TO THE EDITOR:
Despite the exceptional initial response rates with chimeric antigen receptor (CAR) T-cell therapy in large B-cell lymphoma (LBCL), the duration of response (DOR) is short and more than half the patients relapse.1,2 Patients who fail to respond or relapse after CAR T-cell therapy have a poor prognosis, especially those who relapse within 30 days.3 Treatment options after CAR T-cell therapy are limited and persistent cytopenias can limit the use of salvage therapy or participation in clinical trials.4 In a study by Spiegel et al, 26% of the patients who progressed after CAR T-cell received supportive care whereas 74% received various therapies.5 The overall response rate (ORR) in more commonly used therapies was 29%, with only 17% of the patients achieving a complete response (CR) while median progression-free survival (PFS) and overall survival (OS) were 55 days and 180 days, respectively.
Although multiple new agents are available for relapsed LBCL, durable remissions in the post-CAR T-cell therapy setting are uncommon. In the pre–CAR T-cell therapy era, for patients who relapsed after autologous stem cell transplant (auto-HCT), allogeneic transplant (allo-HCT) was a curative intent procedure offered to chemosensitive patients. This procedure has fallen out of favor in light of novel immunotherapies, targeted agents, and cell therapies (Figure 1A). However, in patients with lymphoma failing CAR T-cell therapy, allo-HCT may benefit selected patients.6-9 We report below our experience using polatuzumab as a salvage agent and a bridge to allo-HCT, in patients with non-Hodgkin lymphoma (NHL) who failed CD19-based CAR T-cell therapy, as an approach to achieve long term remission.
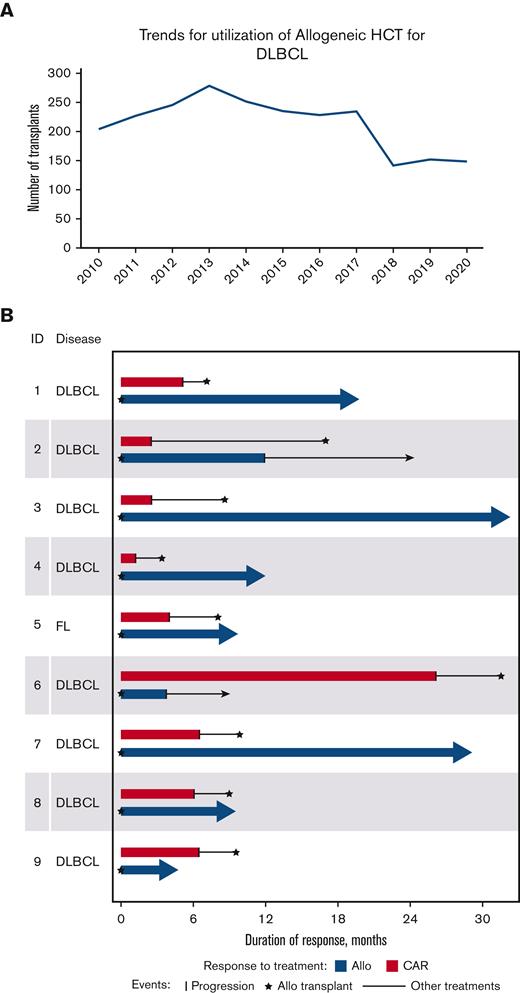
Decreasing trends of allo-HCT over the last decade (A) and duration of response of patients after CAR T (red) and after polatuzumab followed by allo-HCT (blue). (A) Decreasing trend of Utilization of Allogeneic Transplant in DLBCL provided by the Center for International Blood and Marrow Transplant Research (CIBMTR), (B) Swimmers Plot reporting duration of response after CAR T-cell therapy and after allo-HCT by subject.
Decreasing trends of allo-HCT over the last decade (A) and duration of response of patients after CAR T (red) and after polatuzumab followed by allo-HCT (blue). (A) Decreasing trend of Utilization of Allogeneic Transplant in DLBCL provided by the Center for International Blood and Marrow Transplant Research (CIBMTR), (B) Swimmers Plot reporting duration of response after CAR T-cell therapy and after allo-HCT by subject.
