Key Points
The increased risk of thromboembolic events, bleeding, and mortality among patients with CVT depends on sex and age.
Clinicians should be aware of the importance of age and sex heterogeneity in the prognosis of CVT.
Abstract
Cerebral venous thrombosis (CVT) predominantly affects young to middle-aged women. Scarce data exist regarding the long-term prognosis. We examined the clinical course of patients with CVT overall and according to their age and sex. Using Danish registries, we identified all patients with a first-time primary inpatient diagnosis of CVT from 1996-2018 (N = 653; median age, 41 years; 67% women) and individuals from the general population matched for age, sex, and calendar year (N = 65 300). Patients with CVT were at an increased risk of venous thromboembolism (VTE) at other sites, ischemic stroke, major bleeding, and mortality. For both sexes, the increased risks of VTE at other sites were most prominent among younger patients (18-54 years), whereas the increased risks of ischemic stroke, major bleeding, and mortality were most prominent among older patients (≥55 years). Among young women, the 10-year risks of VTE at other sites for patients with CVT compared with members of the matched cohort were 2.2% vs 0.4% (risk difference, 1.8%; 95% confidence interval [CI], 0.0-3.6). Among older women, compared with members of the matched cohort, the 10-year risks were 12.8% vs 3.1% (risk difference, 9.7%; 95% CI, 1.6-17.9) for ischemic stroke, 11.1% vs 4.6% (risk difference, 6.5%; 95% CI, −1.0 to 14.1) for major bleeding, and 43.1% vs 26.7% (risk difference, 16.4%; 95% CI, 3.7-29.1) for all-cause mortality. The risk of myocardial infarction was not elevated. Clinicians should be aware of the importance of age and sex heterogeneity in the prognosis of CVT.
Introduction
Cerebral venous thrombosis (CVT) is an unusual venous thrombosis, with an incidence of 2 to 20 per million people annually, predominantly affecting young to middle-aged women.1 CVT is often triggered by traditional risk factors for venous thromboembolism (VTE), for example, cancer, central nervous system infections, thrombophilia, trauma, pregnancy, and use of oral contraceptives. Most guidelines of CVT have focused mainly on the diagnosis and treatment of the disease and less on its long-term prognosis.2 It is well established that deep vein thrombosis in the legs and pulmonary embolism are associated with a high risk of recurrence and higher mortality than among age- and sex-matched individuals from the general population (GP),3 and there is mounting evidence that extended anticoagulant treatment reduces this risk.4 In contrast, clear international guidelines on the treatment of patients with CVT are not available.2 This reflects the lack of data on the long-term risks of thromboembolic events among these patients.5-13
To expand our knowledge of the clinical course of CVT, we conducted a population-based cohort study examining the long-term risks of thromboembolic events, bleeding, and mortality, compared with a matched comparison cohort from the GP. Because CVT disproportionally affects young to middle-aged women, and because the prevalence and burden of predisposing factors for CVT with important prognostic impact differ across sex and age groups,1 we examined these risks in an overall CVT cohort, and also separately in women aged 18 to 54 years, women aged ≥55 years, men aged 18 to 54 years, and men aged ≥55 years.
Methods
Design and setting
This nationwide, population-based, matched cohort study was based on routinely collected Danish health and administrative registry data.14 All Danish residents were assigned a unique personal identification number that allowed unambiguous linkage across registries at an individual level.15 Denmark has a state-funded healthcare system ensuring free access to health care for all residents.14 We obtained data from the Danish Civil Registration System,15 the Danish National Patient Registry (DNPR),16 the Danish National Prescription Registry,17 and the Danish Registry of Causes of Death18 (detailed descriptions in supplemental Table 1). Ethical approval was not required for the registry–based studies in Denmark. The study was approved by the Danish Data Protection Agency at Aarhus University (number 2016-051-000001-1502) and was performed according to the Declaration of Helsinki.
CVT cohort
We assembled a cohort of patients aged ≥18 years with a first-time primary inpatient diagnosis of CVT (International Classification of Disease, 10th revision, codes I636, I676, G08, O225, and O873) between 1 January 1996 (allowing a minimum of 1 year of prescription data for all patients) and 31 December 2018, recorded in the DNPR. The index date was defined as the hospital admission date for CVT. We excluded patients with a history of any study outcome before the index date.
GP comparison cohort
We used the Civil Registration System to assemble a comparison cohort consisting of members of the GP without CVT. We matched, with replacement, up to 100 persons from the GP by birth year and sex to each patient with CVT. Comparators were required to have no CVT diagnosis before the index date of the matched patient with CVT and to be alive and living in Denmark.19 Comparators were assigned an index date corresponding to that of their matched patient with CVT. If a comparator suffered a CVT during follow-up, he or she then contributed risk time to both cohorts, thereby avoiding informative censoring.19 We applied the same exclusion criteria to the comparison cohort as we did for the CVT cohort.
Outcomes
The outcomes were (1) recurrent CVT, (2) VTE at other sites (ie, a composite endpoint of deep vein thrombosis and pulmonary embolism), (3) myocardial infarction, (4) ischemic stroke, (5) major bleeding (ie, a composite endpoint of hemorrhagic stroke, respiratory tract bleeding, gastrointestinal tract bleeding, urinary tract bleeding, and bleeding-associated anemia),20 and (6) all-cause mortality. Nonfatal outcomes were identified from the primary inpatient diagnoses in the DNPR. Recurrent CVT was defined as an inpatient diagnosis occurring at least 90 days after the index date.21 In a sensitivity analysis, we altered the blanking period to 180 days, aligning with the duration of guideline-recommended treatment with oral anticoagulation.22 Because only patients with CVT were at risk of CVT recurrence, this outcome did not pertain to GP comparators. As secondary outcomes, we examined the individual components of the composite outcomes. We also examined cause–specific mortality: death from diseases of the circulatory system, death from diseases of the respiratory system, and death from neoplasms.3 Information on all-cause and cause–specific mortality were obtained from the Danish Civil Registration System and the Danish Registry of Causes of Death, respectively. Complete data on cause–specific mortality were available until the end of 2016; thus, for these analyses, only patients diagnosed between 1 January 1995 and 31 December 2016 were included.
Covariates
We identified known risk factors for CVT that occurred before the index date for patients with CVT and GP comparators. These encompassed cancer, recent infections, recent fracture/trauma, surgery, or pregnancy, thrombophilia (only applicable for patients with CVT), systemic lupus erythematosus, Behçet disease, inflammatory bowel disease, dehydration, and thyroid disease.22 We also compiled information on ischemic heart disease, atrial fibrillation/flutter, hypertension, diabetes, obesity, heart failure, peripheral artery disease, chronic liver disease, and chronic pulmonary disease. Finally, we obtained data on the recent use of hormonal contraceptives (including combined contraceptives, progesterone-only contraceptives, and the generation of hormonal contraceptives), selective estrogen receptor modulators, other hormonal treatments (excluding hormonal contraceptives and selective estrogen receptor modulators), anticoagulants, antiplatelets, statins, and antihypertensives. Supplemental Table 2 lists all codes and definitions used in this study.
Statistical analyses
We computed the incidence rate (age standardized to the year 2018) of a first-time CVT diagnosis overall and during 5 calendar periods as the number of events divided by the entire Danish midyear population. For this analysis, we specifically applied a broader CVT definition (ie, including secondary and outpatient clinic diagnoses and no exclusion criteria), because we prioritized sensitivity over specificity in estimating absolute rates. To contextualize the analyses of outcomes among patients with CVT, we computed the Aalen-Johansen estimate of redeeming a prescription for a secondary preventive drug within 180 days of CVT diagnosis. Death was considered a competing event.23
For outcome analyses, members of the CVT and comparison cohorts were followed from the index date until the occurrence of an outcome, death, emigration, 10 years of follow-up (as only a few study members were at risk thereafter), or 31 December 2018, whichever occurred first. Separately, for each outcome, we provided the 1-, 5-, and 10-year absolute risks and absolute risk differences between the 2 cohorts. For nonfatal outcomes and cause–specific mortality, we used the Aalen-Johansen estimator, with death (death from other causes for cause–specific mortality) considered a competing event.23 For all-cause mortality, we applied the one minus Kaplan-Meier estimator. As a measure of the relative effect, we used stratified Cox regression models to provide the overall (ie, 0-10 years), 0 to 1 year, >1 to 5 years, and >5 to 10 years unadjusted and adjusted cause–specific hazard ratios (HRs). Unadjusted comparisons between the 2 cohorts were controlled for age, sex, and calendar period per design. Based on previous studies,3 the multivariable Cox models included diagnoses of cancer, recent infection, fracture/trauma, surgery, pregnancy, ischemic heart disease, atrial fibrillation, hypertension, diabetes, obesity, peripheral artery disease, chronic liver disease, and chronic pulmonary disease. Pregnancy was omitted as a covariate in the analyses of men. To align with recommendations for studies with multiple outcomes,24 we considered a single model for all outcomes. The proportional hazards assumption was deemed to be satisfied after examining the log (−log[survival probability]) curves. In further subgroup analyses, we examined the primary outcomes according to the presence of cancer before the index date and the previous use of any hormonal treatment. The analysis of hormonal treatment was restricted to women aged 18 to 54 years owing to sparse data in other strata.
In compliance with Danish data protection regulations, table cells were masked (eg, <5) whenever applicable to prevent identification of unique individuals.
Additional analyses
We performed 2 additional analyses. First, in a CVT subcohort, we examined the risk of primary outcomes in patients who initiated and discontinued treatment with anticoagulants. The purpose of this analysis was to provide clinical guidance for decisions regarding the duration of anticoagulant treatment and follow-up. Therefore, patients with permanent risk factors for recurrence and the need for continued anticoagulant treatment (eg, antiphospholipid syndrome and severe thrombophilia) were excluded. In this analysis, we identified all patients with CVT who initiated anticoagulant treatment within 180 days of their CVT admission date and then discontinued these drugs. The date of discontinuation, set as 90 days after redemption of the last prescription, was defined as the “index date” for this subcohort. Switching between anticoagulant agents was allowed. Patients who continued treatment, those diagnosed with recurrent CVT, VTE at other sites, stroke of any subtype, major bleeding, and those who died or emigrated between CVT admission and discontinuation dates were excluded. Second, we identified patients with CVT who had a previous diagnosis of an outcome. These patients were excluded from the primary analyses to isolate the CVT-specific effect. However, patients with a previous diagnosis of an outcome also represent a potentially high-risk population, for whom knowledge of the clinical course is important.
