Key Points
Complement inhibitor–naive patients with PNH had greater hemoglobin stabilization and LDH reduction with pegcetacoplan vs control.
Pegcetacoplan’s comprehensive control of hemolysis and favorable safety profile in these patients may help expand the treatment population.
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare disease characterized by complement-mediated hemolysis. Pegcetacoplan is the first C3-targeted therapy approved for adults with PNH (United States), adults with PNH with inadequate response or intolerance to a C5 inhibitor (Australia), and adults with anemia despite C5-targeted therapy for ≥3 months (European Union). PRINCE was a phase 3, randomized, multicenter, open-label, controlled study to evaluate the efficacy and safety of pegcetacoplan vs control (supportive care only; eg, blood transfusions, corticosteroids, and supplements) in complement inhibitor–naive patients with PNH. Eligible adults receiving supportive care only for PNH were randomly assigned and stratified based on their number of transfusions (<4 or ≥4) 12 months before screening. Patients received pegcetacoplan 1080 mg subcutaneously twice weekly or continued supportive care (control) for 26 weeks. Coprimary end points were hemoglobin stabilization (avoidance of >1-g/dL decrease in hemoglobin levels without transfusions) from baseline through week 26 and lactate dehydrogenase (LDH) change at week 26. Overall, 53 patients received pegcetacoplan (n = 35) or control (n = 18). Pegcetacoplan was superior to control for hemoglobin stabilization (pegcetacoplan, 85.7%; control, 0; difference, 73.1%; 95% confidence interval [CI], 57.2-89.0; P < .0001) and change from baseline in LDH (least square mean change: pegcetacoplan, −1870.5 U/L; control, −400.1 U/L; difference, −1470.4 U/L; 95% CI, −2113.4 to −827.3; P < .0001). Pegcetacoplan was well tolerated. No pegcetacoplan-related adverse events were serious, and no new safety signals were observed. Pegcetacoplan rapidly and significantly stabilized hemoglobin and reduced LDH in complement inhibitor–naive patients and had a favorable safety profile. This trial was registered at www.clinicaltrials.gov as NCT04085601.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare disease characterized by complement-mediated hemolysis and thrombophilia and is often associated with bone marrow failure syndromes.1 It is typically acquired in adulthood because of somatic mutations within the PIG-A gene in hematopoietic stem cells. These mutations affect the biosynthesis of glycosylphosphatidylinositol (GPI),2 a membrane anchor added posttranslationally to cell-surface proteins. Two cell-surface proteins CD55 and CD59 use GPI anchors to regulate the complement system. Hematopoietic cells propagated from stem cells deficient in GPI biosynthesis lack these complement-regulating proteins, making them susceptible to complement-mediated lysis. Red blood cells (RBCs) are highly susceptible to complement-mediated lysis. People with PNH typically have anemia secondary to hemolysis, an increased incidence of thrombotic events, and end organ damage.3-5 Because of anemia they may also experience debilitating symptoms, such as fatigue, dyspnea, abdominal pain, erectile dysfunction, and dysphagia, which impair their overall quality of life (QoL).6,7
The introduction of C5 complement protein inhibitors (C5is) transformed PNH from a life-threatening disease to a chronic disease.8 The first Cis approved for PNH were eculizumab and ravulizumab. Both treatments target the C5 complement protein, inhibiting terminal complement activation and subsequent intravascular hemolysis.9-13 However, patients who receive C5is may develop extravascular hemolysis,8,14 which is mediated by the proximal C3 complement protein. Fragments of C3 (eg, C3b) opsonize RBCs, targeting them for destruction via phagocytosis in the liver and spleen (ie, extravascular hemolysis),15 which may lead to an incomplete response in patients receiving C5is.8 Therefore, hemoglobin normalization (ie, levels above the lower limit of normal [LLN]) cannot always be achieved with C5is.13,16 Up to 88% of patients treated with a C5i have persistent anemia due to chronic, low-grade hemolysis, and more than half are transfusion dependent.17,18 In countries where Cis are not available, supportive care, including blood transfusions, prophylactic anticoagulants, corticosteroids, and supplements (eg, iron, folate, and vitamin B12), is used to address the symptoms of PNH in patients.19,20
Pegcetacoplan is the first C3-targeting therapy for PNH. In 2 early-phase studies, pegcetacoplan improved hemoglobin levels to within the normal range by day 85 and maintained them for up to 1 year in complement inhibitor–naive patients with PNH receiving supportive care only.21 To confirm these results, we conducted a phase 3 study in complement inhibitor–naive patients with PNH that compared the efficacy and safety of pegcetacoplan with those of the control (supportive care).
Methods
Study design
PRINCE (NCT04085601; EudraCT, 2018-004220-11) was a phase 3, randomized, multicenter, open-label, controlled study. It was conducted in 22 centers (Hong Kong, Malaysia, the Philippines, Singapore, Thailand, Colombia, Mexico, and Peru) where Cis were not approved or widely available (ie, patients were receiving supportive care only for PNH treatment, including transfusions, anticoagulants, corticosteroids, and supplements [iron, folate, and vitamin B12]). The study comprised a ≤4-week screening period followed by a 26-week randomized controlled period (RCP). Investigators are listed in Appendix 1.
The study protocol was designed and monitored in accordance with the ethical principles of good clinical practice and the Declaration of Helsinki. An institutional review board or independent ethics committee at each center approved the protocol. Each patient provided written informed consent before undergoing study-related procedures.
Patients
Eligible patients met the following 5 criteria: (1) ≥18 years of age, (2) PNH diagnosis confirmed by high-sensitivity flow cytometry, (3) hemoglobin level below the LLN (male: <13.6 g/dL and female: <12.0 g/dL), (4) lactate dehydrogenase (LDH) level ≥1.5 times the upper limit of normal (ULN ≥339 U/L), and (5) vaccinated against Streptococcus pneumoniae, Neisseria meningitidis (types A, C, W, Y, and B), and Haemophilus influenzae (type B). Patients treated with the C5is eculizumab or ravulizumab, within 3 months of screening were excluded from the study. Appendix 2.1 lists all inclusion and exclusion criteria.
