Abstract
Autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy has recently been added to the armamentarium in the battle against B-cell acute lymphoblastic leukemia (B-ALL). In this review, we discuss the trials that led to US Food and Drug Administration approval of CAR T-cell therapies in patients with B-ALL. We evaluate the evolving role of allogeneic hematopoietic stem cell transplant in the CAR T-cell era and discuss lessons learned from the first steps with CAR T-cell therapy in ALL. Upcoming innovations in CAR technology, including combined and alternative targets and off-the-shelf allogeneic CAR T-cell strategies are presented. Finally, we envision the role that CAR T cells could take in the management of adult patients with B-ALL in the near future.
Introduction
Historically, adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) have faced a dismal prognosis, with only ∼20% or 40% of the patients achieving remission, with an overall survival (OS) of only 6 months.1,2 In recent years, the introduction of novel targeted therapies has improved the outcomes of patients with R/R B-cell ALL.3,4 Inotuzumab ozogamicin is a humanized anti-CD22 monoclonal antibody conjugated to the cytotoxic antibiotic calicheamicin. In the phase 3 trial (INO-VATE trial), single-agent inotuzumab improved complete remission (CR) rates in comparison with standard salvage intensive chemotherapy (80.7% vs 29.4%). Progression-free survival (PFS) and OS in the inotuzumab group were superior compared with the chemotherapy arm (5 months vs 1.8 months and 7.7 months vs 6.7 months, respectively).3 The bispecific T-cell engager blinatumomab binds CD3 on T cells and CD19 on B cells simultaneously, thus, culminating in the activation of T cells and elimination of CD19+ ALL blasts. Blinatumomab was shown to improve outcomes compared with standard chemotherapy in the phase 3 TOWER trial, with a higher CR rate (44% vs 25%), 6-month event-free survival (EFS; 31% vs 12%), and median OS (7.7 months vs 4 months).4 Despite improvements in the PFS conferred by inotuzumab or blinatumomab monotherapy, durable remissions are uncommon without allogeneic stem cell transplant (allo-SCT) consolidation. Subsequent analyses of the INO-VATE and TOWER trials revealed inferior outcomes in patients who received treatment in later lines of therapy: the 12-month PFS with inotuzumab as second line salvage treatment was ∼20%,5 and the 12-month EFS with blinatumomab as second or further line treatment was 10%.6 The combination of lower dose chemotherapy with targeted therapies (such as mini-CVD chemotherapy with inotuzumab with sequential blinatumomab) has led to improved outcomes with median OS and relapse-free survival of 13.4 months and 9.2 months, respectively; encouraging results were noted among patients with 1 prior line of therapy for whom median OS was 16.5 months.7 These data indicate unmet medical need for effective therapies for R/R ALL, especially in those beyond first relapse.
Chimeric antigen receptor (CAR) T cells are genetically engineered T cells constructed by the fusion of an extracellular antigen-binding portion derived from an antibody and an intracellular signaling portion derived from T-cell signaling proteins, with 1 or more costimulatory domains.8 CAR T-cell design and production processes vary widely among CAR T-cell constructs in clinical trials, with differences with regard to the targeted antigen, binding affinity, costimulatory molecules, manufacturing systems, and transduction techniques, among others. These factors in turn influence availability of viable T cells, transduction efficiency, manufacturing time, and T-cell expansion and persistence, thereby affecting the success in killing leukemic cells.9 The role of CAR T cells has been rapidly evolving in the therapeutic landscape of various hematologic malignancies.
CAR T cells in B-ALL
Adolescent and young adults
Tisagenlecleucel (tisa-cel) was the first CAR T-cell therapy approved for B-ALL by the US Food and Drug Administration after the results of the phase 2 ELIANA trial in which 79 pediatric and young adult patients (median age, 11 years; range, 3-24 years) with CD19+ R/R B-cell ALL were treated.10 A total of 65 of 79 (82%) achieved CR/CR with incomplete count recovery (CRi) and all were measurable residual disease (MRD)-negative via flow cytometry. At 6 months, the EFS and OS were 73% and 90%, respectively. Cytokine release syndrome (CRS) developed in 77% of the patients (grade ≥3, 49%), and neurological toxicities developed in 39% (grade 3, 13% and grade 4, none). In an updated analysis with a median follow-up of >5 years, the investigators reported a median EFS of 15 months with most relapses occurring within the first 18 months; 5-year EFS and OS were 36% and 55%, respectively.11 Notably, only 10 of 65 responding patients underwent a subsequent allo-SCT as a consolidation strategy in ongoing remission; EFS with or without censoring for transplant was similar. These updated results indicate that a subset of patients can achieve long-term remission after CAR T-cell therapy. Based on the ELIANA trial, tisa-cel was approved in August 2017 for patients aged ≤25 years who have B-ALL that is refractory or who are in second or later relapse.