We performed a retrospective, single-center analysis of patients with relapsed or refractory B-cell NHL who relapsed after CD19 based CAR T-cell therapy and subsequently received polatuzumab based salvage chemotherapy. This was approved by the Medical College of Wisconsin Institutional Review Board and informed consent was waived. Data were obtained from electronic medical record. Descriptive statistics were used to detail baseline characteristics. The Kaplan-Meier methodology was used to calculate OS and PFS graphs. Statistical analyses were performed using STATA v13.1 and SAS v9.4.
We identified 17 patients with B-cell NHL who relapsed after CAR T-cell therapy and received polatuzumab based treatment for the first time. The ORR was 71% (2, partial response [PR]; 10, CR). Two patients received only polatuzumab and rituximab whereas bendamustine or lenalidomide was added to 12 and 3 patients, respectively.
Out of these 17 patients, 9 patients underwent allo-HCT (Table 1). The median age of these patients was 66 years (range, 41-72); 8 had diffuse LBCL whereas 1 had grade 3 follicular lymphoma. For these 9 patients, the day-28 ORR to initial CAR T-cell therapy on positron emission tomography (PET) was 89% (CR = 7; PR = 1) whereas the median DOR was 5 months (range, 1-26). For relapse after CAR T-cell therapy, the median lines of therapy were 1 (range, 1-4). Median cycles of polatuzumab were 3 (range, 2-4), and 6 out of 9 patients received polatuzumab as their first salvage treatment. After polatuzumab and before allo-HCT, 8 patients were in CR, whereas 1 had a PR. All patients then received reduced intensity or nonmyeloablative conditioning (RIC/NMA) followed by peripheral blood mobilized stem cells, 5 of which were matched as related, 3 were matched unrelated, and 1 was a haploidentical transplant. All patients received calcineurin inhibitor-based prophylaxis for GVHD while the 1 patient with haploidentical allo-HCT also received post-transplant cyclophosphamide. The median times to neutrophil and platelet recovery were 16 days (range, 4-18) and 13 days (range, 8-29), respectively. Five patients had acute GVHD, 1 of which was grade 4. Only 1 patient had moderate chronic GVHD. At day 90 after allo-HCT, the ORR was 89% on PET (CR = 8; PR = 0). Seven of the 9 patients remain in CR to date with a median follow-up of 12 months (range, 4.7-32.4). Similarly, 7 of the 9 patients have experienced remission inversion with a longer remission after allo-HCT than that of CAR T-cell therapy (Figure 1B). Median PFS was not reached (supplemental Figure 1), and all patients remain alive (supplemental Figure 2) and are currently without evidence of disease (including the 2 patients who relapsed).
Baseline characteristics of patients receiving allo-HCT after polatuzumab bridging (n = 9)
| Patient No. . | Histologic diagnosis, COO . | Age at Allo-HCT/Sex . | CAR T-cell Therapy Administered . | No. lines of therapy prior to CAR . | Response to CAR . | DOR to CAR . | No. of therapies after CAR relapse . | LDH prior to Pola (U/L) . | No. of Pola cycles . | Disease status before allo-HCT/ Best response to Pola . | Days to neutrophil/ platelet recovery . | Acute GVHD grade . | Chronic GVHD/ severity . | Relapse after Allo-HCT . | Survival/ Disease status at last follow-up . | DOR to allo-HCT (mo) . | Follow-up (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DLBCL, GCB | 46/F | LV20.19 CAR | 3 | CR | 5.1 | 1 | 323 | 2 | CR | 4/19 | 1 | 0 | N | Alive/CR | 19.5 | 19.5 |