Validation substudy
We performed a validation substudy of the CVT diagnosis in the DNPR, identifying all patients with CVT from Aarhus University Hospital from January 2018 to November 2021. We restricted the substudy to first-time diagnoses by excluding patients with any previous diagnoses of CVT in the Central Denmark Region up to 2012. An experienced physician (K.A.) adjudicated the patients’ diagnoses using medical records as the reference standard. Following international guidelines,2 the diagnosis of CVT was confirmed by the presence of thrombosis on a computed tomography or magnetic resonance imaging venogram of the cerebral venous system and symptoms suggestive of CVT.2 We then computed the positive predictive value as the proportion of diagnoses from the DNPR that was confirmed in the medical records.
Results
We identified 653 patients with a first-time CVT diagnosis and 65 300 age-, sex-, and calendar year–matched, CVT-free individuals from the GP (Table 1). Overall, 67% of the patients were women, with a median age of 41 years. Young women aged 18 to 54 years disproportionally made up 52% of the overall patient population. In comparison, this group accounted for 30% of the Danish GP in 2007. Comparing the CVT cohort with the comparison cohort, the baseline prevalence of cancer was particularly elevated in older women (27% vs 12%) and older men (15% vs 10%), the prevalence of recent infections was particularly elevated in young women (11% vs 2%) and young men (9% vs 1%), and the prevalence of surgery was elevated across all age and sex strata. Similarly, for young women, recent pregnancy and the use of hormonal contraceptives, mostly combined contraceptives with estrogen and progestogen, were more prevalent among patients with CVT than among comparators (10% vs 3% for recent pregnancy and 61% vs 34% for hormonal contraceptive use). A similar pattern was observed for most comorbidities of cardiovascular origin, for example, hypertension, which was particularly elevated among older women (32% vs 23%) and older men (30% vs 23%).
Characteristics (N, %) of patients with a first-time CVT and matched individuals from the GP according to sex and age group, Denmark, 1996-2018
| . | CVT cohort . | GP comparison cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All . | Women 18-54 y . | Women ≥55 y . | Men 18-54 y . | Men ≥55 y . | All . | Women 18-54 y . | Women ≥55 y . | Men 18-54 y . | Men ≥55 y . | |
| Overall, n | 653 | 343 | 95 | 128 | 87 | 65 300 | 34 285 | 9 515 | 12 682 | 8 818 |
| Age, y, median (IQR) | 41 (27-58) | 31 (24-41) | 67 (62-75) | 39 (28-47) | 66 (60-72) | 41 (27-58) | 31 (24-41) | 67 (62-75) | 39 (28-47) | 66 (59-72) |
| Calendar period of diagnosis, n (%) | ||||||||||
| 1996-2000 | 81 (12.4) | 44 (12.8) | 17 (17.9) | 12 (9.4) | 8 (9.2) | 8 100 (12.4) | 4 442 (13.0) | 1 658 (17.4) | 1 167 (9.2) | 833 (9.4) |
| 2001-2005 | 105 (16.1) | 62 (18.1) | 15 (15.8) | 19 (14.8) | 9 (10.3) | 10 500 (16.1) | 6 211 (18.1) | 1 489 (15.6) | 1 863 (14.7) | 937 (10.6) |
| 2016-2010 | 163 (25.0) | 78 (22.7) | 20 (21.1) | 46 (35.9) | 19 (21.8) | 16 300 (25.0) | 7 813 (22.8) | 1 987 (20.9) | 4 552 (35.9) | 1 948 (22.1) |
| 2011-2014 | 121 (18.5) | 68 (19.8) | 14 (14.7) | 19 (14.8) | 20 (23.0) | 12 100 (18.5) | 6 719 (19.6) | 1 481 (15.6) | 1 900 (15.0) | 2 000 (22.7) |
| 2015-2018 | 183 (28.0) | 91 (26.5) | 29 (30.5) | 32 (25.0) | 31 (35.6) | 18 300 (28.0) | 9 100 (26.5) | 2 900 (30.5) | 3 200 (25.2) | 3 100 (35.2) |
| Hospital-recorded comorbidity (any time before, unless stated otherwise), n (%) | ||||||||||
| Cancer | 57 (8.7) | 10 (2.9) | 26 (27.4) | 8 (6.2) | 13 (14.9) | 2 670 (4.1) | 483 (1.4) | 1 182 (12.4) | 164 (1.3) | 841 (9.5) |
| Infections (within 180 d before)∗ | 67 (10.3) | 37 (10.8) | 8 (8.4) | 11 (8.6) | 11 (12.6) | 1 282 (2.0) | 744 (2.2) | 209 (2.2) | 171 (1.3) | 158 (1.8) |
| Fracture/trauma (within 90 d before) | 37 (5.7) | 13 (3.8) | 8 (8.4) | 6 (4.7) | 10 (11.5) | 1 459 (2.2) | 753 (2.2) | 180 (1.9) | 377 (3.0) | 149 (1.7) |
| Surgery (within 90 d before)† | 103 (15.8) | 57 (16.6) | 15 (15.8) | 14 (10.9) | 17 (19.5) | 1 849 (2.8) | 1 062 (3.1) | 301 (3.2) | 191 (1.5) | 295 (3.3) |
| Pregnancy (within 90 d before) | 33 (5.1) | 33 (9.6) | — | — | — | 854 (1.3) | 854 (2.5) | — | — | — |
| Thrombophilia (any time before or within 1 y after) | 38 (5.8) | 23 (6.7) | <5 | 10 (7.8) | <5 | — | — | — | — | — |
| Systemic lupus erythematosus | <5 | <5 | <5 | <5 | <5 | <70 | 47 (0.1) | 14 (0.1) | <5 | 5 (0.1) |
| Behçet disease | 7 (1.1) | <5 | <5 | <5 | <5 | 339 (0.5) | 156 (0.5) | 63 (0.7) | 43 (0.3) | 77 (0.9) |
| Inflammatory bowel disease | <20 | 13 (3.8) | <5 | <5 | <5 | 891 (1.4) | 512 (1.5) | 97 (1.0) | 173 (1.4) | 109 (1.2) |
| Dehydration | 14 (2.1) | 6 (1.7) | <5 | <5 | <5 | 455 (0.7) | 184 (0.5) | 148 (1.6) | 42 (0.3) | 81 (0.9) |
| Thyroid disease | 32 (4.9) | 18 (5.2) | 12 (12.6) | <5 | <5 | 2 669 (4.1) | 1 195 (3.5) | 1 169 (12.3) | 92 (0.7) | 213 (2.4) |
| Ischemic heart disease (excluding myocardial infarction) | 21 (3.2) | <5 | 5 (5.3) | <5 | 9 (10.3) | 1 499 (2.3) | 115 (0.3) | 582 (6.1) | 112 (0.9) | 690 (7.8) |
| Atrial fibrillation/flutter | 14 (2.1) | <5 | 7 (7.4) | <5 | 5 (5.7) | 923 (1.4) | 53 (0.2) | 332 (3.5) | 62 (0.5) | 476 (5.4) |
| Hypertension | 85 (13.0) | 19 (5.5) | 30 (31.6) | 10 (7.8) | 26 (29.9) | 5 378 (8.2) | 729 (2.1) | 2 213 (23.3) | 380 (3.0) | 2 056 (23.3) |
| Diabetes | 24 (3.7) | 6 (1.7) | <5 | 5 (3.9) | 9 (10.3) | 2 228 (3.4) | 525 (1.5) | 630 (6.6) | 226 (1.8) | 847 (9.6) |
| Obesity | 43 (6.6) | 36 (10.5) | <5 | <5 | <5 | 2 591 (4.0) | 1 874 (5.5) | 381 (4.0) | 145 (1.1) | 191 (2.2) |
| Heart failure | 11 (1.7) | <5 | <5 | <5 | 6 (6.9) | 451 (0.7) | 31 (0.1) | 179 (1.9) | 35 (0.3) | 206 (2.3) |
| Peripheral artery disease | <10 | 5 (1.5) | <5 | <5 | <5 | 698 (1.1) | 117 (0.3) | 232 (2.4) | 45 (0.4) | 304 (3.4) |
| Chronic liver disease | <5 | <5 | <5 | <5 | <5 | 206 (0.3) | 57 (0.2) | 54 (0.6) | 22 (0.2) | 73 (0.8) |
| Chronic pulmonary disease | 54 (8.3) | 27 (7.9) | 11 (11.6) | <5 | 12 (13.8) | 3 298 (5.1) | 1 271 (3.7) | 929 (9.8) | 382 (3.0) | 716 (8.1) |
| Filled prescriptions (prior 180 d), n (%) | ||||||||||
| Hormonal contraceptives | 209 (32.0) | 209 (60.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 747 (18.0) | 11 698 (34.1) | 32 (0.3) | 9 (0.1) | 8 (0.1) |
| Combined contraceptives (estrogen and progestogen) | 204 (31.2) | 204 (59.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 903 (16.7) | 10 887 (31.8) | 16 (0.2) | 0 (0.0) | 0 (0.0) |
| Progestogen-only contraceptives | 7 (1.1) | 7 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 885 (1.4) | 852 (2.5) | 16 (0.2) | 9 (0.1) | 8 (0.1) |
| First-generation hormonal contraceptives | <5 | <5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 146 (0.2) | 146 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Second-generation hormonal contraceptives | 62 (9.5) | 62 (18.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <3 545 | 3 536 (10.3) | <5 | 0 (0.0) | 0 (0.0) |
| Third-generation hormonal contraceptives | 112 (17.2) | 112 (32.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 791 (8.9) | 5 786 (16.9) | 5 (0.1) | 0 (0.0) | 0 (0.0) |
| Fourth-generation hormonal contraceptives | 22 (3.4) | 22 (6.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 815 (1.2) | 815 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Selective estrogen receptor modulators | <5 | <5 | <5 | <5 | <5 | 13 (0.1) | <5 | 10 (0.1) | 0 (0.0) | 0 (0.0) |