Studies reporting real-world data have demonstrated findings that are similar to those of pivotal clinical trials. In a Center for International Blood and Marrow Transplant Research (CIBMTR) registry analysis of 255 pediatric patients with B-ALL who received tisa-cel, the CR rate was 85.5%, and 12-month EFS was 52.4%.12 An updated analysis of the CIBMTR registry data, presented at the 2021 American Society of Hematology annual meeting, reported the outcomes of 451 children/young adults who received commercial tisa-cel for R/R B-ALL.13 Overall, efficacy outcomes were similar to those observed in the ELIANA trial. With a median follow-up of 21.5 months, the overall response rate (ORR) was 86.8%, and 12-month relapse-free survival (RFS) was 62.5%. Grade ≥3 CRS (17.8%) and immune effector cell–associated neurotoxicity syndrome (ICANS; 10%) were less common compared with those reported in the ELIANA trial10 (49% and 13%, respectively), perhaps reflecting the lower burden of disease in patients receiving tisa-cel in the real-world setting. In a recent multicenter pooled analysis of 200 children/young adults from 15 centers in the United States in whom leukapheresis for tisa-cel was performed, the CR rate was 85% among the 185 of the 200 patients who received the cell infusion. The 12-month EFS and OS in the cohort were 50% and 72%, respectively.14 Rates of grade ≥3 CRS and neurotoxicity in the entire cohort were 21% and 7%, respectively. Unlike the ELIANA trial, which required ≥5% blasts at enrollment and reported a median blast of 74% (range, 5%-99%), in this real-world setting, patients with low disease burden were enrolled. Investigators categorized patients as those with (1) high disease burden (≥5% marrow blasts; any peripheral blood blasts; central nervous system 3 (CNS3); non-CNS extramedullary disease), which constituted ∼52% of the cohort; (2) low disease burden (MRD positive group), which constituted 23% of the patients; and (3) undetectable disease (MRD negative group), which constituted 25% of the patients. Not unexpectedly, those with high-burden disease had inferior 12-month EFS (31%) and OS (58%) and experienced higher rates of grade ≥3 CRS (35%) and neurotoxicity (9%) compared with those with low disease burden or undetectable disease.
A trial from the National Institutes of Health reported long-term outcomes of 50 pediatric and young adult patients (median age, 13.5 years; range, 4-30 years) who received a CD19-directed CAR T-cell therapy that contained a CD28 costimulatory domain in a phase 1 trial.15 A total of 56% of participants achieved MRD-negative CR. With a median follow-up of 4.8 years, the median EFS and OS for the entire cohort were 3.1 and 10.5 months, respectively. Notably, outcomes were worse if patients had ≥5% blasts in the marrow before cell infusion (median OS, 6 months; median EFS, 0.9 months). In this study, 42 of 50 patients received fludarabine/cyclophosphamide lymphodepletion (LD); the remaining 8 patients received either fludarabine, cytarabine, filgrastim (n = 6), or ifosfamide/etoposide (n = 2). Notably, patients receiving fludarabine/cyclophosphamide LD had higher CR rate than those receiving alternative LD regimens, with the caveat that alternative LD regimens were reserved for patients with high tumor burden in this trial.
In a recent report, investigators reported 3-year follow-up data for the ZUMA-4 trial, which is a phase 1 trial of KTE-X19 (CD28 costimulatory domain) in children with R/R B-ALL (median age, 13.5 years; range, 3-20 years).16 A total of 31 patients were enrolled, and they underwent leukapheresis; 24 patients received cell infusion. The median pre-LD marrow blast count was 37%. Two dose levels were investigated: 1 × 106 CAR T cells per kg and 2 × 106 CAR T cells per kg. Grade 3 CRS occurred in 33% of the patients; no grade 4 or 5 CRS events occurred. Grade 3 or 4 ICANS occurred in 21% of patients. The ORR was 67%, with a median duration of response of 7.2 months.