Randomization
Centralized Interactive Response Technology randomly assigned patients using a 2:1 ratio of pegcetacoplan treatment to control (supportive care only). Randomization was stratified based on the number of packed RBC (PRBC) transfusions (<4 or ≥4) received within the 12 months before screening.
Procedures
Patients visited the study clinic every 2 weeks for efficacy and safety assessments through week 26. A central laboratory conducted objective end point laboratory measurements; however, certified local laboratories were used under extenuating circumstances (eg, COVID-19 travel restrictions and medical emergencies). Units and normal ranges for values obtained at certified local laboratories were normalized for consistency with the study’s central laboratory (Appendix 2.2).
Patients randomly assigned to the pegcetacoplan group self-administered subcutaneous infusions of pegcetacoplan 1080 mg twice weekly (treatment details in Appendix 2.3). Information required for actual administration of the treatment regimen in practice is provided in the supplemental file. Patients randomly assigned to the control group continued supportive care but could escape to pegcetacoplan treatment if their hemoglobin level decreased ≥2 g/dL below their baseline measurement or if they had a qualifying thromboembolic event secondary to PNH. The transfusion eligibility threshold was hemoglobin levels <7 or ≥7 g/dL and <9 g/dL with signs or symptoms warranting transfusion. Hematologic laboratory values from certified local laboratories were collected before transfusion administration.
Outcomes
The coprimary end points for the study were hemoglobin stabilization (defined as the avoidance of a >1-g/dL decrease in hemoglobin levels from baseline to week 26) and change from baseline (CFB) in LDH levels at week 26. Nine secondary end points were tested in a hierarchical manner at week 26: (1) hemoglobin response (defined as hemoglobin increase ≥1 g/dL from baseline), (2) CFB in absolute reticulocyte count (ARC), (3) CFB in hemoglobin level, (4) patients (in percentage) who received transfusion and/or had a >2-g/dL decrease from baseline in hemoglobin level, (5) transfusion avoidance (defined as no transfusions during the 26-week RCP), (6) number of PRBC units transfused during the 26-week RCP; (7) CFB in the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale scores, (8) CFB in global health status/QoL scores using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) instrument, and (9) ARC normalization (defined as ARC < ULN [male: from 10 × 109 to 140 × 109 cells per L; and female: from 10 × 109 to 120 × 109 cells per L]). Three additional secondary end points at week 26 were included (1) patients (%) who had a clinically meaningful improvement in FACIT-Fatigue score (≥3-point increase22), (2) hemoglobin normalization (defined as hemoglobin levels ≥ LLN [male: ≥13.6 g/dL; and female: ≥12.0 g/dL]), and (3) LDH normalization (defined as LDH levels ≤ ULN [226 U/L]).
Post hoc analyses
Post hoc CFB analyses for hematologic parameters (LDH, hemoglobin, and ARC) and EORTC QLQ-C30 and FACIT-Fatigue scores were conducted for the pegcetacoplan treatment group only (ie, excluding escape patients). Normalization of D-dimer, defined as D-dimer level < ULN (0.5 μg/mL), was analyzed for patients randomly assigned to pegcetacoplan and supportive care. Post hoc analyses were conducted to determine the time-aligned CFBs (ie, time of the switch to pegcetacoplan for the escape group) in LDH and hemoglobin levels among patients who escaped from control (supportive care) to pegcetacoplan.
Safety
Safety was evaluated during clinical site visits by monitoring adverse events (AEs).
Statistical analysis
A sample size of 48 patients (32 pegcetacoplan and 16 control) was required to achieve 90% power at the 5% significance level (two-sided), using a two-group Fisher exact test and a 2:1 allocation (pegcetacoplan:control), to detect the effect of pegcetacoplan on the first coprimary end point of hemoglobin stabilization vs that of the control. This calculation assumes a 45% increase in the percentage of patients in the pegcetacoplan group who experience hemoglobin stabilization compared with that in the control group (eg, a change from 5% [control] to 50% [pegcetacoplan]). The same sample size provided 96% power (assuming an effect size of ≥1.2) to detect the effect of pegcetacoplan on the second coprimary end point of CFB in LDH levels at week 26. To account for loss of power because of discontinuations, the researchers attempted to randomly assign ≥54 participants.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Coprimary and secondary efficacy end points were evaluated with the intent-to-treat analysis set; all patients were analyzed based on their original treatment group, and patients who escaped to pegcetacoplan treatment were set as missing in the control group. Patients who escaped from the control group were considered nonresponders (ie, patients for whom treatment failed to achieve the first coprimary end point of hemoglobin stabilization). Efficacy data for escape patients were evaluated separately in post hoc analyses.
All statistical testing was performed at the 5% level of significance (two-sided), and all point estimates for the comparison between treatment groups were accompanied by adjusted odds ratios and two-sided 95% confidence intervals (CIs). Continuous end points were analyzed using an analysis of covariance model with a multiple imputation approach for handling missing data. Continuous end point summary values included mean, median, range, standard deviation, and standard error. Categorical end points were analyzed by tabulating the number and percentage of patients based on the treatment group and comparing them using a stratified Cochran-Mantel-Haenszel χ2 test. Secondary efficacy end points were tested in a hierarchical manner after statistical significance was reached for the 2 coprimary end points to preserve the type 1 error rate. If a patient received a transfusion during their treatment period, the pretransfusion values for hemoglobin, LDH, ARC, FACIT-Fatigue score, and EORTC QLQ-C30 score were used in the model. Patients were categorized as nonresponders for the hematologic stabilization, response, normalization and for clinically meaningful improvements in the FACIT-Fatigue score end points if they received a transfusion until week 26, escaped from the control to the pegcetacoplan group, withdrew from the study before week 26, or were lost to follow-up. Transfusions were defined as any transfusion of RBCs, leukocyte-depleted PRBCs, leukocyte-poor PRBCs, leukocyte-poor blood, or whole blood. Appendix 2.4 provides additional details regarding statistical analyses.
Post hoc analyses of mean (standard deviation and two-sided 95% CI) CFB to week 26 for hematologic values (hemoglobin, LDH, and ARC) and FACIT-Fatigue and EORTC QLQ-C30 scale scores were conducted for the pegcetacoplan treatment arm only. These parameters were statistically tested using Student t test. P < .05 was considered to be statistically significant, meaning that the mean CFB was statistically different from zero. D-dimer normalization was summarized descriptively using number and frequency over time. Patients with a D-dimer value <0.5 were considered to have reached D-dimer normalization. Descriptive statistics were used to generate time-aligned mean LDH and hemoglobin levels from baseline (ie, time of the switch to pegcetacoplan for the escape group) to the end of the study for patients who escaped to pegcetacoplan.