| Other hormone treatment, excluding hormonal contraceptive and selective estrogen receptor modulators | 31 (4.7) | 11 (3.2) | 20 (21.1) | 0 (0.0) | 0 (0.0) | 2 410 (3.7) | 864 (2.5) | 1 540 (16.2) | 0 (0.0) | 6 (0.1) |
| Anticoagulants | <20 | 6 (1.7) | 5 (5.3) | <5 | 5 (5.7) | 613 (0.9) | 30 (0.1) | 211 (2.2) | 21 (0.2) | 351 (4.0) |
| Aspirin | <30 | <5 | 12 (12.6) | 5 (3.9) | 10 (11.5) | 2 487 (3.8) | 129 (0.4) | 1 097 (11.5) | 108 (0.9) | 1 153 (13.1) |
| Adenosine diphosphate receptor inhibitors | 7 (1.1) | <5 | <5 | <5 | <5 | 324 (0.5) | 17 (0.0) | 123 (1.3) | 24 (0.2) | 160 (1.8) |
| Statins | <40 | <5 | 19 (20.0) | 6 (4.7) | 11 (12.6) | 3 704 (5.7) | 314 (0.9) | 1 474 (15.5) | 266 (2.1) | 1 650 (18.7) |
| Antihypertensives | 104 (15.9) | 30 (8.7) | 34 (35.8) | 9 (7.0) | 31 (35.6) | 9 098 (13.9) | 1 494 (4.4) | 3 655 (38.4) | 665 (5.2) | 3 284 (37.2) |
| . | CVT cohort . | GP comparison cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All . | Women 18-54 y . | Women ≥55 y . | Men 18-54 y . | Men ≥55 y . | All . | Women 18-54 y . | Women ≥55 y . | Men 18-54 y . | Men ≥55 y . | |
| Overall, n | 653 | 343 | 95 | 128 | 87 | 65 300 | 34 285 | 9 515 | 12 682 | 8 818 |
| Age, y, median (IQR) | 41 (27-58) | 31 (24-41) | 67 (62-75) | 39 (28-47) | 66 (60-72) | 41 (27-58) | 31 (24-41) | 67 (62-75) | 39 (28-47) | 66 (59-72) |
| Calendar period of diagnosis, n (%) | ||||||||||
| 1996-2000 | 81 (12.4) | 44 (12.8) | 17 (17.9) | 12 (9.4) | 8 (9.2) | 8 100 (12.4) | 4 442 (13.0) | 1 658 (17.4) | 1 167 (9.2) | 833 (9.4) |
| 2001-2005 | 105 (16.1) | 62 (18.1) | 15 (15.8) | 19 (14.8) | 9 (10.3) | 10 500 (16.1) | 6 211 (18.1) | 1 489 (15.6) | 1 863 (14.7) | 937 (10.6) |
| 2016-2010 | 163 (25.0) | 78 (22.7) | 20 (21.1) | 46 (35.9) | 19 (21.8) | 16 300 (25.0) | 7 813 (22.8) | 1 987 (20.9) | 4 552 (35.9) | 1 948 (22.1) |
| 2011-2014 | 121 (18.5) | 68 (19.8) | 14 (14.7) | 19 (14.8) | 20 (23.0) | 12 100 (18.5) | 6 719 (19.6) | 1 481 (15.6) | 1 900 (15.0) | 2 000 (22.7) |
| 2015-2018 | 183 (28.0) | 91 (26.5) | 29 (30.5) | 32 (25.0) | 31 (35.6) | 18 300 (28.0) | 9 100 (26.5) | 2 900 (30.5) | 3 200 (25.2) | 3 100 (35.2) |
| Hospital-recorded comorbidity (any time before, unless stated otherwise), n (%) | ||||||||||
| Cancer | 57 (8.7) | 10 (2.9) | 26 (27.4) | 8 (6.2) | 13 (14.9) | 2 670 (4.1) | 483 (1.4) | 1 182 (12.4) | 164 (1.3) | 841 (9.5) |
| Infections (within 180 d before)∗ | 67 (10.3) | 37 (10.8) | 8 (8.4) | 11 (8.6) | 11 (12.6) | 1 282 (2.0) | 744 (2.2) | 209 (2.2) | 171 (1.3) | 158 (1.8) |
| Fracture/trauma (within 90 d before) | 37 (5.7) | 13 (3.8) | 8 (8.4) | 6 (4.7) | 10 (11.5) | 1 459 (2.2) | 753 (2.2) | 180 (1.9) | 377 (3.0) | 149 (1.7) |
| Surgery (within 90 d before)† | 103 (15.8) | 57 (16.6) | 15 (15.8) | 14 (10.9) | 17 (19.5) | 1 849 (2.8) | 1 062 (3.1) | 301 (3.2) | 191 (1.5) | 295 (3.3) |
| Pregnancy (within 90 d before) | 33 (5.1) | 33 (9.6) | — | — | — | 854 (1.3) | 854 (2.5) | — | — | — |
| Thrombophilia (any time before or within 1 y after) | 38 (5.8) | 23 (6.7) | <5 | 10 (7.8) | <5 | — | — | — | — | — |
| Systemic lupus erythematosus | <5 | <5 | <5 | <5 | <5 | <70 | 47 (0.1) | 14 (0.1) | <5 | 5 (0.1) |
| Behçet disease | 7 (1.1) | <5 | <5 | <5 | <5 | 339 (0.5) | 156 (0.5) | 63 (0.7) | 43 (0.3) | 77 (0.9) |
| Inflammatory bowel disease | <20 | 13 (3.8) | <5 | <5 | <5 | 891 (1.4) | 512 (1.5) | 97 (1.0) | 173 (1.4) | 109 (1.2) |
| Dehydration | 14 (2.1) | 6 (1.7) | <5 | <5 | <5 | 455 (0.7) | 184 (0.5) | 148 (1.6) | 42 (0.3) | 81 (0.9) |
| Thyroid disease | 32 (4.9) | 18 (5.2) | 12 (12.6) | <5 | <5 | 2 669 (4.1) | 1 195 (3.5) | 1 169 (12.3) | 92 (0.7) | 213 (2.4) |
| Ischemic heart disease (excluding myocardial infarction) | 21 (3.2) | <5 | 5 (5.3) | <5 | 9 (10.3) | 1 499 (2.3) | 115 (0.3) | 582 (6.1) | 112 (0.9) | 690 (7.8) |
| Atrial fibrillation/flutter | 14 (2.1) | <5 | 7 (7.4) | <5 | 5 (5.7) | 923 (1.4) | 53 (0.2) | 332 (3.5) | 62 (0.5) | 476 (5.4) |
| Hypertension | 85 (13.0) | 19 (5.5) | 30 (31.6) | 10 (7.8) | 26 (29.9) | 5 378 (8.2) | 729 (2.1) | 2 213 (23.3) | 380 (3.0) | 2 056 (23.3) |
| Diabetes | 24 (3.7) | 6 (1.7) | <5 | 5 (3.9) | 9 (10.3) | 2 228 (3.4) | 525 (1.5) | 630 (6.6) | 226 (1.8) | 847 (9.6) |
| Obesity | 43 (6.6) | 36 (10.5) | <5 | <5 | <5 | 2 591 (4.0) | 1 874 (5.5) | 381 (4.0) | 145 (1.1) | 191 (2.2) |
| Heart failure | 11 (1.7) | <5 | <5 | <5 | 6 (6.9) | 451 (0.7) | 31 (0.1) | 179 (1.9) | 35 (0.3) | 206 (2.3) |
| Peripheral artery disease | <10 | 5 (1.5) | <5 | <5 | <5 | 698 (1.1) | 117 (0.3) | 232 (2.4) | 45 (0.4) | 304 (3.4) |
| Chronic liver disease | <5 | <5 | <5 | <5 | <5 | 206 (0.3) | 57 (0.2) | 54 (0.6) | 22 (0.2) | 73 (0.8) |
| Chronic pulmonary disease | 54 (8.3) | 27 (7.9) | 11 (11.6) | <5 | 12 (13.8) | 3 298 (5.1) | 1 271 (3.7) | 929 (9.8) | 382 (3.0) | 716 (8.1) |
| Filled prescriptions (prior 180 d), n (%) | ||||||||||
| Hormonal contraceptives | 209 (32.0) | 209 (60.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 747 (18.0) | 11 698 (34.1) | 32 (0.3) | 9 (0.1) | 8 (0.1) |
| Combined contraceptives (estrogen and progestogen) | 204 (31.2) | 204 (59.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 903 (16.7) | 10 887 (31.8) | 16 (0.2) | 0 (0.0) | 0 (0.0) |
| Progestogen-only contraceptives | 7 (1.1) | 7 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 885 (1.4) | 852 (2.5) | 16 (0.2) | 9 (0.1) | 8 (0.1) |
| First-generation hormonal contraceptives | <5 | <5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 146 (0.2) | 146 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Second-generation hormonal contraceptives | 62 (9.5) | 62 (18.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <3 545 | 3 536 (10.3) | <5 | 0 (0.0) | 0 (0.0) |
| Third-generation hormonal contraceptives | 112 (17.2) | 112 (32.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 791 (8.9) | 5 786 (16.9) | 5 (0.1) | 0 (0.0) | 0 (0.0) |
| Fourth-generation hormonal contraceptives | 22 (3.4) | 22 (6.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 815 (1.2) | 815 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Selective estrogen receptor modulators | <5 | <5 | <5 | <5 | <5 | 13 (0.1) | <5 | 10 (0.1) | 0 (0.0) | 0 (0.0) |
| Other hormone treatment, excluding hormonal contraceptive and selective estrogen receptor modulators | 31 (4.7) | 11 (3.2) | 20 (21.1) | 0 (0.0) | 0 (0.0) | 2 410 (3.7) | 864 (2.5) | 1 540 (16.2) | 0 (0.0) | 6 (0.1) |
| Anticoagulants | <20 | 6 (1.7) | 5 (5.3) | <5 | 5 (5.7) | 613 (0.9) | 30 (0.1) | 211 (2.2) | 21 (0.2) | 351 (4.0) |
| Aspirin | <30 | <5 | 12 (12.6) | 5 (3.9) | 10 (11.5) | 2 487 (3.8) | 129 (0.4) | 1 097 (11.5) | 108 (0.9) | 1 153 (13.1) |
| Adenosine diphosphate receptor inhibitors | 7 (1.1) | <5 | <5 | <5 | <5 | 324 (0.5) | 17 (0.0) | 123 (1.3) | 24 (0.2) | 160 (1.8) |
| Statins | <40 | <5 | 19 (20.0) | 6 (4.7) | 11 (12.6) | 3 704 (5.7) | 314 (0.9) | 1 474 (15.5) | 266 (2.1) | 1 650 (18.7) |
| Antihypertensives | 104 (15.9) | 30 (8.7) | 34 (35.8) | 9 (7.0) | 31 (35.6) | 9 098 (13.9) | 1 494 (4.4) | 3 655 (38.4) | 665 (5.2) | 3 284 (37.2) |
Among the 67 patients with CVT with any infections within 180 days before diagnosis, there were a total of 101 hospital–based diagnoses of infections. Of these, 21 were central nervous system infections (21%), 11 were pneumonia (11%), 9 were urinary tract infections (9%), 7 were gastrointestinal tract infections (7%), and the remaining were miscellaneous.