A novel CAR T-cell construct containing a humanized anti-CD19 single-chain variable fraction domain has been developed with the aim to avoid rejection of traditional murine-based domains, thereby potentially prolonging CAR T-cell persistence and duration of response. A pilot trial of humanized CD19 CAR T-cell (huCART19) included 74 children and young adults, 33 of whom had previously received a CAR T-cell treatment.17 Response rates were 98% and 64%, in the CAR-naive and retreatment cohorts, respectively. In 73% of patients who were CAR naive, CAR T cells were still detected after 6 months from infusion, compared with 52% for patients who were retreated. In the 2 cohorts, the 24-month RFS was 74% and 58%, respectively.
Adults
In a phase 1 trial conducted at the Memorial Sloan Kettering Cancer Center (MSKCC), 53 adult patients (median age, 44 years; range, 23-74 years) with R/R B-ALL were treated with CD19-directed CAR T cells containing a CD28 costimulatory domain.18 In total, 68% of the patients had received at least 2 prior lines of treatment, and 36% had received ≥4 prior treatments. Notably, 81% (43 of 53) of patients received cyclophosphamide alone as LD chemotherapy. A total of 83% patients achieved CR and 60% achieved MRD-negative remission. Median EFS was 6.1 months and median OS 12.9 months. Outcomes were better for patients who received CAR T-cell therapy for MRD positive (marrow blasts < 5%) disease.
Another phase 1/2 trial at the Fred Hutchinson Cancer Center investigated CD19-directed CAR T-cell therapy for adult patients with B-ALL using a construct with a 4-1BB costimulatory domain. Similar to the previous trial at MSKCC, patients were heavily pretreated, with a median of 3 prior lines of therapy.19 Of the 53 evaluable patients (median age, 39 years; range, 20-76 years), 85% achieved MRD-negative CR. Grade ≥3 CRS occurred in 19%, and grade ≥3 ICANS occurred in 23% of patients. For patients who achieved MRD-negative CR, the median EFS was 7.6 months. CAR T-cell expansion correlated with response, and patients who did not receive fludarabine as part of their lymphodepletion regimen had worse outcomes.
The results of ZUMA-3, a phase 2 trial for CD19-directed CAR T-cell therapy for adult patients with R/R B-ALL, were recently published.20 Of the 71 patients who had enrolled and underwent leukapheresis, 55 were infused with brexucabtagene autoleucel (brexu-cel, previously KTE-X19). The median time from apheresis to product release was 13 days for US-based patients and 14.5 days for patients from Europe. The LD consisted of fludarabine 25 mg/m2 daily for 3 days and cyclophosphamide 900 mg/m2 on the second day of the lymphodepletion. The infused CAR T-cell dose was 1 × 106 CAR T cells per kg. A total of 55 of the 71 enrolled patients received CAR T-cell infusion. The median patient age was 40 years (interquartile range, 28-52 years), with a median of 2 prior therapies. A total of 45% patients had received prior blinatumomab, 22% had received prior inotuzumab ozogamicin, and 42% had a prior allo-SCT. Among the 55 patients who received the CAR T cells, 39 (71%) patients achieved CR/CRi, with 38 of 39 achieving MRD-negative remission. In an updated analysis presented at the American Society of Clinical Oncology 2022 annual meeting, the median OS for all treated patients was 25.4 months.21 The median duration of remission among responders was 14.6 months. Among the responding patients, 10 patients had a subsequent allo-SCT; the median duration of remission was similar with or without censoring for allo-SCT. CRS occurred in 89% of patients, with 24% being grade 3 or 4 events. The median time to CRS onset was 5 days, and the median duration of CRS was 7.5 days. ICANS occurred in 60% of the patients, with 25% being of grade 3 to 5 (1 patient died of brain herniation on day 8 after CAR T-cell infusion). The median time of onset of ICANS was 9 days, and the median duration was 7 days. Overall, tocilizumab was given to 80% of patients and steroids to 75% patients. The median time to peak blood CAR T-cell level was 15 days. In 79% of the 28 patients with available samples, CAR T cells were no longer detectable in the blood at 6 months (while the patients were in ongoing remission). CAR T-cell expansion was associated with continued CR as well as MRD negativity. CD19 negative relapse was observed in 3 of 9 (33%) patients with available samples at the time of disease relapse. In October 2021, based on the results of the ZUMA-3 trial, the US Food and Drug Administration granted approval for brexu-cel for adult patients with R/R B-ALL.