Pegcetacoplan safety was evaluated using the safety analysis set, with patients categorized into 2 groups: (1) overall pegcetacoplan, which included all patients who received ≥1 dose of pegcetacoplan after being randomly assigned to the pegcetacoplan group and those who escaped the control group while receiving pegcetacoplan and (2) patients in the control group who received supportive care only throughout the study or before escape. No formal statistical analyses were performed to evaluate AEs, and an external, independent data monitoring committee periodically assessed safety data. Safety measures were descriptively summarized based on the treatment group from baseline to week 26, and absolute values are presented. AEs of special interest (injection site reactions, infections [including sepsis], hemolytic disorders, thrombosis events, and hypersensitivity) were selected based on their relevance to PNH and the mechanism of action or route of administration of pegcetacoplan.
Results
The study was conducted from 27 August 2019 to 23 June 2021. A total of 53 complement inhibitor–naive patients were randomly assigned to pegcetacoplan treatment (n = 35) or continued supportive care (control; n = 18; Figure 1). The actual number of patients randomly assigned exceeded the sample size requirement of 48 patients to account for potential loss of power because of discontinuations. Over a median of 10.2 weeks (range, 5.3-21.0 weeks), 11 control patients escaped to pegcetacoplan treatment after a qualifying event; none of the qualifying events were PNH-related thrombosis. All escape patients completed the study on pegcetacoplan treatment until week 26. Three patients did not complete the trial. One pegcetacoplan-treated patient was lost to follow-up, and 2 patients died (1 in each group). Neither death was considered related to the treatment: 1 pegcetacoplan-treated patient died of septic shock in the context of bone marrow failure, and 1 supportive care–treated patient died from respiratory failure and septic shock.
Trial profile. ∗ indicates the control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]); †, patients assigned to the control group could escape to pegcetacoplan therapy if their hemoglobin levels were ≥2 g/dL below their baseline measurement or presented with a qualifying thromboembolic event secondary to PNH; ‡, 1 death occurred in the pegcetacoplan group related to septic shock in the context of bone marrow failure (both events were deemed unrelated to pegcetacoplan); and §, 1 death occurred in the control group related to respiratory failure and septic shock.
Trial profile. ∗ indicates the control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]); †, patients assigned to the control group could escape to pegcetacoplan therapy if their hemoglobin levels were ≥2 g/dL below their baseline measurement or presented with a qualifying thromboembolic event secondary to PNH; ‡, 1 death occurred in the pegcetacoplan group related to septic shock in the context of bone marrow failure (both events were deemed unrelated to pegcetacoplan); and §, 1 death occurred in the control group related to respiratory failure and septic shock.
The mean baseline characteristics were similar between the groups, except the pegcetacoplan group was ∼7 years younger, had a slightly lower percentage of patients with a history of aplastic anemia, and had a lower percentage of patients receiving ≥4 transfusions within 12 months before the study; the control group had a higher percentage of Asian patients (Table 1). Prior and concomitant medications are listed in Appendix 3.1. Appendix 3.2 describes pegcetacoplan exposure and incorrect dose adjustment in 2 patients.
Baseline demographic and clinical characteristics
| Characteristics . | Pegcetacoplan (n = 35) . | Control, supportive care only∗ (n = 18) . |
|---|---|---|
| Age, mean (range), y | 42.2 (22-67) | 49.1 (20-74) |
| ≥65 y, n (%) | 2 (5.7) | 4 (22.2) |
| Female, n (%) | 16 (45.7) | 8 (44.4) |
| Race, n (%) | ||
| Asian | 23 (65.7) | 16 (88.9) |
| American Indian or Alaska native | 9 (25.7) | 2 (11.1) |
| Black or African American | 2 (5.7) | 0 |
| White | 0 | 0 |
| Other | 1 (2.9) | 0 |
| Clinical characteristics | ||
| Body mass index, kg/m2, mean (SD) | 24.0 (4.4) | 23.1 (2.9) |
| History of aplastic anemia, n (%) | 5 (14.3) | 5 (27.8) |
| Time since PNH diagnosis, median (range), y | 3.4 (0.1-27.0) | 4.7 (0.1-15.1) |
| Transfusion in previous 12 mo, n (%) | 29 (82.9) | 14 (77.8) |
| <4 transfusions, n (%) | 21 (60.0) | 8 (44.4) |
| ≥4 transfusions, n (%) | 14 (40.0) | 10 (55.6) |
| Laboratory measurements, mean (SD) | ||
| Hemoglobin, g/dL† | 9.4 (1.4) | 8.7 (0.8) |
| LDH, U/L‡ | 2151.0 (909.4) | 1945.9 (1003.7) |
| ARC, ×109 cells per L§ | 230.2 (81.0) | 180.3 (109.1) |
| Total bilirubin, μmol/L‖ | 39.4 (20.5) | 35.5 (15.0) |
| Indirect bilirubin, μmol/L¶ | 34.1 (18.5) | 30.5 (13.5) |
| FACIT-Fatigue score# | 36.3 (10.7) | 37.1 (9.3) |
| EORTC QLQ-C30 global health status/QoL score∗∗ | 63.3 (19.7) | 62.0 (15.8) |
| Characteristics . | Pegcetacoplan (n = 35) . | Control, supportive care only∗ (n = 18) . |
|---|---|---|
| Age, mean (range), y | 42.2 (22-67) | 49.1 (20-74) |
| ≥65 y, n (%) | 2 (5.7) | 4 (22.2) |
| Female, n (%) | 16 (45.7) | 8 (44.4) |
| Race, n (%) | ||
| Asian | 23 (65.7) | 16 (88.9) |
| American Indian or Alaska native | 9 (25.7) | 2 (11.1) |
| Black or African American | 2 (5.7) | 0 |
| White | 0 | 0 |
| Other | 1 (2.9) | 0 |
| Clinical characteristics | ||
| Body mass index, kg/m2, mean (SD) | 24.0 (4.4) | 23.1 (2.9) |
| History of aplastic anemia, n (%) | 5 (14.3) | 5 (27.8) |
| Time since PNH diagnosis, median (range), y | 3.4 (0.1-27.0) | 4.7 (0.1-15.1) |
| Transfusion in previous 12 mo, n (%) | 29 (82.9) | 14 (77.8) |
| <4 transfusions, n (%) | 21 (60.0) | 8 (44.4) |
| ≥4 transfusions, n (%) | 14 (40.0) | 10 (55.6) |
| Laboratory measurements, mean (SD) | ||
| Hemoglobin, g/dL† | 9.4 (1.4) | 8.7 (0.8) |
| LDH, U/L‡ | 2151.0 (909.4) | 1945.9 (1003.7) |
| ARC, ×109 cells per L§ | 230.2 (81.0) | 180.3 (109.1) |
| Total bilirubin, μmol/L‖ | 39.4 (20.5) | 35.5 (15.0) |
| Indirect bilirubin, μmol/L¶ | 34.1 (18.5) | 30.5 (13.5) |
| FACIT-Fatigue score# | 36.3 (10.7) | 37.1 (9.3) |
| EORTC QLQ-C30 global health status/QoL score∗∗ | 63.3 (19.7) | 62.0 (15.8) |
SD, standard deviation.