Among the 103 patients with CVT with any surgery within 90 days before diagnosis, there were a total 203 surgeries. Of these, 99 were cranial or intracranial operations (48%).
Rate of first-time CVT
The incidence rate of a first-time CVT diagnosis per 100 000 person-years was 1.24 (95% confidence interval [CI], 1.17-1.31) overall, 1.73 (95% CI, 1.59-1.88) among young women, 1.15 (95% CI, 1.01-1.32) among older women, 0.75 (95% CI, 0.66-0.85) among young men, and 1.37 (95% CI, 1.20-1.56) among older men. The rate per 100 000 person-years increased over time overall and in all examined age and sex strata; however, this was mainly in young women (Figure 1).
Incidence rates of a first-time diagnosis of CVT between 1996-2018 in Denmark, overall and according to sex and age group.
Incidence rates of a first-time diagnosis of CVT between 1996-2018 in Denmark, overall and according to sex and age group.
Use of preventive drugs
During the entire study period, 68% of patients with CVT filled a prescription for a vitamin K antagonist within 180 days of their index date. The corresponding percentages were 5.9% for factor Xa inhibitors, 0.8% for dabigatran, and 9.8% for low-molecular weight heparins. In general, young women and men were less likely to fill a prescription for these drugs. The percentages of use of other preventive drugs are presented in supplemental Table 3.
Primary outcomes
We observed 37 hospitalizations with a repeated code of CVT that occurred at least 90 days after the index date, defined as CVT recurrence; 26 (70%) of these occurred in young women (Table 2). The overall 1-year absolute risk of CVT recurrence was 3.9% (supplemental Table 3), increasing to 6.3% after 10 years. The 10-year risk was among young women (8.3%) and in young men (8.0%). When the blanking period was extended to 180 days, the overall 1- and 10-year risks were 1.4% and 4.2%, respectively. Notably, 7 individuals from the comparison cohort had their first CVT during the follow-up.
Numbers, events, person-years, 10-year risks, and overall (0-10 years) adjusted HRs of CVT recurrence, VTE at other sites, myocardial infarction, ischemic stroke, and all-cause mortality, comparing patients with a first-time diagnosis of CVT and matched individuals from the GP
| . | N . | Events, N . | Person-years, n . | Risk, % . | Adjusted HR (95% CI)∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVT . | GP . | CVT . | GP . | CVT . | GP . | CVT . | GP . | Difference (95% CI) . | ||
| CVT recurrence | ||||||||||
| All | 653 | 65 300 | 37 | — | 3 745 | — | 6.3 | — | — | — |
| Women, aged 18-54 y | 343 | 34 285 | 26 | — | 2 070 | — | 8.3 | — | — | — |
| Women, aged ≥55 y | 95 | 9 515 | 0 | — | 480 | — | - | — | — | — |
| Men, aged 18-54 y | 128 | 12 682 | <10 | — | 819 | — | 8.0 | — | — | — |
| Men, aged ≥55 y | 87 | 8 818 | <5 | — | 375 | — | 2.4 | — | — | — |
| VTE | ||||||||||
| All | 653 | 65 300 | 12 | 346 | 3 934 | 434 537 | 2.4 | 0.8 | 1.6 (0.2-3.0) | 3.95 (2.20-7.11) |
| Women, aged 18-54 y | 343 | 34 285 | 6 | 94 | 2 212 | 235 970 | 2.2 | 0.4 | 1.8 (0.0-3.6) | 6.55 (2.79-15.35) |
| Women, aged ≥55 y | 95 | 9 515 | <5 | 102 | 476 | 57 791 | 2.2 | 1.6 | 0.6 (−2.5 to 3.7) | 2.65 (0.64-10.97) |
| Men, aged 18-54 y | 128 | 12 682 | <5 | 40 | 857 | 90 556 | 3.3 | 0.5 | 2.9 (−0.9 to 6.6) | 7.34 (2.14-25.23) |
| Men, aged ≥55 y | 87 | 8 818 | <5 | 110 | 389 | 50 219 | 1.3 | 2.2 | −0.9 (−3.4 to 1.5) | 1.13 (0.15-8.31) |
| Myocardial infarction | ||||||||||
| All | 653 | 65 300 | 5 | 615 | 3 974 | 433 444 | 0.9 | 1.4 | −0.5 (−1.3, 0.3) | 0.93 (0.38-2.26) |
| Women, aged 18-54 y | 343 | 34 285 | <5 | 69 | 2 245 | 236 093 | 0.4 | 0.3 | 0.0 (−0.7 to 0.8) | 0.50 (0.04-6.12) |
| Women, aged ≥55 y | 95 | 9 515 | <5 | 157 | 480 | 57 565 | 1.1 | 2.4 | −1.2 (−3.5 to 1.0) | 0.99 (0.14-7.12) |
| Men, aged 18-54 y | 128 | 12 682 | <5 | 111 | 865 | 90 242 | 0.0 | 1.3 | −1.3 (−1.5 to −1.0) | — |
| Men, aged ≥55 y | 87 | 8 818 | <5 | 278 | 384 | 49 545 | 3.9 | 5.1 | −1.2 (−5.6 to 3.2) | 1.46 (0.46-4.63) |
| Ischemic stroke | ||||||||||
| All | 653 | 65 300 | 29 | 519 | 3 852 | 434 040 | 5.4 | 1.2 | 4.2 (2.2-6.2) | 7.40 (5.02-10.91) |
| Women, aged 18-54 y | 343 | 34 285 | 5 | 57 | 2 212 | 236 163 | 1.5 | 0.3 | 1.2 (−0.1 to 2.6) | 6.47 (2.27-18.41) |
| Women, aged ≥55 y | 95 | 9 515 | 9 | 198 | 445 | 57 505 | 12.8 | 3.1 | 9.7 (1.6-17.9) | 6.63 (3.32-13.23) |
| Men, aged 18-54 y | 128 | 12 682 | 9 | 54 | 817 | 90 505 | 8.8 | 0.6 | 8.1 (2.5-13.8) | 20.20 (9.51-42.91) |
| Men, aged ≥55 y | 87 | 8 818 | 6 | 210 | 379 | 49 866 | 7.0 | 3.9 | 3.1 (−2.3 to 8.5) | 3.86 (1.66-8.99) |
| Major bleeding | ||||||||||
| All | 653 | 65 300 | 27 | 850 | 3 900 | 432 952 | 5.3 | 1.9 | 3.4 (1.3-5.5) | 3.69 (2.49-5.47) |
| Women, aged 18-54 y | 343 | 34 285 | <5 | 158 | 2 230 | 235 645 | 1.8 | 0.7 | 1.1 (−0.7 to 2.8) | 1.77 (0.63-4.99) |
| Women, aged ≥55 y | 95 | 9 515 | <10 | 289 | 464 | 57 323 | 11.1 | 4.6 | 6.5 (−1.0 to 14.1) | 3.97 (1.93-8.18) |
| Men, aged 18-54 y | 128 | 12 682 | 10 | 101 | 819 | 90 269 | 9.8 | 1.1 | 8.6 (2.6-14.7) | 9.75 (4.87-19.51) |
| Men, aged ≥55 y | 87 | 8 818 | 5 | 302 | 387 | 49 715 | 6.1 | 5.7 | 0.3 (−4.9 to 5.5) | 2.29 (0.92-5.70) |
| All-cause mortality | ||||||||||
| All | 653 | 65 300 | 82 | 3 221 | 3 985 | 435 586 | 15.8 | 7.5 | 8.2 (4.9-11.6) | 3.22 (2.57-4.04) |
| Women, aged 18-54 y | 343 | 34 285 | 17 | 182 | 2 246 | 236 335 | 5.3 | 0.8 | 4.5 (2.0-7.0) | 5.99 (3.45-10.38) |
| Women, aged ≥55 y | 95 | 9 515 | 31 | 1 671 | 480 | 58 062 | 43.1 | 26.7 | 16.4 (3.7-29.1) | 2.82 (1.96-4.05) |
| Men, aged 18-54 y | 128 | 12 682 | 10 | 172 | 865 | 90 696 | 9.7 | 2.1 | 7.6 (1.7-13.5) | 3.96 (1.96-7.99) |
| Men, aged ≥55 y | 87 | 8 818 | 24 | 1 196 | 394 | 50 494 | 39.1 | 23.7 | 15.3 (1.4-29.3) | 2.50 (1.65-3.79) |