In an attempt to decrease CAR T-cell–related toxicity and prolong CAR T-cell persistence, a novel CD19-directed CAR T-cell therapy was recently reported. The AUTO1 construct was designed with a 4-1BB costimulatory domain and a fast off rate, designed to produce a low affinity anti-CD19 CAR T-cell,22 reducing cytokine release by more physiological T-cell activation. Fast off rate CAR T cells were previously shown to produce better in vitro proliferation and cytotoxicity, and early clinical data showed a favorable toxicity profile.23 Of the 20 adult patients who received AUTO1, none experienced grade ≥3 CRS.22 Three patients (20%) experienced grade 3 ICANS that improved to grade 1 or resolved within 72 hours. Seventeen patients (85%) achieved MRD-negative CR, and the 12-month EFS was 48.3%. The CAR T cells persisted in the peripheral blood for a median of 5.5 months.
Major selected trials of autologous CD19 CAR T cells for B-ALL are summarized in Table 1.
Characteristics and outcomes of selected autologous CD19-directed CAR T-cell trials for patients with B-ALL
| Reference . | Patient age (median; range) y . | Number of patients . | Previous lines of therapy (median; range) . | Costimulatory domain . | CR + CRi with MRD-negative remission, (%) . | EFS/RFS/LFS . | Grade ≥3 CRS, (%) . | Grade ≥3 ICANS, (%) . |
|---|---|---|---|---|---|---|---|---|
| Maude et al 201810 | 11 (3-23) | 75 | 3 (1-8) | 4-1BB | 81 | 50% EFS at 12 months | 46 | 13 |
| Shah NN et al 202115 | 13.5 (4.3-30.4) | 50 | NA | CD28 | 62 | 38% EFS at 6 months | 22 | 8 |
| Myers et al 202117∗ | 10.3 (1.7-29.1) | 41 | NA | 4-1BB† | 98 | 82% EFS at 12 months | 15 | 7 |
| Park et al 201818 | 44 (23-64) | 53 | NA‡ | CD28 | 83 | Median EFS 6.1 months | 26 | 42 |
| Hay et al 201919 | 39 (20-76) | 53 | 3 (1-11) | 4-1BB | 85§ | NA | 19 | 23 |
| Shah BD et al 202121 | 40 (28-52) | 55 | 2 (2-3) | CD28 | 71 | 58% RFS at 6 months | 24 | 26 |
| Rodie et al 202122 | 41.5 (18-62) | 20 | 3 (2-6) | 4-1BB | 85§ | 44% EFS at 12 months | 0 | 15 |
| Zhang et al 202124 | 12 (2-61) | 110 | NA | 4-1BB/CD28ε | 87 | 58% LFS at 12 months | 16 | 14ǁ |
| Reference . | Patient age (median; range) y . | Number of patients . | Previous lines of therapy (median; range) . | Costimulatory domain . | CR + CRi with MRD-negative remission, (%) . | EFS/RFS/LFS . | Grade ≥3 CRS, (%) . | Grade ≥3 ICANS, (%) . |
|---|---|---|---|---|---|---|---|---|
| Maude et al 201810 | 11 (3-23) | 75 | 3 (1-8) | 4-1BB | 81 | 50% EFS at 12 months | 46 | 13 |
| Shah NN et al 202115 | 13.5 (4.3-30.4) | 50 | NA | CD28 | 62 | 38% EFS at 6 months | 22 | 8 |
| Myers et al 202117∗ | 10.3 (1.7-29.1) | 41 | NA | 4-1BB† | 98 | 82% EFS at 12 months | 15 | 7 |
| Park et al 201818 | 44 (23-64) | 53 | NA‡ | CD28 | 83 | Median EFS 6.1 months | 26 | 42 |
| Hay et al 201919 | 39 (20-76) | 53 | 3 (1-11) | 4-1BB | 85§ | NA | 19 | 23 |
| Shah BD et al 202121 | 40 (28-52) | 55 | 2 (2-3) | CD28 | 71 | 58% RFS at 6 months | 24 | 26 |
| Rodie et al 202122 | 41.5 (18-62) | 20 | 3 (2-6) | 4-1BB | 85§ | 44% EFS at 12 months | 0 | 15 |
| Zhang et al 202124 | 12 (2-61) | 110 | NA | 4-1BB/CD28ε | 87 | 58% LFS at 12 months | 16 | 14ǁ |
NA, not available.
CAR T-cell–naive cohort.
With a humanized anti-CD19 single-chain variable fragment domain.
Previous therapies: 2, 40%; 3, 25%; and ≥4, 36%.
Rate of MRD-negative CR.