Control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]).
Normal reference range: male, 13.6 to 18 g/dL; and female, 12 to 16 g/dL.
Normal reference range: 113 to 226 U/L.
Normal reference range: male, 10 × 109 to 140 × 109 cells per L; and female, 10 × 109 to 120 ×109 cells per L.
Normal reference range: 1.7 to 18.8 μmol/L.
Normal reference range: 1.7 to 15.4 μmol/L.
General population norm: 43.6. Defined by Cella et al.23
General population norm: 75.7. Defined by Hinz et al.24
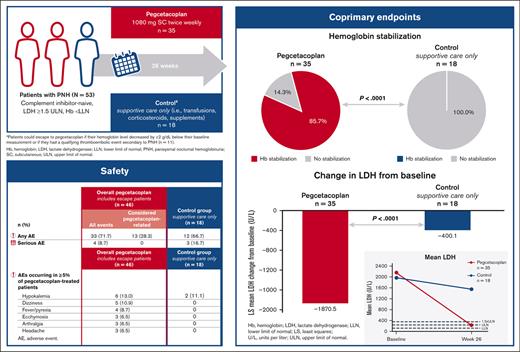
Pegcetacoplan was superior to the control for meeting the coprimary end points of hemoglobin stabilization through week 26 and the CFB in LDH levels at week 26 (Figure 2A). Most pegcetacoplan-treated patients (85.7%) and none of the supportive care–treated patients (0%) reached hemoglobin stabilization at week 26. The difference between groups was statistically significant (Figure 2A; P < .0001). Pegcetacoplan-treated patients had statistically improved least squares (LSs) mean CFB in LDH levels compared with the control group at week 26 (difference, −1470.4 U/L; 95% CI, –2113.4 to −827.3 U/L; P < .0001; Figure 2A).
Coprimary end points and hematologic parameters over time. (A) Coprimary end points of hemoglobin stabilization (defined as avoidance of >1-g/dL decrease in hemoglobin levels from baseline through week 26) and CFB in LDH levels at week 26 in patients in the pegcetacoplan group or control group. Reference range for normal LDH levels: 113 to 226 U/L. (B) Mean hemoglobin levels (±standard error [SE]) and (C) mean LDH over time (±SE) for patients in the pegcetacoplan group or control group. Reference range for normal hemoglobin levels: male, 13.6 to 18 g/dL; female, 12 to 16 g/dL. SE bars are not visible on data points representing mean values with small SE. ∗ indicates control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]) and †, avoidance of >1-g/dL decrease in hemoglobin levels from baseline through week 26. Patients who received a transfusion, escaped from the control arm to pegcetacoplan treatment, withdrew from the study before week 26, or were lost to follow-up were categorized as failing to achieve hemoglobin stabilization. ‡ indicates from baseline to week 26.
Coprimary end points and hematologic parameters over time. (A) Coprimary end points of hemoglobin stabilization (defined as avoidance of >1-g/dL decrease in hemoglobin levels from baseline through week 26) and CFB in LDH levels at week 26 in patients in the pegcetacoplan group or control group. Reference range for normal LDH levels: 113 to 226 U/L. (B) Mean hemoglobin levels (±standard error [SE]) and (C) mean LDH over time (±SE) for patients in the pegcetacoplan group or control group. Reference range for normal hemoglobin levels: male, 13.6 to 18 g/dL; female, 12 to 16 g/dL. SE bars are not visible on data points representing mean values with small SE. ∗ indicates control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]) and †, avoidance of >1-g/dL decrease in hemoglobin levels from baseline through week 26. Patients who received a transfusion, escaped from the control arm to pegcetacoplan treatment, withdrew from the study before week 26, or were lost to follow-up were categorized as failing to achieve hemoglobin stabilization. ‡ indicates from baseline to week 26.
Pegcetacoplan treatment increased mean hemoglobin levels to 12.0 g/dL by week 6. Mean hemoglobin continued to increase until week 26 (12.8 g/dL; Figure 2B). Pegcetacoplan treatment resulted in mean LDH levels ≤226 U/L (ULN) by week 4 that were maintained throughout 26 weeks (Figure 2C). Supportive care in the control group demonstrated no meaningful improvements in any hematologic end point throughout 26 weeks. For changes in the mean and median hemoglobin and LDH levels over time, see Appendix 3.3.
Pegcetacoplan was superior to the control in the first 5 secondary end points, per the predefined hierarchical testing strategy (Table 2). The percentage of patients with a hemoglobin response at week 26 was higher in the pegcetacoplan-treated group than in the supportive care–treated group (71.4% vs 5.6%, P < .0001). Pegcetacoplan was also superior compared with control with respect to the CFB in ARC (LS mean, −123.3 × 109 cells per L vs −19.4 × 109 cells per L, P = .0002). Patients who received pegcetacoplan had superior CFB in hemoglobin levels (2.9 g/dL vs 0.3 g/dL, P = .0019) at week 26 compared with those who received control treatment. More than 90% of pegcetacoplan-treated patients were transfusion-free for 26 weeks (P < .0001) vs <6% in the control group. Appendix 3.4 includes additional data regarding the total transfusion units and the percentage of patients who received transfusions or had decreased hemoglobin >2 g/dL from baseline to week 26.