| 2 | Transformed DLBCL, non-GCB | 66/F | Axi-cel | 3 | CR | 2.8 | 4 | 244 | 3 | CR | 17/14 | 0 | 0 | Y | Alive/CR | 12 | 24.7 |
| 3 | DLBCL, GCB | 70/M | LV20.19 CAR | 3 | PR | 2.3 | 3 | 306 | 4 | CR | 14/29 | 1 | 0 | N | Alive/CR | 32.4 | 32.4 |
| 4 | DLBCL, GCB (triple hit) | 62/M | LV20.19 CAR | 2 | PD | 0 | 1 | 283 | 3 | CR | 18/12 | 0 | 0 | N | Alive/CR | 12 | 12 |
| 5 | FL, grade 3A | 68/M | LV20.19 CAR | 3 | CR | 4.0 | 1 | 277 | 4 | PR | 17/15 | 1 | 0 | N | Alive/CR | 9.7 | 9.7 |
| 6 | DLBCL, non-GCB | 41/M | LV20.19 CAR | 3 | CR | 26.2 | 2 | 286 | 3 | CR | 16/12 | 4 | 0 | N | Alive/PR | 3.5 | 9.0 |
| 7 | DLBCL, GCB | 65/F | Axi-cel | 2 | CR | 6.5 | 1 | 192 | 3 | CR | 16/8 | 1 | Moderate | Y | Alive/CR | 29.2 | 29.2 |
| 8 | DLBCL, GCB | 72/M | LV20.19 CAR | 3 | CR | 5.9 | 1 | 213 | 3 | CR | 15/11 | 0 | 0 | N | Alive/CR | 9.6 | 9.6 |
| 9 | DLBCL, non-GCB | 66/F | Axi-cel | 3 | CR | 6.5 | 1 | 1067 | CR | 16/10 | 0 | 0 | N | Alice/CR | 4.7 | 4.7 | 9 |
| Patient No. . | Histologic diagnosis, COO . | Age at Allo-HCT/Sex . | CAR T-cell Therapy Administered . | No. lines of therapy prior to CAR . | Response to CAR . | DOR to CAR . | No. of therapies after CAR relapse . | LDH prior to Pola (U/L) . | No. of Pola cycles . | Disease status before allo-HCT/ Best response to Pola . | Days to neutrophil/ platelet recovery . | Acute GVHD grade . | Chronic GVHD/ severity . | Relapse after Allo-HCT . | Survival/ Disease status at last follow-up . | DOR to allo-HCT (mo) . | Follow-up (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DLBCL, GCB | 46/F | LV20.19 CAR | 3 | CR | 5.1 | 1 | 323 | 2 | CR | 4/19 | 1 | 0 | N | Alive/CR | 19.5 | 19.5 |
| 2 | Transformed DLBCL, non-GCB | 66/F | Axi-cel | 3 | CR | 2.8 | 4 | 244 | 3 | CR | 17/14 | 0 | 0 | Y | Alive/CR | 12 | 24.7 |
| 3 | DLBCL, GCB | 70/M | LV20.19 CAR | 3 | PR | 2.3 | 3 | 306 | 4 | CR | 14/29 | 1 | 0 | N | Alive/CR | 32.4 | 32.4 |
| 4 | DLBCL, GCB (triple hit) | 62/M | LV20.19 CAR | 2 | PD | 0 | 1 | 283 | 3 | CR | 18/12 | 0 | 0 | N | Alive/CR | 12 | 12 |
| 5 | FL, grade 3A | 68/M | LV20.19 CAR | 3 | CR | 4.0 | 1 | 277 | 4 | PR | 17/15 | 1 | 0 | N | Alive/CR | 9.7 | 9.7 |
| 6 | DLBCL, non-GCB | 41/M | LV20.19 CAR | 3 | CR | 26.2 | 2 | 286 | 3 | CR | 16/12 | 4 | 0 | N | Alive/PR | 3.5 | 9.0 |
| 7 | DLBCL, GCB | 65/F | Axi-cel | 2 | CR | 6.5 | 1 | 192 | 3 | CR | 16/8 | 1 | Moderate | Y | Alive/CR | 29.2 | 29.2 |
| 8 | DLBCL, GCB | 72/M | LV20.19 CAR | 3 | CR | 5.9 | 1 | 213 | 3 | CR | 15/11 | 0 | 0 | N | Alive/CR | 9.6 | 9.6 |
| 9 | DLBCL, non-GCB | 66/F | Axi-cel | 3 | CR | 6.5 | 1 | 1067 | CR | 16/10 | 0 | 0 | N | Alice/CR | 4.7 | 4.7 | 9 |
Axi-cel, Axicabtagene ciloleucel; COO, cell of origin; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; GCB, germinal center B; GVHD, graft-versus-host-disease; LV20.19, lentiviral anti-CD20/anti-CD19 CAR; Pola, polatuzumab; PD, progressive disease.
