The phenotype observed in Par1R41Q mice indicates that thrombin-mediated activation of PAR1 is deleterious in doxorubicin-induced cardiac injury. We previously found that broad inhibition of PAR1 activation by vorapaxar reduced doxorubicin-induced cardiac injury in mice.6 Given that thrombin is an important activator of PAR1 mediated Gαq signaling we sought to evaluate the effect of selectively inhibiting this signaling using the small molecule antagonist Q94.17,45,46 Interestingly, Q94 has previously been shown to protect against doxorubicin-induced renal injury.47 Consistent with this previous finding Q94 significantly reduced cardiac injury in Par1+/+ mice subject to the acute model of doxorubicin-induced cardiac injury. The protective effects of Q94 in an acute model of doxorubicin-induced cardiac injury were similar to those observed in Par1-/- and Par1R41Q mice. This suggests that the deleterious effects of PAR1 activation in this setting are likely driven by downstream Gαq-mediated signaling. This was supported by the lack of a protective effect of Q94 in the Par1R41Q mice. Further studies are warranted to determine the molecular mechanism by which PAR1 mediated Gαq signaling contributes to cardiac injury.
In this study conscious mouse echocardiography was used as the primary measure of cardiac injury. Echocardiography-based measurements of percentage FS provide a sensitive and quantitative assessment of cardiac function after doxorubicin-induced injury.48 Importantly, the reduced cardiac injury observed in echocardiographic assessments of doxorubicin-treated Par1-/- and Par1R41Q mice was complemented by both reduced plasma levels of cTnI, a sensitive and specific marker of cardiac injury.49
In summary, PAR1 activation in cardiomyocytes and cardiac fibroblasts contributes to doxorubicin-induced cardiac injury. Doxorubicin administration leads to TF-dependent thrombin generation. Thrombin-mediated activation of PAR1 that requires Arg41, the cleavage site for thrombin, in turn enhances doxorubicin-induced cardiac injury. However, the cardiotoxic effects of doxorubicin can be abrogated by selective inhibition of Gαq signaling downstream of PAR1.
Acknowledgments
The authors thank Ying Zhang for expert technical assistance and Xiao Xu for production of the Par1R41Q and Par1R46Q mice.
This work was supported by an American Heart Association postdoctoral fellowship under award number 19POST34370026 (S.P.G.) and the National Heart Lung and Blood Institute of the National Institutes of Health under award R01HL142975 (J.H.G.) and R01HL148432 (S.A.).
Authorship
Contribution: S.P.G., S.A., and N.M. designed the experiment; S.P.G., V.B., and J.J.P. conducted experiments and analyzed data; S.P.G. wrote the manuscript; J.H.G. provided genetically PAR1-modified mice; S.A., N.M., J.J.P., J.S.P., and J.H.G. interpreted the data and edited the manuscript; S.A. provided overall study supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvio Antoniak, UNC Blood Research Center, Department of Pathology and Laboratory Medicine, 116 Manning Drive, 8004A Mary Ellen Jones Building CB#7035, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: silvio_antoniak@med.unc.edu.
References
Author notes
Data are available upon request from the corresponding author Silvio Antoniak (silvio_antoniak@med.unc.edu).