Secondary end points
| Secondary end points . | Pegcetacoplan (n = 35) . | Control, supportive care only∗ (n = 18) . | Difference (95% CI) . | P value . |
|---|---|---|---|---|
| Hemoglobin response, n (%)†,‡ | 25 (71.4) | 1 (5.6) | 54.1 (33.9-74.3) | < .0001 |
| CFB in ARC at wk 26, LS mean (SD), ×109 cells per L | −123.3 (9.2) | −19.4 (25.2) | −103.8 (–158.9 to −48.7) | .0002 |
| Hemoglobin CFB at wk 26, LS mean (SE), g/dL | 2.9 (0.4) | 0.3 (0.8) | 2.7 (1.0-4.4) | .0019 |
| Transfusion avoidance through wk 26,‡ n (%) | 32 (91.4) | 1 (5.6) | 72.4 (55.8-89.0) | < .0001 |
| FACIT-Fatigue score CFB at wk 26, LS mean (SE) | 7.8 (1.2) | 3.3 (2.1) | 4.5 (−0.2 to 9.2) | .0610 |
| Global health status/QoL score (EORTC QLQ-C30) CFB at wk 26, LS mean (SE) | 18.9 (2.9) | −2.9 (5.7) | 21.8 (9.4-34.2) | Nominal .0006 |
| ARC normalization, n (%)‡,§ | 21 (60.0) | 1 (5.6) | 46.4 (25.3-67.5) | Nominal .0002 |
| Patients with a clinically meaningful‖ improvement in FACIT-Fatigue score,‡ n (%) | 21 (60.0) | 2 (11.1) | 48.9 (27.1-70.7) | Nominal .0007 |
| Hemoglobin normalization at wk 26,‡,¶ n (%) | 16 (45.7) | 0 (0) | 36.5 (16.5-56.4) | Nominal .0010 |
| LDH normalization,‡,# n (%) | 23 (65.7) | 0 (0) | 55.9 (36.8-75.0) | Nominal < .0001 |
| Secondary end points . | Pegcetacoplan (n = 35) . | Control, supportive care only∗ (n = 18) . | Difference (95% CI) . | P value . |
|---|---|---|---|---|
| Hemoglobin response, n (%)†,‡ | 25 (71.4) | 1 (5.6) | 54.1 (33.9-74.3) | < .0001 |
| CFB in ARC at wk 26, LS mean (SD), ×109 cells per L | −123.3 (9.2) | −19.4 (25.2) | −103.8 (–158.9 to −48.7) | .0002 |
| Hemoglobin CFB at wk 26, LS mean (SE), g/dL | 2.9 (0.4) | 0.3 (0.8) | 2.7 (1.0-4.4) | .0019 |
| Transfusion avoidance through wk 26,‡ n (%) | 32 (91.4) | 1 (5.6) | 72.4 (55.8-89.0) | < .0001 |
| FACIT-Fatigue score CFB at wk 26, LS mean (SE) | 7.8 (1.2) | 3.3 (2.1) | 4.5 (−0.2 to 9.2) | .0610 |
| Global health status/QoL score (EORTC QLQ-C30) CFB at wk 26, LS mean (SE) | 18.9 (2.9) | −2.9 (5.7) | 21.8 (9.4-34.2) | Nominal .0006 |
| ARC normalization, n (%)‡,§ | 21 (60.0) | 1 (5.6) | 46.4 (25.3-67.5) | Nominal .0002 |
| Patients with a clinically meaningful‖ improvement in FACIT-Fatigue score,‡ n (%) | 21 (60.0) | 2 (11.1) | 48.9 (27.1-70.7) | Nominal .0007 |
| Hemoglobin normalization at wk 26,‡,¶ n (%) | 16 (45.7) | 0 (0) | 36.5 (16.5-56.4) | Nominal .0010 |
| LDH normalization,‡,# n (%) | 23 (65.7) | 0 (0) | 55.9 (36.8-75.0) | Nominal < .0001 |
LS, least squares; SE, standard error.
Control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]).
Defined as ≥1-g/dL increase from baseline to week 26.
Patients who received a transfusion, escaped from the control group to pegcetacoplan treatment, withdrew from study before week 26, or were lost to follow-up were categorized as nonresponders.
ARC < sex-specific ULN (male, 10 × 109 to 140 × 109 cells per L; and female, 10 × 109 to 120 × 109 cells per L) at week 26.
Clinically meaningful improvement is defined as a ≥3-point FACIT-Fatigue score increase22 from baseline to week 26.
Hemoglobin levels ≥ sex-specific LLN: male, 13.6 g/dL; and female, 12.0 g/dL.
LDH levels ≤ ULN (226 U/L) at week 26.
FACIT-Fatigue scores (Table 2) also improved with pegcetacoplan treatment. At week 26, mean scores were above the standard score of the general population (43.6)23 in the pegcetacoplan group. The LS mean CFB in FACIT-Fatigue scores at week 26 was greater in the pegcetacoplan group vs the control group (n = 28 vs 5; 7.8 vs 3.3, respectively); however, the difference was not statistically significant (P = .0610). Therefore, additional secondary end points were only tested for a nominal P value. A significantly higher percentage of pegcetacoplan-treated patients had a clinically meaningful improvement (≥3-point increase22) in their FACIT-Fatigue scores at week 26 compared with control-treated patients (60.0% vs 11.1%; nominal P = .0007; Table 2). Pegcetacoplan-treated patients also had significantly improved LS mean EORTC QLQ-C30 global health status/QoL scores vs control-treated patients (18.9 vs −2.9; nominal P = .0006; Table 2). In addition, the improvement in EORTC QLQ-C30 global health status/QoL scores for the pegcetacoplan group at week 26 was above the standard score of the general population (75.7).24 Control-treated patients did not have improved EORTC QLQ-30 scores.