In this study, we report durable remissions with allo-HCT in selected patients who achieved a deep response with polatuzumab based salvage chemotherapy after CAR T-cell therapy. Seven out of 9 patients experienced a longer remission after allo-HCT than with CAR T-cell therapy, and 5 patients are now >1 year in remission, demonstrating allo-HCT is feasible and facilitates sustained remissions. The median PFS was not reached, and all patients remain alive to date which is likely related to our usage of RIC regimens for B-cell lymphoma, limiting nonrelapse mortality (NRM). Outcomes of polatuzumab after CAR T-cell therapy relapse were recently reported by Gouni et al.10 Among 54 patients who received polatuzumab, the ORR was 44%. Although our outcomes are superior, we acknowledge that most patients in this report were responding to CAR T-cell therapy who later relapsed and not patients who were primarily refractory to CAR-directed therapy. More importantly, in the study by Gouni et al, the median DOR to polatuzumab was only 11 weeks, suggesting that responses to polatuzumab are short lived without an appropriate consolidation such as allo-HCT as used in our study. To facilitate timely allo-HCT, we did not administer 6 cycles of polatuzumab as reported by Sehn et al.11 The median number of polatuzumab doses was 3. Additional cycles were forgone to facilitate allo-HCT consolidation, demonstrating that early allo-HCT intensifies and prolongs responses because polatuzumab alone does not lead to durable response in this patient population.4
Previous studies have reported on allo-HCT outcomes after CAR T-cell therapy failure with various induction regimens.12,13 In a recent study, Zurko et al reported 1-year PFS, OS, and NRM of 40%, 51%, and 27%, respectively, in 65 patients with LBCL after a median follow-up of 14 months.12 The higher NRM reported in the study by Zurko et al may be because of variable conditioning including myeloablative (MAC) regimens in 25% of patients. As prior studies have demonstrated unclear benefit and a higher NRM with MAC in lymphoma patients, our approach of RIC regimens limited the NRM, which is 0% to date.14,15 Shadman et al also reported outcomes of allo-HCT in 13 patients with NHL and chronic lymphocytic leukemia who relapsed after CAR T-cell therapy.13 The 1-year OS and NRM were 59% and 33%, respectively. Again, the higher NRM may be attributed to the higher use of MAC that was administered in 38% of patients. Gerhardt et al reported on 2 patients with aggressive lymphomas who received salvage therapy with 3 cycles of polatuzumab, bendamustine, and rituximab followed by MAC and allo-HCT, who were disease-free at last follow-up.16
In conclusion, albeit small, our study suggests that polatuzumab is an effective salvage therapy that can lead to deep responses facilitating a curative intent allo-HCT in patients who have failed CAR T-cell therapy. This approach led to durable remissions, with most patients (78%) achieving a longer disease-free period after allo-HCT than that after CAR T-cell therapy. Given the limited response duration to salvage therapy in general for patients who progress after CD19 based CAR T-cell therapy, our data emphasize the role of early consolidation with allo-HCT after clinical response. With the expected adaptation of polatuzumab in the frontline, its effectiveness in the post-CAR T-cell therapy setting will evolve. However, we speculate with other emerging treatments, which if able to induce a response, that consolidation with allo-HCT may similarly facilitate a durable remission for post-CAR T-cell therapy relapses.
Contribution: F.F. and N.N.S. conceptualized and designed the study, and prepared the first draft; F.F., M.M., and N.N.S. collected and assembled the data; F.F., A.S., and N.N.S. analyzed the data; and all authors interpreted the data, helped revise the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.S.F. consults and/or is in communication with Adaptive Biotechologies, AstraZeneca, Beigene, Kite (Gilead), MorphoSys, Pharmacyclics (AbbVie), Sanofi, SeaGen, Servier Pharmaceuticals, and TG Therapeutics. M.H. consults with Incyte Corporation, ADC Therapeutics, Pharmacyclics, Omeros, Genmab, Morphosys, Kadmon, Kite, Novartis, AbbVie, Legend, Gamida Cell, and SeaGen, and is in the speaker’s bureau of Sanofi Genzyme, AstraZeneca, BeiGene, and ADC Therapeutics. N.N.S is on the advisory board and/or consults with Kite Pharma, BMS, TG therapeutics, Miltenyi Biotec, Lilly, Epizyme, Legend, Incyte, Novartis, and Umoja, and has research funding and honoraria from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Nirav N. Shah, Bone Marrow Transplant & Cellular Therapy Program, Department of Medicine, Medical College of Wisconsin, 200 West Wisconsin Ave, Milwaukee, WI 53226; e-mail: nishah@mcw.edu.
References
Author notes
Data are available on request from the corresponding author, Nirav N Shah (nishah@mcw.edu).
The full-text version of this article contains a data supplement.