| . | N . | Events, N . | Person-years, n . | Risk, % . | Adjusted HR (95% CI)∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVT . | GP . | CVT . | GP . | CVT . | GP . | CVT . | GP . | Difference (95% CI) . | ||
| CVT recurrence | ||||||||||
| All | 653 | 65 300 | 37 | — | 3 745 | — | 6.3 | — | — | — |
| Women, aged 18-54 y | 343 | 34 285 | 26 | — | 2 070 | — | 8.3 | — | — | — |
| Women, aged ≥55 y | 95 | 9 515 | 0 | — | 480 | — | - | — | — | — |
| Men, aged 18-54 y | 128 | 12 682 | <10 | — | 819 | — | 8.0 | — | — | — |
| Men, aged ≥55 y | 87 | 8 818 | <5 | — | 375 | — | 2.4 | — | — | — |
| VTE | ||||||||||
| All | 653 | 65 300 | 12 | 346 | 3 934 | 434 537 | 2.4 | 0.8 | 1.6 (0.2-3.0) | 3.95 (2.20-7.11) |
| Women, aged 18-54 y | 343 | 34 285 | 6 | 94 | 2 212 | 235 970 | 2.2 | 0.4 | 1.8 (0.0-3.6) | 6.55 (2.79-15.35) |
| Women, aged ≥55 y | 95 | 9 515 | <5 | 102 | 476 | 57 791 | 2.2 | 1.6 | 0.6 (−2.5 to 3.7) | 2.65 (0.64-10.97) |
| Men, aged 18-54 y | 128 | 12 682 | <5 | 40 | 857 | 90 556 | 3.3 | 0.5 | 2.9 (−0.9 to 6.6) | 7.34 (2.14-25.23) |
| Men, aged ≥55 y | 87 | 8 818 | <5 | 110 | 389 | 50 219 | 1.3 | 2.2 | −0.9 (−3.4 to 1.5) | 1.13 (0.15-8.31) |
| Myocardial infarction | ||||||||||
| All | 653 | 65 300 | 5 | 615 | 3 974 | 433 444 | 0.9 | 1.4 | −0.5 (−1.3, 0.3) | 0.93 (0.38-2.26) |
| Women, aged 18-54 y | 343 | 34 285 | <5 | 69 | 2 245 | 236 093 | 0.4 | 0.3 | 0.0 (−0.7 to 0.8) | 0.50 (0.04-6.12) |
| Women, aged ≥55 y | 95 | 9 515 | <5 | 157 | 480 | 57 565 | 1.1 | 2.4 | −1.2 (−3.5 to 1.0) | 0.99 (0.14-7.12) |
| Men, aged 18-54 y | 128 | 12 682 | <5 | 111 | 865 | 90 242 | 0.0 | 1.3 | −1.3 (−1.5 to −1.0) | — |
| Men, aged ≥55 y | 87 | 8 818 | <5 | 278 | 384 | 49 545 | 3.9 | 5.1 | −1.2 (−5.6 to 3.2) | 1.46 (0.46-4.63) |
| Ischemic stroke | ||||||||||
| All | 653 | 65 300 | 29 | 519 | 3 852 | 434 040 | 5.4 | 1.2 | 4.2 (2.2-6.2) | 7.40 (5.02-10.91) |
| Women, aged 18-54 y | 343 | 34 285 | 5 | 57 | 2 212 | 236 163 | 1.5 | 0.3 | 1.2 (−0.1 to 2.6) | 6.47 (2.27-18.41) |
| Women, aged ≥55 y | 95 | 9 515 | 9 | 198 | 445 | 57 505 | 12.8 | 3.1 | 9.7 (1.6-17.9) | 6.63 (3.32-13.23) |
| Men, aged 18-54 y | 128 | 12 682 | 9 | 54 | 817 | 90 505 | 8.8 | 0.6 | 8.1 (2.5-13.8) | 20.20 (9.51-42.91) |
| Men, aged ≥55 y | 87 | 8 818 | 6 | 210 | 379 | 49 866 | 7.0 | 3.9 | 3.1 (−2.3 to 8.5) | 3.86 (1.66-8.99) |
| Major bleeding | ||||||||||
| All | 653 | 65 300 | 27 | 850 | 3 900 | 432 952 | 5.3 | 1.9 | 3.4 (1.3-5.5) | 3.69 (2.49-5.47) |
| Women, aged 18-54 y | 343 | 34 285 | <5 | 158 | 2 230 | 235 645 | 1.8 | 0.7 | 1.1 (−0.7 to 2.8) | 1.77 (0.63-4.99) |
| Women, aged ≥55 y | 95 | 9 515 | <10 | 289 | 464 | 57 323 | 11.1 | 4.6 | 6.5 (−1.0 to 14.1) | 3.97 (1.93-8.18) |
| Men, aged 18-54 y | 128 | 12 682 | 10 | 101 | 819 | 90 269 | 9.8 | 1.1 | 8.6 (2.6-14.7) | 9.75 (4.87-19.51) |
| Men, aged ≥55 y | 87 | 8 818 | 5 | 302 | 387 | 49 715 | 6.1 | 5.7 | 0.3 (−4.9 to 5.5) | 2.29 (0.92-5.70) |
| All-cause mortality | ||||||||||
| All | 653 | 65 300 | 82 | 3 221 | 3 985 | 435 586 | 15.8 | 7.5 | 8.2 (4.9-11.6) | 3.22 (2.57-4.04) |
| Women, aged 18-54 y | 343 | 34 285 | 17 | 182 | 2 246 | 236 335 | 5.3 | 0.8 | 4.5 (2.0-7.0) | 5.99 (3.45-10.38) |
| Women, aged ≥55 y | 95 | 9 515 | 31 | 1 671 | 480 | 58 062 | 43.1 | 26.7 | 16.4 (3.7-29.1) | 2.82 (1.96-4.05) |
| Men, aged 18-54 y | 128 | 12 682 | 10 | 172 | 865 | 90 696 | 9.7 | 2.1 | 7.6 (1.7-13.5) | 3.96 (1.96-7.99) |
| Men, aged ≥55 y | 87 | 8 818 | 24 | 1 196 | 394 | 50 494 | 39.1 | 23.7 | 15.3 (1.4-29.3) | 2.50 (1.65-3.79) |
Controlled for matching factors (age, sex, and calendar period) by design and adjusted for cancer, recent infection, fracture/trauma, surgery, or pregnancy, previous VTE, myocardial infarction, ischemic and hemorrhagic stroke, major bleeding, other ischemic heart disease, atrial fibrillation, hypertension, diabetes, obesity, peripheral artery disease, chronic liver disease, and chronic pulmonary disease.
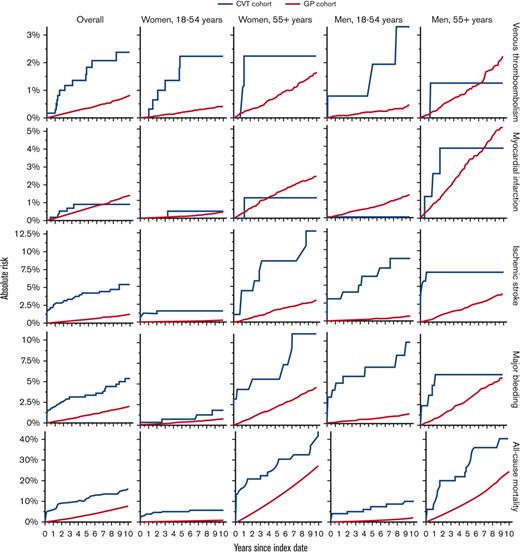
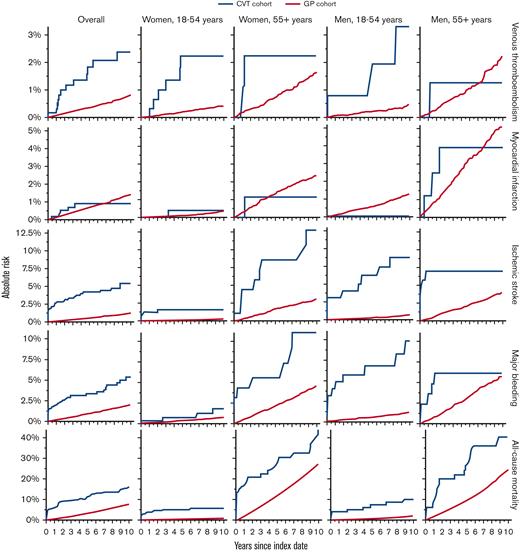
A similar pattern was observed for VTE at other sites (Table 2; Figure 2). Among the patients with CVT, we observed 12 events overall, 6 (50%) of which occurred among young women. The 10-year risks for patients with CVT compared with the GP cohort were overall 2.4% vs 0.8% (risk difference, 1.6%; 95% CI, 0.2-3.0) (adjusted HR, 3.95; 95% CI, 2.20-7.11) and among young women 2.2% vs 0.4% (risk difference, 1.8%; 95% CI, 0.0-3.6) (adjusted HR, 6.55; 95% CI, 2.79-15.35).
Absolute risks of VTE in other sites, myocardial infarction, ischemic stroke, major bleeding, and all-cause mortality, comparing patients with a first-time diagnosis of CVT with matched individuals from the GP. Recurrent CVT not depicted, as no contrast with GP comparators was performed.
Absolute risks of VTE in other sites, myocardial infarction, ischemic stroke, major bleeding, and all-cause mortality, comparing patients with a first-time diagnosis of CVT with matched individuals from the GP. Recurrent CVT not depicted, as no contrast with GP comparators was performed.
Based on 5 myocardial infarction events among patients with CVT, the risk of this outcome appeared similar among patients and comparators. Overall, the 10-year risks were 0.9% vs 1.4% in the 2 cohorts (risk difference, −0.5%; 95% CI, −1.3 to 0.3) (adjusted HR, 0.93; 95% CI, 0.38-2.26). In contrast, based on 29 events among patients with CVT, the risk of ischemic stroke was elevated overall and across all age and sex strata compared with the GP cohort, although most noticeably in older women. The 10-year risks were overall 5.4% vs 1.2% (risk difference, 4.2%; 95% CI, 2.2-6.2) (adjusted HR, 7.40; 95% CI, 5.02-10.91) and among older women 12.8% vs 3.1% (risk difference, 9.7%; 95% CI, 1.6-17.9) (adjusted HR, 6.63; 95% CI, 3.32-13.23).