A high percentage of pegcetacoplan-treated patients reached normalized hematologic parameters. ARC was significantly normalized (ie, less than the sex-specific ULN [male, 10 × 109 to 140 × 109 cells per L; and female, 10 × 109 to 120 × 109 cells per L]) with pegcetacoplan compared with control (60.0% vs 5.6%; nominal P = .0002; Table 2). Nearly half of pegcetacoplan-treated patients had normalized hemoglobin (ie, greater than the sex-specific LLN [male, 13.6 g/dL; and female, 12.0 g/dL]) at week 26 vs none in the control group (nominal P = .0010). Levels of LDH were normalized in >50% and ∼90% of pegcetacoplan-treated patients by weeks 2 and 4, respectively (Appendix 3.5). At week 26, 65.7% of pegcetacoplan-treated patients had normalized LDH (ie, ULN ≤226 U/L); none of the control patients had normalized LDH (nominal P < .0001; Table 2).
Post hoc analyses conducted among patients randomly assigned to the pegcetacoplan group showed statistically significant improvements in the mean CFB to week 26 for all hematologic parameters (hemoglobin, LDH, and ARC) and FACIT-Fatigue and EORTC QLQ-C30 scores (Appendix 3.6). In an additional post hoc analysis, the percentage of patients with D-dimer levels within the normal range increased from 51.4% at baseline to 67.9% at week 26 in the pegcetacoplan group (Appendix 3.6). In the supportive care group, the percentage of patients with D-dimer levels within the normal range decreased from 71.4% at baseline to 60.0% at week 26.
Post hoc analyses of time-aligned data for patients who escaped from supportive care to pegcetacoplan (n = 11) showed a rapid response to pegcetacoplan, with a significant decrease in LDH levels by 1 week after escape and mean LDH levels within the normal range by week 2 (Appendix 3.6). Seven of the 11 patients who escaped showed a significant increase in hemoglobin levels by week 2 of pegcetacoplan.
Pegcetacoplan was well tolerated in all patients who received it, including those who escaped from the control group (n = 11; Table 3). The percentage of patients with AEs was similar in both treatment groups at week 26. Most AEs were mild or moderate. Pegcetacoplan-related AEs occurred in 13 patients; none were considered to be serious by the investigators. The most common AEs in pegcetacoplan group were hypokalemia, dizziness, fever, ecchymosis, arthralgia, and headache. There were no acute hemolytic events reported among patients who received pegcetacoplan. No events of thrombosis were reported in either treatment group. No AEs led to discontinuation. Two patients died, 1 in each study group; both deaths were considered by investigators to be unrelated to treatment.
AEs that occurred during the 26-week RCP
| Overall AEs and severity, n (%) . | Overall pegcetacoplan includes escape patients∗ (n = 46) . | Control group, supportive care only† (n = 18) . |
|---|---|---|
| Any AE | 33 (71.7) | 12 (66.7) |
| AEs considered related to pegcetacoplan | 13 (28.3) | NA |
| SAE | 4 (8.7) | 3 (16.7) |
| Related to pegcetacoplan | 0 | NA |
| AEs based on severity | ||
| Mild | 16 (34.8) | 7 (38.9) |
| Moderate | 13 (28.3) | 3 (16.7) |
| Severe | 4 (8.7) | 2 (11.1) |
| AEs occurring in ≥5% of pegcetacoplan-treated patients | ||
| Hypokalemia | 6 (13.0) | 2 (11.1) |
| Dizziness | 5 (10.9) | 0 |
| Fever/pyrexia | 4 (8.7) | 0 |
| Ecchymosis | 3 (6.5) | 0 |
| Arthralgia | 3 (6.5) | 0 |
| Headache | 3 (6.5) | 0 |
| AEs of special interest‡ | ||
| Injection site reactions§ | 14 (30.4) | NA |
| Infections‖ | 9 (19.6) | 5 (27.8) |
| Sepsis | 0 | 0 |
| Hypersensitivity¶ | 9 (19.6) | 1 (5.6) |
| Hemolytic disorders | 0 | 0 |
| Thrombosis | 0 | 0 |
| Overall AEs and severity, n (%) . | Overall pegcetacoplan includes escape patients∗ (n = 46) . | Control group, supportive care only† (n = 18) . |
|---|---|---|
| Any AE | 33 (71.7) | 12 (66.7) |
| AEs considered related to pegcetacoplan | 13 (28.3) | NA |
| SAE | 4 (8.7) | 3 (16.7) |
| Related to pegcetacoplan | 0 | NA |
| AEs based on severity | ||
| Mild | 16 (34.8) | 7 (38.9) |
| Moderate | 13 (28.3) | 3 (16.7) |
| Severe | 4 (8.7) | 2 (11.1) |
| AEs occurring in ≥5% of pegcetacoplan-treated patients | ||
| Hypokalemia | 6 (13.0) | 2 (11.1) |
| Dizziness | 5 (10.9) | 0 |
| Fever/pyrexia | 4 (8.7) | 0 |
| Ecchymosis | 3 (6.5) | 0 |
| Arthralgia | 3 (6.5) | 0 |
| Headache | 3 (6.5) | 0 |
| AEs of special interest‡ | ||
| Injection site reactions§ | 14 (30.4) | NA |
| Infections‖ | 9 (19.6) | 5 (27.8) |
| Sepsis | 0 | 0 |
| Hypersensitivity¶ | 9 (19.6) | 1 (5.6) |
| Hemolytic disorders | 0 | 0 |
| Thrombosis | 0 | 0 |
NA, not applicable.
Patients randomly assigned to the control group could escape to the pegcetacoplan group if their hemoglobin levels decreased ≥2 g/dL below their baseline measurement or had a qualifying thromboembolic event secondary to PNH.
Control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]).
AEs of special interest are either relevant to PNH or to the mechanism of action or route of administration of pegcetacoplan.
Injection site reactions included bruising, hemorrhage, induration, inflammation, rash, peripheral swelling, puncture site reaction, vaccination site reaction, ecchymosis, erythema, and hematoma.
Infections included viral infection, arthritis reactive, COVID-19, COVID-19 pneumonia, cellulitis, nasopharyngitis, pharyngitis, tuberculosis, upper respiratory tract infection, urinary tract infection enterococcal, vaginal infection, influenza, Pneumocystis jirovecii pneumonia, and urinary tract infection.