Grade 2-4 neurotoxicity.
Eighty-nine patients received 4-1BB construct; 21 patients received CD28 construct.
Lessons learned
Management of CRS and ICANS
CRS and ICANS are 2 of the most prominent toxicities related to CAR T-cell therapy, with potential for life-threatening clinical sequelae. Although corticosteroids can suppress the cytokine storm associated with CRS, concern for hampered CAR T-cell function and expansion after steroid use was supported by early evidence in clinical trials.25 Therefore, initial recommendations cautioned against the use of steroids in the setting of CRS and suggested their use only in severe cases after failure of tocilizumab or cases with associated neurotoxicity.26,27 However, recent data suggest that earlier initiation of steroid administration may help prevent severe CRS/ICANS and may not have deleterious effects on the treatment efficacy.28 Tocilizumab, the humanized monoclonal antibody against interleukin-6, appears to have no effect on CAR T-cell efficacy when administered in the setting of CRS. Gardner et al demonstrated that early intervention with tocilizumab and corticosteroids reduced the transition from mild to severe CRS without negatively affecting efficacy outcomes in patients with B-ALL.29 Similarly, an analysis of the cohort 6 of the ZUMA-1 trial in patients with non-Hodgkin lymphoma showed that prophylactic corticosteroids resulted in no grade ≥3 CRS and low rates of ICANS while maintaining high response rates with the limitation that patients in this cohort had lower tumor burden than previously enrolled cohorts.30 Efforts to mitigate development of severe CRS and ICANS in earlier stages are increasingly implemented into clinical trial protocols and clinical practice. The interleukin-1 receptor inhibitor, anakinra, has been shown to decrease rates of severe CRS and ICANS, without hindering short-term CAR T-cell efficacy when given as prophylaxis in adult patients with lymphoma.31,32 Prophylactic tocilizumab has been shown to be safe and reduce the incidence and severity of CRS.33 Clinicians should follow the guidelines for CRS and ICANS management per the prescribing guidelines for approved CAR T-cell products and those listed in individual clinical trial protocols. The prophylactic use of steroids, tocilizumab, or anakinra is currently not indicated outside of a clinical trial.
Frey et al evaluated protocol modifications for optimization of tisa-cel administration. Thirty-five adult patients with R/R B-ALL received either low- (n = 6) or high- (n = 29) dose of CAR T cells.34 The high-dose CAR T cells were administered as a single infusion (n = 6) or fractionated infusions (n = 20). A total of 65% of the patients in the high-dose fractionated cohort had >5% blasts at baseline, compared with 84% and 100% in the high-dose single infusion cohort and low-dose cohort, respectively. Patients who received the high-dose fractionated infusions had 90% CR and manageable CRS as well as the best 2-year OS rates of all cohorts, at 73%. Therefore, fractionation of cell infusions and dose modifications could potentially be used to optimize the safety of CAR T cells while preserving its efficacy, although, in this trial, there was imbalance in the baseline disease burden between the different study arms. Fractionation of CAR T-cell infusions was also used in the aforementioned AUTO1 trial that demonstrated a favorable toxicity profile, including no grade ≥3 CRS.22
Effect of CD19 expression and blinatumomab
Concern has been raised regarding the possible effect of prior exposure to blinatumomab on CD19-directed CAR T-cell therapy. In the ZUMA-3 trial, 45% of the patients had previously received blinatumomab. Patients with prior blinatumomab exposure had numerically lower rates of CR/CRi (60% in blinatumomab-exposed vs 80% in blinatumomab-naive); however, it did not affect RFS and OS.20 In a multicenter analysis of 420 children and young adults who received either tisa-cel or another CD19 CAR T-cell treatment, patients who did not respond to prior blinatumomab had inferior CR rates and 6 month RFS after anti-CD19 CAR T-cell infusion, compared with those who previously responded to blinatumomab or did not receive blinatumomab before CAR T-cell therapy.35 Of note, there were some differences between the blinatumomab-treated and blinatumomab-naive groups in baseline disease burden as well as prior therapies (including more patients in the blinatumomab-treated group than in the blinatumomab-naive group who received a prior allo-SCT).
The evolving role of allo-SCT in the era of CAR T cells
CAR T-cell therapy have provided remarkable response rates in pivotal trials, as described earlier, and ∼60% of responding patients are alive and disease free at 1 year after infusion.10,20 Allo-SCT is 1 of the options to further consolidate responses after CAR T-cell therapy, and the role of transplant in the current era is a subject of ongoing research and debate.