Based on 27 events, the risk of major bleeding was higher among patients with CVT than among comparators across all age and sex strata, although less pronounced in young women. The 10-year risks were overall 5.3% vs 1.9% (risk difference, 3.4%; 95% CI, 1.3-5.5) (adjusted HR, 3.69; 95% CI, 2.49, 5.47) and among young women 1.8% vs 0.7% (risk difference, 1.1%; 95% CI, −0.7 to 2.8) (adjusted HR, 1.77; 95% CI, 0.63-4.99).
We observed 82 deaths among the patients with CVT, of which 55 (67%) occurred among older women and men. Mortality increased overall and across all age and sex strata of the CVT cohort compared with the GP cohort, although older women and older men had the highest absolute risks. The 10-year risks were overall 15.8% vs 7.5% (risk difference, 8.2%; 95% CI, 4.9-11.6) (adjusted HR, 3.22; 95% CI, 2.57-4.04), among older women 43.1% vs 26.7% (risk difference, 16.4%; 95% CI, 3.7-29.1) (adjusted HR, 2.82; 95% CI, 1.96-4.05), and among older men 39.1% vs 23.7% (risk difference, 15.3%; 95% CI, 1.4-29.3) (adjusted HR, 2.50; 95% CI, 1.65-3.79).
Risk differences and HRs for different follow-up periods are shown in supplemental Tables 4 and 5, respectively.
The results of the further subgroup analyses are shown in supplemental Table 6. Risk differences for most outcomes tended to be higher among those with a history of cancer than among those without this history, as well as in young women without a history of hormonal treatment than in those with this history.
Secondary outcomes
The association between deep vein thrombosis and pulmonary embolism was broadly similar (supplemental Table 7). Of the 27 major bleeding events, 15 (56%) had hemorrhagic strokes and 6 (22%) had gastrointestinal tract bleeding (supplemental Table 7). The increased mortality associated with CVT was driven by conditions of the circulatory system, although mortality associated with both respiratory system conditions and neoplasms also was increased (Table 3). As in the case of all-cause mortality, absolute risks and differences were highest among older women and older men.
Numbers, events, person-years, 10-year risks, and overall (0-10 years) adjusted HRs of mortality owing to conditions of the circulatory system, respiratory system, and neoplasms, comparing patients with a first-time diagnosis of CVT with matched individuals from the GP
| . | N . | Events, N . | Person-years, n . | Risk, % . | Adjusted HR∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVT . | GP . | CVT . | GP . | CVT . | GP . | CVT . | GP . | Difference . | ||
| Mortality, circulatory system | ||||||||||
| All | 612 | 63 349 | 26 | 590 | 3 816 | 423 016 | 5.4 | 1.5 | 4.0 (1.8-6.1) | 8.69 (5.73-13.16) |
| Women, aged 18-54 y | 333 | 34 136 | 5 | 19 | 2 201 | 235 222 | 1.6 | 0.1 | 1.5 (0.1-2.8) | 26.01 (7.58-89.24) |
| Women, aged ≥55 y | 77 | 8 619 | 9 | 348 | 398 | 52 552 | 17.9 | 6.6 | 11.3 (−0.3 to 22.8) | 7.01 (3.50-14.06) |
| Men, aged 18-54 y | 123 | 12 520 | 5 | 20 | 849 | 89 449 | 5.3 | 0.2 | 5.0 (0.3-9.7) | 22.37 (7.02-71.30) |
| Men, aged ≥55 y | 79 | 8 074 | 7 | 203 | 368 | 45 792 | 10.5 | 4.8 | 5.8 (−2.0 to 13.5) | 5.17 (2.34-11.41) |
| Mortality, respiratory system | ||||||||||
| All | 612 | 63 349 | 7 | 523 | 3 816 | 423 016 | 1.6 | 1.3 | 0.3 (−0.9 to 1.5) | 1.74 (0.81-3.72) |
| Women, aged 18-54 y | 333 | 34 136 | 0 | 29 | 2 201 | 235 222 | 0.0 | 0.1 | −0.1 (−0.2 to −0.1) | — |
| Women, aged ≥55 y | 77 | 8 619 | <5 | 282 | 398 | 52 552 | 5.3 | 5.4 | −0.1 (−6.1 to 6.0) | 1.72 (0.54-5.46) |
| Men, aged 18-54 y | 123 | 12 520 | 0 | 10 | 849 | 89 449 | 0.0 | 0.1 | −0.1 (−0.2 to −0.0) | — |
| Men, aged ≥55 y | 79 | 8 074 | <5 | 202 | 368 | 45 792 | 9.8 | 5.0 | 4.8 (−5.6 to 15.2) | 2.64 (0.94-7.37) |
| Mortality, neoplasms | ||||||||||
| All | 612 | 63 349 | 13 | 638 | 3 816 | 423 016 | 2.5 | 1.5 | 1.0 (−0.4 to 2.4) | 1.98 (1.12-3.49) |
| Women, aged 18-54 y | 333 | 34 136 | <5 | 55 | 2 201 | 235 222 | 1.4 | 0.3 | 1.2 (−0.3 to 2.6) | 3.87 (1.21-12.43) |
| Women, aged ≥55 y | 77 | 8 619 | <5 | 292 | 398 | 52 552 | 6.1 | 5.4 | 0.7 (−5.3 to 6.6) | 1.78 (0.65-4.86) |
| Men, aged 18-54 y | 123 | 12 520 | <5 | 28 | 849 | 89 449 | 2.0 | 0.3 | 1.7 (−1.1 to 4.5) | 6.56 (1.41-30.58) |
| Men, aged ≥55 y | 79 | 8 074 | <5 | 263 | 368 | 45 792 | 4.9 | 5.7 | −0.8 (−6.4 to 4.8) | 0.97 (0.30-3.14) |
| . | N . | Events, N . | Person-years, n . | Risk, % . | Adjusted HR∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVT . | GP . | CVT . | GP . | CVT . | GP . | CVT . | GP . | Difference . | ||
| Mortality, circulatory system | ||||||||||
| All | 612 | 63 349 | 26 | 590 | 3 816 | 423 016 | 5.4 | 1.5 | 4.0 (1.8-6.1) | 8.69 (5.73-13.16) |
| Women, aged 18-54 y | 333 | 34 136 | 5 | 19 | 2 201 | 235 222 | 1.6 | 0.1 | 1.5 (0.1-2.8) | 26.01 (7.58-89.24) |
| Women, aged ≥55 y | 77 | 8 619 | 9 | 348 | 398 | 52 552 | 17.9 | 6.6 | 11.3 (−0.3 to 22.8) | 7.01 (3.50-14.06) |
| Men, aged 18-54 y | 123 | 12 520 | 5 | 20 | 849 | 89 449 | 5.3 | 0.2 | 5.0 (0.3-9.7) | 22.37 (7.02-71.30) |
| Men, aged ≥55 y | 79 | 8 074 | 7 | 203 | 368 | 45 792 | 10.5 | 4.8 | 5.8 (−2.0 to 13.5) | 5.17 (2.34-11.41) |
| Mortality, respiratory system | ||||||||||
| All | 612 | 63 349 | 7 | 523 | 3 816 | 423 016 | 1.6 | 1.3 | 0.3 (−0.9 to 1.5) | 1.74 (0.81-3.72) |
| Women, aged 18-54 y | 333 | 34 136 | 0 | 29 | 2 201 | 235 222 | 0.0 | 0.1 | −0.1 (−0.2 to −0.1) | — |
| Women, aged ≥55 y | 77 | 8 619 | <5 | 282 | 398 | 52 552 | 5.3 | 5.4 | −0.1 (−6.1 to 6.0) | 1.72 (0.54-5.46) |
| Men, aged 18-54 y | 123 | 12 520 | 0 | 10 | 849 | 89 449 | 0.0 | 0.1 | −0.1 (−0.2 to −0.0) | — |
| Men, aged ≥55 y | 79 | 8 074 | <5 | 202 | 368 | 45 792 | 9.8 | 5.0 | 4.8 (−5.6 to 15.2) | 2.64 (0.94-7.37) |
| Mortality, neoplasms | ||||||||||
| All | 612 | 63 349 | 13 | 638 | 3 816 | 423 016 | 2.5 | 1.5 | 1.0 (−0.4 to 2.4) | 1.98 (1.12-3.49) |
| Women, aged 18-54 y | 333 | 34 136 | <5 | 55 | 2 201 | 235 222 | 1.4 | 0.3 | 1.2 (−0.3 to 2.6) | 3.87 (1.21-12.43) |
| Women, aged ≥55 y | 77 | 8 619 | <5 | 292 | 398 | 52 552 | 6.1 | 5.4 | 0.7 (−5.3 to 6.6) | 1.78 (0.65-4.86) |
| Men, aged 18-54 y | 123 | 12 520 | <5 | 28 | 849 | 89 449 | 2.0 | 0.3 | 1.7 (−1.1 to 4.5) | 6.56 (1.41-30.58) |
| Men, aged ≥55 y | 79 | 8 074 | <5 | 263 | 368 | 45 792 | 4.9 | 5.7 | −0.8 (−6.4 to 4.8) | 0.97 (0.30-3.14) |
Controlled for matching factors (age, sex, and calendar period) by design and adjusted for cancer, recent infection, fracture/trauma, surgery, or pregnancy, previous VTE, myocardial infarction, ischemic and hemorrhagic stroke, major bleeding, other ischemic heart disease, atrial fibrillation, hypertension, diabetes, obesity, peripheral artery disease, chronic liver disease, and chronic pulmonary disease.
Additional analyses
In the CVT subcohort of 488 patients (75%) who were prescribed an anticoagulant within 180 days after their index date, 32 (7%) continued treatment beyond 31 December 2018, 143 (29%) were diagnosed with recurrent CVT, VTE at other sites, stroke of any subtype, major bleeding, and <5 (<1%) died. The remaining 309 patients (63%) discontinued treatment after a median duration of 236 days (7.8 months). In general, patients with CVT who discontinued anticoagulant therapy remained at an increased risk of recurrent VTE events, bleeding, and mortality, with largely the same age and sex patterns as observed in the main analyses, although estimates were less precise (Table 4).