Hypersensitivity was defined as erythema, rash, allergic cough, dermatitis, dermatitis contact, eyelid edema, injection site rash, rash maculopapular, and rhinitis allergic.
For AEs of special interest, there were no serious infections (eg, meningococcal infections), treatment-related sepsis, or PNH-relevant hemolytic events or thrombosis (Table 3).
Serious AEs (SAEs) occurred in 4 pegcetacoplan-treated patients. None were considered related to treatment. Three patients each had 1 of the following SAEs: neutropenia on treatment-day 1, dermoid cysts, or symptomatic anemia requiring 1 unit of PRBC. One patient had the following 3 SAEs: severe pancytopenia on treatment, febrile neutropenia, and septic shock.
Discussion
In this trial of complement inhibitor–naive adults with PNH, pegcetacoplan was superior to supportive care with respect to the coprimary end points of hemoglobin stabilization and reduction in mean LDH levels at week 26. Treatment with pegcetacoplan led to significant improvements in the secondary end points for hematologic parameters, normalization of hematologic values, fatigue, and QoL compared with supportive care. The favorable safety profile in PRINCE was consistent with previous clinical trials;21,25,26 no new safety risks were identified. No acute hemolytic events were reported in patients treated with pegcetacoplan.
Notable in the current study was the high rate of hemoglobin stabilization (86%) and normalization (∼50%) in pegcetacoplan-treated patients at week 26. The high rate of stabilization is remarkable because we used a more stringent criterion for hemoglobin stabilization (ie, a decline of 1 rather than 2 g/dL) compared with previous clinical trials.9,11,27 Mean hemoglobin increased 3.4 g/dL from baseline to a mean of 12.8 g/dL at week 26. The high rates of improved and stabilized hemoglobin allowed nearly all (91%) patients who received pegcetacoplan to avoid transfusions throughout the 26-week study. It is also noteworthy that patients who escaped from the control group because of acute hemolysis (ie, hemoglobin decrease of ≥2 g/dL from baseline) had rapid improvement in hemoglobin levels after initiating pegcetacoplan.
The findings this study compare favorably with those of the PADDOCK (NCT02588833) and PALOMINO (NCT03593200) trials.21 In these phase 1b and 2a trials, respectively, complement inhibitor–naive patients self-administered daily 270 mg subcutaneous infusions of pegcetacoplan for up to 1 year. The mean CFB in hemoglobin (3.7 g/dL and 5.3 g/dL in PADDOCK and PALOMINO, respectively) increased to nearly normal levels by treatment-day 85 and was sustained for 1 year (12.1 and 13.0 g/dL in PADDOCK and PALOMINO, respectively). Patients also had rapid and sustained decreases in LDH. From mean baseline levels ≥8 × ULN, 80% of patients had LDH within the normal range by day 22 in PADDOCK and to ≤1.5 × ULN in 82% of PALOMINO patients at 1 year. Patients felt better with pegcetacoplan treatment because of decreased fatigue. In both studies, their mean FACIT-Fatigue scores improved to approximately the standard score of the general population by day 43 in PADDOCK and day 15 in PALOMINO.23
A second phase 3 study of pegcetacoplan, PEGASUS (NCT03500549), was conducted concurrently with PRINCE and was the based on US Food and Drug Administration, Australian Department of Health and Aged Care, and European Medicines Agency approvals.25,28-30 PEGASUS was a randomized, active-controlled study in patients with PNH and hemoglobin <10.5 g/dL despite stable eculizumab treatment (≥3 months).25 In PEGASUS, pegcetacoplan was superior to eculizumab for improved hemoglobin levels and noninferior to eculizumab for improved levels of other hematologic parameters. Pegcetacoplan had a favorable safety profile in patients with previous exposure to a C5i. Taken together, the 2 phase 3 trials demonstrate the ability of pegcetacoplan to effectively and safely treat a wide range of patients with PNH, both treatment-naive and C5i-experienced.
Studies of eculizumab and ravulizumab in complement inhibitor–naive patients with PNH showed improved LDH levels and better recovery from fatigue,9-11,27 and hemoglobin stabilized to avoid a ≥2-g/dL decrease from baseline. However, normalized hemoglobin, an important parameter for assessing PNH treatment response,8,31 was not directly assessed for patients who received C5i treatment, although the hemoglobin levels reported suggest that hemoglobin normalization was not common for these patients.9,11,13,16,27 Additionally, a recent survey-based study found C5i treatment associated with high rates of persistent anemia, fatigue, and poor QoL (decreased work productivity and impaired ability to perform daily activities).18 Potential PNH treatments that target factor D and factor B are being studied.20
This study was limited by the small sample size of the control group. More than half of patients in the control group receiving only supportive care escaped to the pegcetacoplan group, limiting direct comparisons for safety and some efficacy end points between treatment groups. The post hoc analyses of the pegcetacoplan group showed statistically significant CFBs in all mean hematologic parameters and QoL and fatigue scores, increasing our confidence in the accuracy of our primary analyses. An extension study (APL2-307; NCT03531255) enrolling patients from PRINCE and 4 other pegcetacoplan studies is underway to evaluate the long-term safety and efficacy of pegcetacoplan for up to 4 years.32 The current study could not assess the effects of pegcetacoplan on thrombosis risk in treatment-naive patients with PNH because no AEs of thrombosis were reported in either treatment group.
Overall, the PRINCE trial showed that pegcetacoplan comprehensively controlled hemolysis in complement inhibitor–naive patients and rapidly stabilized and normalized hematologic parameters, decreased fatigue, and improved QoL compared with the control treatment. These findings show that pegcetacoplan is a better alternative for existing C5i therapies that stabilize but cannot normalize hematologic parameters.
Acknowledgments
The authors thank the patients who participated in the PRINCE study and their caregivers; the institution staff and investigators who participated in this study for their valuable contributions; and the members of the independent review committee and data monitoring committee and Matthew Ferenc, Jinny Min, and Michael Yeh of Apellis Pharmaceuticals Inc. Writing and editorial support was provided by Amanda Agazi, Francie Moehring, and Apeksha Shenoy at Boston Strategic Partners Inc (funded by Apellis Pharmaceuticals Inc), Kathryn Fogarty, Kay Square Scientific, Newtown Square, PA), and Tracey Fine of Apellis Pharmaceuticals Inc.