In the ELIANA trial, a small subset (10 patients, ie, ∼15% of the patients who achieved a response) underwent subsequent allo-SCT; EFS with or without censoring for transplant was similar.10,11 In contrast, in the National Institutes of Health pediatric trial using CAR T-cell constructs with CD28 costimulatory domain,15 75% of patients with MRD-negative CR (n = 21) proceeded to undergo allo-SCT, and had a 5-year posttransplant EFS of 62% and median OS of 70.2 months. The remaining 7 patients with MRD-negative CR who did not undergo subsequent allo-SCT relapsed. A retrospective analysis of 52 children and young adult patients who underwent allo-SCT with reduced intensity conditioning after achieving CR with a CD19 or CD22 CAR T cells showed promising results, with 1-year OS, EFS, and transplant-related mortality (TRM) rates of 87.7%, 73%, and 2.2%, respectively.38
Examining the differential role of allo-SCT consolidation after CAR T-cell infusion in adults and children should take into consideration the differences in transplant outcomes between the 2 populations. Analysis of the CIBMTR data set showed that for children who underwent transplantation for ALL in first CR, the 3-year OS was 79%, and for adults it was 64%.39 In general, adults undergoing allo-SCT (especially older adults with more comorbidities) have higher nonrelapse mortality compared with pediatric patients.40 A total of 11of 55 (20%) patients treated in the ZUMA-3 trial underwent subsequent allo-SCT (10 patients in CR/CRi, and 1 patient had blast-free aplastic marrow) after a median of 98 days after the CAR T-cell infusion.20 Duration of remission and RFS were similar with and without censoring at subsequent transplant. In an updated analysis with 2-year follow-up data, the 2-year OS was ∼60% for those who underwent allo-SCT.21 These data should be interpreted with caution, because there is inherent bias, as patients selected to undergo allo-SCT were in sustained remission (or with aplastic marrow) and deemed fit enough to undergo the transplant.
In the MSKCC trial in adult patients,18 17 patients (39%) who achieved CR proceeded to allo-SCT and had similar EFS and survival outcomes compared with those who did not. In contrast, in the trial at the Fred Hutchison Cancer Center, post–CAR T-cell allo-SCT was associated with improved EFS (HR = 0.39) for patients who achieved MRD– CR.19
It is difficult to extrapolate allo-SCT data from 1 CAR T-cell construct to another. In the ELIANA trial in children and young adults treated with 4-1BB costimulatory domain, very few patients had a subsequent allo-SCT, and the transplant did not seem to influence outcomes.10,11 However, in the National Institutes of Health pediatric trial using a CD28 costimulatory domain, consolidative allo-SCT significantly improved outcomes.15 In the adult patient population, the ZUMA-3 trial20,21 and the MSKCC trial18 (both using CD28 costimulatory domain) showed no benefit of consolidative allo-SCT; however, the Fred Hutchison trial19 with 4-1BB costimulatory domain supported consolidative allo-SCT.
It is important to note that none of the aforementioned prospective trials were designed to evaluate the role of allo-SCT after CAR T-cell therapy. Allo-SCT has been used for several decades and has known efficacy in patients with R/R ALL, providing a possibility for cure for a significant minority of the patients.41 The pivotal trials for approved CAR T cells in children and adults (ELIANA and ZUMA-3 trials, respectively) did not show benefit of consolidative allo-SCT. Therefore, at the present time, the role of allo-SCT after CAR T-cell treatment is not clear. Advanced MRD detection technologies might help delineate the patients who would benefit from allo-SCT after CAR T-cell treatment. Next-generation sequencing MRD detection after tisa-cel was recently shown to be highly predictive of subsequent relapse in children and young adults with B-ALL.42 Whether next-generation sequencing MRD can be used to identify patients at risk of relapse after CAR T-cell therapy and therefore identify potential candidates for allo-SCT as consolidation remains to be determined.