Numbers, events, person-years, 10-year risks, and overall (0-10 years) adjusted HRs of CVT recurrence, VTE in other sites, myocardial infarction, ischemic stroke, and all-cause mortality comparing patients with a first-time diagnosis of CVT and matched comparators from the GP; and additional analyses of patients with CVT initiation and discontinuing anticoagulants
| . | N . | Events, N . | Person-years, n . | Risk, % . | Adjusted HR (95% CI)∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVT . | GP . | CVT . | GP . | CVT . | GP . | CVT . | GP . | Difference (95% CI) . | ||
| CVT recurrence | ||||||||||
| All | 309 | 30 900 | 5 | — | 1 831 | — | 2.4 | — | — | — |
| Women, aged 18-54 y | 174 | 17 483 | <5 | — | 1 102 | — | 3.2 | — | — | — |
| Women, aged ≥55 y | 43 | 4 217 | 0 | — | 214 | — | — | — | — | — |
| Men, aged 18-54 y | 56 | 5 630 | <5 | — | 352 | — | 2.6 | — | — | — |
| Men, aged ≥55 y | 36 | 3 570 | 0 | — | 164 | — | — | — | — | — |
| VTE | ||||||||||
| All | 309 | 30 900 | <5 | 135 | 1 848 | 190 135 | 2.5 | 0.7 | 1.8 (−0.8 to 4.3) | 2.33 (0.82-6.62) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 55 | 1 115 | 115 324 | 3.0 | 0.5 | 2.5 (−1.0 to 6.0) | 4.19 (1.21-14.50) |
| Women, aged ≥55 y | 43 | 4 217 | 0 | 26 | 214 | 20 972 | 0.0 | 1.3 | −1.3 (−1.8 to −0.8) | — |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 24 | 355 | 36 578 | 3.6 | 0.7 | 2.9 (−4.1 to 9.9) | 3.99 (0.44-35.96) |
| Men, aged ≥55 y | 36 | 3 570 | 0 | 30 | 164 | 17 261 | 0.0 | 1.6 | −1.6 (−2.2 to −1.0) | — |
| Myocardial infarction | ||||||||||
| All | 309 | 30 900 | <5 | 212 | 1 853 | 189 831 | 0.7 | 1.1 | −0.4 (−1.4 to 0.7) | 0.83 (0.20-3.37) |
| Women, aged 18-54 y | 174 | 17 483 | 0 | 31 | 1 129 | 115 443 | 0.0 | 0.3 | −0.3 (−0.4 to −0.2) | — |
| Women, aged ≥55 y | 43 | 4 217 | 0 | 61 | 214 | 20 817 | 0.0 | 2.8 | −2.8 (−3.5 to −2.0) | — |
| Men, aged 18-54 y | 56 | 5 630 | 0 | 49 | 356 | 36 457 | 0.0 | 1.3 | −1.3 (−1.7 to −0.9) | — |
| Men, aged ≥55 y | 36 | 3 570 | <5 | 71 | 155 | 17 114 | 6.9 | 4.2 | 2.7 (−6.6 to 12.1) | 2.74 (0.64-11.64) |
| Ischemic stroke | ||||||||||
| All | 309 | 30 900 | 6 | 195 | 1 839 | 190 069 | 2.3 | 1.1 | 1.2 (−0.6 to 3.1) | 3.21 (1.41-7.31) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 24 | 1 127 | 115 460 | 0.6 | 0.2 | 0.4 (−0.8 to 1.6) | 4.53 (0.56-36.83) |
| Women, aged ≥55 y | 43 | 4 217 | <5 | 68 | 196 | 20 895 | 11.0 | 3.6 | 7.5 (−3.0 to 17.9) | 4.67 (1.58-13.81) |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 28 | 352 | 36 543 | 2.5 | 0.8 | 1.7 (−3.1 to 6.6) | 3.16 (0.42-23.60) |
| Men, aged ≥55 y | 36 | 3 570 | 0 | 75 | 164 | 17 171 | 0.0 | 4.3 | −4.3 (−5.4 to −3.3) | — |
| Major bleeding | ||||||||||
| All | 309 | 30 900 | 8 | 307 | 1 849 | 189 674 | 5.1 | 1.7 | 3.4 (−0.2 to 7.0) | 2.56 (1.26-5.20) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 88 | 1 126 | 115 235 | 1.0 | 0.8 | 0.1 (−1.7 to 2.0) | 1.16 (0.16-8.46) |
| Women, aged ≥55 y | 43 | 4 217 | <5 | 76 | 207 | 20 828 | 16.8 | 3.8 | 13.1 (−5.1 to 31.2) | 3.82 (1.18-12.42) |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 48 | 354 | 36 500 | 11.0 | 1.4 | 9.6 (−2.5 to 21.7) | 4.68 (1.36-16.11) |
| Men, aged ≥55 y | 36 | 3 570 | <5 | 95 | 162 | 17 110 | 3.1 | 5.5 | −2.3 (−8.5 to 3.8) | 1.11 (0.15-8.24) |
| All-cause mortality | ||||||||||
| All | 309 | 30 900 | 18 | 876 | 1 863 | 190 623 | 10.3 | 4.8 | 5.5 (0.7-10.3) | 2.01 (1.25-3.24) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 107 | 1 129 | 115 553 | 2.2 | 1.0 | 1.1 (−1.4 to 3.6) | 1.82 (0.53-6.18) |
| Women, aged ≥55 y | 43 | 4 217 | 7 | 358 | 214 | 21 054 | 41.7 | 18.4 | 23.3 (−1.9 to 48.5) | 1.86 (0.86-4.01) |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 75 | 356 | 36 663 | 9.6 | 2.1 | 7.4 (−3.1 to 18.0) | 2.27 (0.64-8.03) |
| Men, aged ≥55 y | 36 | 3 570 | 5 | 336 | 164 | 17 353 | 24.9 | 19.9 | 5.0 (−15.2 to 25.2) | 1.93 (0.79-4.71) |
| . | N . | Events, N . | Person-years, n . | Risk, % . | Adjusted HR (95% CI)∗ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVT . | GP . | CVT . | GP . | CVT . | GP . | CVT . | GP . | Difference (95% CI) . | ||
| CVT recurrence | ||||||||||
| All | 309 | 30 900 | 5 | — | 1 831 | — | 2.4 | — | — | — |
| Women, aged 18-54 y | 174 | 17 483 | <5 | — | 1 102 | — | 3.2 | — | — | — |
| Women, aged ≥55 y | 43 | 4 217 | 0 | — | 214 | — | — | — | — | — |
| Men, aged 18-54 y | 56 | 5 630 | <5 | — | 352 | — | 2.6 | — | — | — |
| Men, aged ≥55 y | 36 | 3 570 | 0 | — | 164 | — | — | — | — | — |
| VTE | ||||||||||
| All | 309 | 30 900 | <5 | 135 | 1 848 | 190 135 | 2.5 | 0.7 | 1.8 (−0.8 to 4.3) | 2.33 (0.82-6.62) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 55 | 1 115 | 115 324 | 3.0 | 0.5 | 2.5 (−1.0 to 6.0) | 4.19 (1.21-14.50) |
| Women, aged ≥55 y | 43 | 4 217 | 0 | 26 | 214 | 20 972 | 0.0 | 1.3 | −1.3 (−1.8 to −0.8) | — |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 24 | 355 | 36 578 | 3.6 | 0.7 | 2.9 (−4.1 to 9.9) | 3.99 (0.44-35.96) |
| Men, aged ≥55 y | 36 | 3 570 | 0 | 30 | 164 | 17 261 | 0.0 | 1.6 | −1.6 (−2.2 to −1.0) | — |
| Myocardial infarction | ||||||||||
| All | 309 | 30 900 | <5 | 212 | 1 853 | 189 831 | 0.7 | 1.1 | −0.4 (−1.4 to 0.7) | 0.83 (0.20-3.37) |
| Women, aged 18-54 y | 174 | 17 483 | 0 | 31 | 1 129 | 115 443 | 0.0 | 0.3 | −0.3 (−0.4 to −0.2) | — |
| Women, aged ≥55 y | 43 | 4 217 | 0 | 61 | 214 | 20 817 | 0.0 | 2.8 | −2.8 (−3.5 to −2.0) | — |
| Men, aged 18-54 y | 56 | 5 630 | 0 | 49 | 356 | 36 457 | 0.0 | 1.3 | −1.3 (−1.7 to −0.9) | — |
| Men, aged ≥55 y | 36 | 3 570 | <5 | 71 | 155 | 17 114 | 6.9 | 4.2 | 2.7 (−6.6 to 12.1) | 2.74 (0.64-11.64) |
| Ischemic stroke | ||||||||||
| All | 309 | 30 900 | 6 | 195 | 1 839 | 190 069 | 2.3 | 1.1 | 1.2 (−0.6 to 3.1) | 3.21 (1.41-7.31) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 24 | 1 127 | 115 460 | 0.6 | 0.2 | 0.4 (−0.8 to 1.6) | 4.53 (0.56-36.83) |
| Women, aged ≥55 y | 43 | 4 217 | <5 | 68 | 196 | 20 895 | 11.0 | 3.6 | 7.5 (−3.0 to 17.9) | 4.67 (1.58-13.81) |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 28 | 352 | 36 543 | 2.5 | 0.8 | 1.7 (−3.1 to 6.6) | 3.16 (0.42-23.60) |
| Men, aged ≥55 y | 36 | 3 570 | 0 | 75 | 164 | 17 171 | 0.0 | 4.3 | −4.3 (−5.4 to −3.3) | — |
| Major bleeding | ||||||||||
| All | 309 | 30 900 | 8 | 307 | 1 849 | 189 674 | 5.1 | 1.7 | 3.4 (−0.2 to 7.0) | 2.56 (1.26-5.20) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 88 | 1 126 | 115 235 | 1.0 | 0.8 | 0.1 (−1.7 to 2.0) | 1.16 (0.16-8.46) |
| Women, aged ≥55 y | 43 | 4 217 | <5 | 76 | 207 | 20 828 | 16.8 | 3.8 | 13.1 (−5.1 to 31.2) | 3.82 (1.18-12.42) |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 48 | 354 | 36 500 | 11.0 | 1.4 | 9.6 (−2.5 to 21.7) | 4.68 (1.36-16.11) |
| Men, aged ≥55 y | 36 | 3 570 | <5 | 95 | 162 | 17 110 | 3.1 | 5.5 | −2.3 (−8.5 to 3.8) | 1.11 (0.15-8.24) |
| All-cause mortality | ||||||||||
| All | 309 | 30 900 | 18 | 876 | 1 863 | 190 623 | 10.3 | 4.8 | 5.5 (0.7-10.3) | 2.01 (1.25-3.24) |
| Women, aged 18-54 y | 174 | 17 483 | <5 | 107 | 1 129 | 115 553 | 2.2 | 1.0 | 1.1 (−1.4 to 3.6) | 1.82 (0.53-6.18) |
| Women, aged ≥55 y | 43 | 4 217 | 7 | 358 | 214 | 21 054 | 41.7 | 18.4 | 23.3 (−1.9 to 48.5) | 1.86 (0.86-4.01) |
| Men, aged 18-54 y | 56 | 5 630 | <5 | 75 | 356 | 36 663 | 9.6 | 2.1 | 7.4 (−3.1 to 18.0) | 2.27 (0.64-8.03) |
| Men, aged ≥55 y | 36 | 3 570 | 5 | 336 | 164 | 17 353 | 24.9 | 19.9 | 5.0 (−15.2 to 25.2) | 1.93 (0.79-4.71) |
Controlled for matching factors (age, sex, and calendar period) by design and adjusted for cancer, recent infection, fracture/trauma, surgery, or pregnancy, previous VTE, myocardial infarction, ischemic and hemorrhagic stroke, major bleeding, other ischemic heart disease, atrial fibrillation, hypertension, diabetes, obesity, peripheral artery disease, chronic liver disease, and chronic pulmonary disease.