This study was funded by Apellis Pharmaceuticals Inc. Apellis Pharmaceuticals Inc and Swedish Orphan Biovitrum AB boards reviewed this manuscript.
Authorship
Contribution: R.S.M.W. served as the principal investigator, provided study management/oversight, designed the study protocol, enrolled patients, and collected data; M.A.-A., T.A., J.S., P.D., C.F., and F.G. designed the study protocol and collected data; J.R.N.-C., N.S.C., Y.T.G., D.K., D.G.-A., and T.D. reviewed the study protocol, enrolled patients, and collected data; R.S.M.W., J.R.N.-C., N.S.C., Y.T.G., D.K., D.G.-A., M.A.-A., T.A., P.A., J.S., P.D., C.F., F.G., and T.D. analyzed and interpreted data and reviewed and edited the manuscript; underlying data reported in the manuscript were directly accessed and verified by R.S.M.W., M.A.-A., and T.A; and all authors were responsible for the decision to submit the manuscript for publication, confirm that they had full access to all the data in the study, and accept responsibility to submit it for publication.
Conflict-of-interest disclosure: R.S.M.W. has received consulting fees, honoraria, research funding, and speaker’s bureau fees from Alexion Pharmaceuticals Inc and F. Hoffmann-La Roche Ltd. and research funding and speaker’s bureau fees from Apellis Pharmaceuticals, Inc. D.G.-A. has received speaking and advising roles from Teva, Roche, Amgen, Bristol Myers Squibb, Takeda, Janssen, AbbVie, and Astellas. M.A.-A. is an employee of Apellis Pharmaceuticals Inc. T.A., J.S., P.D., C.F., and F.G. are employees and current equity holders of the publicly traded company Apellis Pharmaceuticals Inc. P.A. reports former consultancy with Apellis Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
Correspondence: Raymond Siu Ming Wong, Sir YK Pao Centre for Cancer & Department of Medicine and Therapeutics, Prince of Wales Hospital, Chinese University of Hong Kong, 30-32 Ngan Shing St, Shatin, NT, Hong Kong, Hong Kong; e-mail: raymondwong@cuhk.edu.hk.
References
Author notes
Presented in abstract and oral forms at the 63rd annual meeting and exposition of the American Society of Hematology, Atlanta, GA, from 11 to 14 December 2021; and in abstract and poster forms at the 27th Congress of the European Hematology Association, Vienna, Austria, from 9-12 June 2022.
Data from the PRINCE trial or the study protocol are available on request from the author, Federico Grossi (federico@apellis.com).
The study protocol will be available with no end date.
All proposals requesting data access will need to specify how the data will be used and will need the approval of the trial investigator team before data release.
Individual participant data will not be shared.
The full-text version of this article contains a data supplement.

![Trial profile. ∗ indicates the control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]); †, patients assigned to the control group could escape to pegcetacoplan therapy if their hemoglobin levels were ≥2 g/dL below their baseline measurement or presented with a qualifying thromboembolic event secondary to PNH; ‡, 1 death occurred in the pegcetacoplan group related to septic shock in the context of bone marrow failure (both events were deemed unrelated to pegcetacoplan); and §, 1 death occurred in the control group related to respiratory failure and septic shock.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/11/10.1182_bloodadvances.2022009129/2/m_blooda_adv-2022-009129-gr1.jpeg?Expires=1768499269&Signature=1FI-X1AhEQ08Lab4afybCMTSZQDNRr3CIdHYVEo2DXDs3G-wO7irktDXqvuvSObgDH6SlXiVLVr7U8dcvvITfecOE01AT9hyTsbPjcOgrDcvCVocGIKcC1YqE1uNuB1iwnXGhvDzrmRRxEyk-bLuJrM0kcT-PZ1~TUOd4-KoNBaG1uuLxOfqBy9O1nYrBA81QmJ3RJVmS27R75T-3Sl-RNb6pnjM~j6AUK9OxzhkeVuhlChBGfKpecRdeuFJBdrECElI9NHTP3Sfkw0HD~GuxD8QuwoRKOv42Xvt9WwSmvgwTpDk4CP67bNJbK9TPz~zOen0OXmnu2A1W6EVuwRc3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Coprimary end points and hematologic parameters over time. (A) Coprimary end points of hemoglobin stabilization (defined as avoidance of >1-g/dL decrease in hemoglobin levels from baseline through week 26) and CFB in LDH levels at week 26 in patients in the pegcetacoplan group or control group. Reference range for normal LDH levels: 113 to 226 U/L. (B) Mean hemoglobin levels (±standard error [SE]) and (C) mean LDH over time (±SE) for patients in the pegcetacoplan group or control group. Reference range for normal hemoglobin levels: male, 13.6 to 18 g/dL; female, 12 to 16 g/dL. SE bars are not visible on data points representing mean values with small SE. ∗ indicates control group patients received supportive care (eg, transfusions, corticosteroids, and supplements [iron, folate, and vitamin B12]) and †, avoidance of >1-g/dL decrease in hemoglobin levels from baseline through week 26. Patients who received a transfusion, escaped from the control arm to pegcetacoplan treatment, withdrew from the study before week 26, or were lost to follow-up were categorized as failing to achieve hemoglobin stabilization. ‡ indicates from baseline to week 26.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/11/10.1182_bloodadvances.2022009129/2/m_blooda_adv-2022-009129-gr2.jpeg?Expires=1768499269&Signature=zlziP4XZviptQtJqwVOhkyxmzXywJa2lTsGJPlRXKFNST~hZUb-N6NXkjzeuWmHFyz4d3Rh01grrxCmEz-PIRfHg2u3MeHXdP38Iv4H2HsSj06rj6NIeNZBLm2VkvYRqP6cE8hFT05Ir9FKdjv8cELODxV-rHA19wN9xSFs-YsX~2p5PAAlxH611FRE0GN6wDZlgaP1tCpWMC09KA6sccbzCNJNhC7VmqDgwnY3PNXd1hzYak5~ZWrUKPQBP5r3Q5nQHr6Ek-DmvjrDNwa~xDM-GGqrF~QRi-wbiX6I4zKqAcRlaK5ZVDLJ~HyE2emz6a7uHbjyfnhrliA9Ec~hAwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)