Relapse patterns after CAR T-cell therapy
Two main modes of relapse have emerged in patients after autologous anti-CD19 CAR T-cell therapy. The first, CD19-positive, relapse has been linked to poor persistence of the CAR T cells in the recipient.19,43 The identity of the costimulatory domain has been implicated as 1 of the determinants of postinfusion persistence of the cells. There is evidence to suggest that the 4-1BB domain confers extended persistence compared with a CD28 domain, which is associated with greater peak but lower long-term persistence, perhaps owing to amelioration of T-cell exhaustion in the 4-1BB construct10,20,44 The second mode of relapse, CD19-negative, has been shown to develop via several mechanisms, including antigen escape,45 lineage switch,46 and genetic mutations.47 Improved understanding of these mechanisms of post–CAR T-cell therapy leukemia progression is driving research to prevent and treat relapse.48
Targets beyond CD19
CD22
CD22 is expressed in ∼90% of both adult and pediatric B-ALL.3,51 Initial results have been reported of a phase 1 trial with 21 children and adults with B-ALL who received an anti-CD22 CAR T-cell treatment.36 Most patients had previously received an anti-CD19 CAR T-cell treatment. CR rate was dose dependent, with 73% of those who received higher doses achieving CR. An updated report37 with a larger cohort of 55 patients (51 had a prior CD19 CAR T-cell therapy) and an improved manufacturing method, yielded a CR rate of 70%. Among patients who achieved CR, the RFS was 6 months. Notably, a third of patients developed hemophagocytic lymphohistiocytosis, which developed an average of 14 days after CAR T-cell infusion. In another study, 34 pediatric and adult patients received CD22-directed CAR T-cell therapy after failure of anti-CD19 CAR T-cell treatment. CR/CRi was achieved in 80% of evaluable patients.52 To address the challenge of reduced expression of CD22 on blasts at relapse after anti-CD22 CAR T-cell infusion, a full humanCD22–CAR T-cell construct with potent activity against CD22low B-ALL has been developed.53
Target combinations
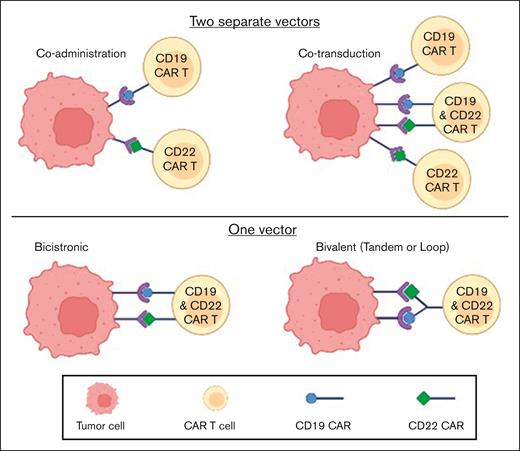
This strategy has been developed to avoid antigen-escape relapse. The most explored combination CAR T-cell therapy in B-ALL has been that of CD19 and CD22. Different strategies have been used to generate a dual anti–CD19/CD22 CAR construct, as depicted in Figure 1. In a phase 1/2 trial with sequential administration of anti-CD19 and anti-CD22 CAR T cells for B-cell malignancies, among 51 patients with R/R B-ALL, 96% achieved MRD-negative CR and median PFS was 13.6 months.54 A similar strategy of sequential administration of CD19 and CD22 CAR T cells was implemented in a trial reported by Liu and colleagues.55 Twenty-seven adult and pediatric patients with B-ALL received a CD19-directed murine CAR T-cell therapy, followed by humanized CD22 CAR T cells a month later. The 18-month OS and EFS rates were 88.5% and 67.5%, respectively. Another strategy has been coadministration of 2 humanized CAR T-cell constructs, 1 targeting CD19 and the other CD22, using a fractionated dosing schedule;56 all 11 evaluable patients achieved MRD-negative CR at 1 month. After a median follow-up of 6.2 months, 10 of these 11 patients were alive and in MRD-negative remission.
Similar positive early experience has been described with other bispecific CAR T-cell constructs,57-59 although there have been some signals of antigen escape at relapse after administration of these products.58,59
In a preliminary report of 36 patients who received a novel tandem CD19/CD22 CART-cell construct with CD28 and OX40 costimulatory domains, outcomes were favorable when retrospectively compared with a matched control cohort of patients who received anti-CD19 CAR T cells.60 An updated report in a 2021 annual meeting24 revealed data on 47 patients who received the tandem construct. With a median follow-up of 21.8 months, the median OS and leukemia-free survival (LFS) for the entire cohort had not been reached. The LFS rate at 1 year was 68.3%.
AUTO3, a dual CD19/CD22 CAR T-cell construct, was investigated in a phase 1 trial in 15 pediatric and young adult patients.61 There were no cases of grade ≥3 CRS or ICANS, and 86% of patients achieved CR/CRi at 1 month. Yet, 9 patients relapsed during follow-up, leading to a 1-year PFS of only 32%. Of the 9 patients that relapsed, 8 had low levels of CAR T cells in their peripheral blood cells at relapse, suggesting impaired persistence hindered long-term response duration. Table 2 displays major trials with the dual targeting of CD19 and CD22 for patients with B-ALL.