Among 192 patients with CVT with a previous outcome before the index date (excluded from the main analyses) and corresponding matched comparators, the all-cause mortality risk was substantially higher than that in the main analyses (supplemental Table 8). For other outcomes, the absolute risk differences were slightly higher than those reported in the main analyses, although the relative risks were slightly lower.
Validation substudy
We identified 64 patients with CVT codes. Among these patients, 56 had relevant symptoms and a positive computed tomography or magnetic resonance imaging venogram–confirmed CVT (positive predictive value, 88%; 95% CI, 77-94). The positive predictive value was higher for primary diagnoses (93%; 95% CI, 82-98) than for secondary diagnoses (60%; 95% CI, 26-88), and it was higher for inpatients (96%; 95% CI, 86-99%) than for outpatients (63%; 95% CI, 35-85).
Discussion
Patients with CVT were at an increased risk of VTE at other sites, ischemic stroke, major bleeding, and mortality compared with individuals from the GP matched on age, sex, and calendar year. The magnitude of these risks depended on sex and age at the time of diagnosis.
Our study builds on the current understanding of the incidence and clinical course of CVT. Our findings regarding the annual CVT incidence rate (1.2 per 100 000 person-years) corroborate those from recent US (1.4-2.0),1 Australian (1.6),25 and Dutch (1.3)26 studies, whereas older studies found lower annual rates (0.2-0.5).27,28 The rising incidence of CVT is likely explained by improved CVT ascertainment and more widespread use of neuroimaging, for example, following headache29 or trauma. Other contributing factors may include an increased prevalence of risk factors for CVT (eg, obesity was found to be associated with CVT in women using hormonal contraceptives)30 and a growing number of cancer survivors. The rising incidence of CVT was not related to female hormones, as the number of younger women per 1000 inhabitants who received a prescription for hormones decreased in the period from 1999-2021 in Denmark.31
Our study also expands the existing knowledge regarding the absolute risk of recurrent CVT and other VTE events among patients with CVT. Previous reports were constrained by their limited sample sizes (approximately n <650),7,10,11 short follow-up periods (1-6 years),6,7,9,13 time period (˃20 years ago),5-7 and failure to consider the competing risk of death.5-13 In addition, previous studies did not stratify their analyses according to age and sex, although the distribution of predisposing risk factors varied substantially across these subgroups. Our results for CVT recurrence were lower than those of a previous study on CVT recurrence risk (1-year risk, 12%),5 whereas our results for the risk of nonfatal VTE also were lower than those of a multicenter study with a median follow-up of 40 months (recurrent CVT, 4.4%; VTE at other sites, 6.5%)10 and in a large US cohort study based on administrative databases (5-year risk of pulmonary embolism, 3.4%).13 In contrast, several other studies have suggested that the risk of recurrent CVT was less frequent, which is largely in line with our results. These include the multicenter International Study on Cerebral Vein and Dural Sinus Thrombosis (CVT recurrence risk, 2% during a median follow-up of 16 months),7 a French study of pregnant women with prior CVT (1 recurrent CVT among 82 pregnancies),12 and the VENOPORT study.6 The reasons for these discrepancies are not completely understood but could be partly related to disparities in outcome definitions, the composition of the study population, and loss to follow-up encountered in many previous studies. Importantly, none of the previous studies have used a comparison cohort from the GP, which permits the contextualization of the risk estimates for patients with CVT concerning the risk expected in the GP. To better understand the excess mortality among patients with CVT, we also investigated the causes of death. We provided evidence that circulatory system–related mortality was the main driver of mortality during 10 years of follow-up, although both respiratory- and neoplasm-related mortality also increased. Thus, consistent with a recent Dutch study,32 CVT may be a marker of cancer, particularly hematologic malignancies. We also included data on arterial thromboembolic events to understand the broad spectrum of cardiovascular conditions. Although previous reports have indicated a link between VTE and subsequent acute arterial events,33 this was not apparent in our study, based on the findings of myocardial infarction. The high risk of ischemic stroke following CVT, as observed in our and other studies,34 likely represents subsequent hospitalizations for complications of CVT. Thus, it is likely that our finding regarding ischemic stroke is partly an artifact of misclassification among the stroke codes.
An understanding of the risk of recurrent CVT, VTE at other sites, and complications following CVT needs to be balanced with the risk of serious bleeding events. To the best of our knowledge, data on bleeding risk among patients with CVT have been sparsely investigated.35 In a case series of 45 patients with cancer-associated CVT, followed for 12 months, major bleeding occurred in 15 patients,35 among whom 13 had an intracerebral hemorrhage. Similarly, our findings suggest a substantially higher risk of major bleeding following hospitalization for CVT compared with the GP, particularly among older women and men.
Although decisions regarding the duration of anticoagulation treatment for CVT should always be individualized, our age- and sex-stratified data may guide clinical decision making. Although the risk of major bleeding was higher than that in the GP, our data also suggest that the risk of VTE events exceeded that of major bleeding, particularly among young women. In an additional analysis of patients who stopped anticoagulant treatment, an increased risk of VTE events persisted, which suggests that these patients may have had a lower threshold for venous events. Notably, our data also suggested a shift toward increasing the use of direct oral anticoagulants during the study period, although only a few studies have compared the efficacy of these drugs with those of dose–adjusted warfarin in preventing recurrent VTE events in patients with CVT.36,37
The strengths of our study include its nationwide, population-based design and a uniform healthcare system with a virtually complete 10-year follow-up. This reduced the potential for selection bias. We used diagnostic codes for cardiovascular and bleeding outcomes that have high positive predictive values in the DNPR.38,39 We are hesitant to provide firm conclusions regarding our findings for CVT recurrence; our validation substudy aimed to validate first-time CVT diagnoses and not recurrences, which would have required a larger sample. Instead, we relied on a previously applied algorithm used for VTE recurrence,21 wherein early second hospitalizations with an International Classification of Disease diagnostic code for VTE were not counted because during the early period of anticoagulation treatment, recurrences are clinically known to be rare. This is also clinically known for CVT; an early second hospitalization with a diagnostic code for CVT is more likely to be attributable to CVT complications. However, it is possible that, in using this definition, we missed some early cases of CVT recurrence, thereby potentially underestimating absolute recurrence risks.20 The prevalence of thrombophilia was lower than that in previous reports,40,41 reflecting a low sensitivity for thrombophilia codes in the DNPR. In Denmark, only patients with CVT aged <50 years are recommended to undergo thrombophilia testing,22 and comparison cohort members from the GP will rarely be tested for thrombophilia. We also lacked data on some risk factors for thromboembolic and bleeding events, such as smoking and alcohol abuse. However, we likely captured some of the effects of these variables indirectly, by adjusting for a large set of correlated comorbidities. Finally, our findings are likely generalizable to populations with similar healthcare systems and societal structures, for example, other Northern European countries; although, the extent to which our findings apply to other populations remains unclear.
In conclusion, patients with CVT were at increased risk of recurrent CVT, VTE at other sites, ischemic stroke, major bleeding, and mortality, however, the magnitude of these risks depended on sex and age at the time of diagnosis.
Acknowledgments
This study was internally funded by the Department of Clinical Epidemiology, Aarhus University and Aarhus University Hospital.
The Department of Clinical Epidemiology, Aarhus University and Aarhus University Hospital, received funding from private and public institutions in the form of research grants to (and administered by) Aarhus University. None of these grants has any relation to the present study.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Authorship
Contribution: N.S., H.T.S., and K.A. acquired the data and directed the analyses; N.S. performed data analyses; N.S. and K.A. wrote the initial draft; and all authors contributed to the study design and the discussion and interpretation of the results, which secured the intellectual content of the manuscript, and approved the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nils Skajaa, Department of Clinical Epidemiology, Aarhus University Hospital, Olof Palmes Allé 43-45, DK, 8200 Aarhus, Denmark; e-mail: nilsskajaa@clin.au.dk.
References
Author notes
Data presented in this study were obtained from Danish registries. Owing to data protection rules, we are not allowed to share individual-level data. Other researchers who fulfill the requirements set by the data providers could obtain similar data.
Data are available on request from the corresponding author, Nils Skajaa (nilsskajaa@clin.au.dk).
The full-text version of this article contains a data supplement.