Selected major trials of dual CD19/CD22 CAR T-cell construct in B-ALL
| Reference . | Type of dual targeting . | Number of patients . | Median age (range), y . | CR + CRi (%) . | EFS/PFS . | Comments . |
|---|---|---|---|---|---|---|
| Wang et al 202054 | Coadministration | 51 | 27 (9-62) | 96 | Median PFS, 13.6 mo | -49% of responders relapsed-Lower efficacy for BM blasts ≥5% or CNS involvement |
| Liu et al 202155 | Coadministration (sequential) | 27 | 21 (1.6-55) | 85 | 18-mo EFS, 68% | -All patient had a prior allo-SCT-23% developed CAR T-cell–associated GVHD |
| Frey et al 202156 | Coadministration | 13 | 46 (28-72) | 85 | 10/11 responders in ongoing remission | 1 patient develop CAR-HLH |
| Annesley et al 202157 | Cotransduction | 23 | 12 (0.5-22) | 89 | — | Best persistence for CD22 CAR (compared with CD19 or dual CARs) |
| Cui et al 202124 | Bivalent (tandem) | 47 | 28 (6-56) | 100 | 12-mo LFS, 68% | 72% underwent subsequent allo-SCT with improved outcomes |
| Dai et al 202058 | Bivalent | 6 | 23.5 (17-44) | 100 | — | 3 of 6 patients relapsed by 12 mo |
| Spiegel et al 202159 | Bivalent (loop) | 17 | 47 (26-68) | 82 | Median PFS, 5.8 mo | |
| Cordoba et al 202161 | Bicistronic | 15 | 8 (4-16) | 86% | 12-mo EFS 32% | -Limited persistence-69% of responders relapsed |
| Reference . | Type of dual targeting . | Number of patients . | Median age (range), y . | CR + CRi (%) . | EFS/PFS . | Comments . |
|---|---|---|---|---|---|---|
| Wang et al 202054 | Coadministration | 51 | 27 (9-62) | 96 | Median PFS, 13.6 mo | -49% of responders relapsed-Lower efficacy for BM blasts ≥5% or CNS involvement |
| Liu et al 202155 | Coadministration (sequential) | 27 | 21 (1.6-55) | 85 | 18-mo EFS, 68% | -All patient had a prior allo-SCT-23% developed CAR T-cell–associated GVHD |
| Frey et al 202156 | Coadministration | 13 | 46 (28-72) | 85 | 10/11 responders in ongoing remission | 1 patient develop CAR-HLH |
| Annesley et al 202157 | Cotransduction | 23 | 12 (0.5-22) | 89 | — | Best persistence for CD22 CAR (compared with CD19 or dual CARs) |
| Cui et al 202124 | Bivalent (tandem) | 47 | 28 (6-56) | 100 | 12-mo LFS, 68% | 72% underwent subsequent allo-SCT with improved outcomes |
| Dai et al 202058 | Bivalent | 6 | 23.5 (17-44) | 100 | — | 3 of 6 patients relapsed by 12 mo |
| Spiegel et al 202159 | Bivalent (loop) | 17 | 47 (26-68) | 82 | Median PFS, 5.8 mo | |
| Cordoba et al 202161 | Bicistronic | 15 | 8 (4-16) | 86% | 12-mo EFS 32% | -Limited persistence-69% of responders relapsed |
BM, bone marrow; CNS, central nervous system.
A strategy targeting 3 antigens (CD19, CD20, and CD22) has been developed as well. Trivalent CAR T-cell constructs with either 3 individually expressed CARs (TriCAR) or a single anti-CD19 CAR and a bispecific CAR for the other 2 antigens in tandem (SideCAR) have shown in vitro efficacy. Moreover, these trivalent CARs, each containing a 4-1BB costimulatory domain, reportedly mitigated CD19-negative relapse in murine models.62 In another preclinical study, optimization of potential trispecific (targeting CD19, CD20, and CD22) CAR T-cell constructs was evaluated.63 These trispecific CAR T cells successfully eliminated tumors in a mouse model of lymphoma cells with variable expression of these 3 antigens, whereas monospecific CAR T cells failed.
Other targets
A report of a patient who received anti-CD38 CAR T cells after failure of a bispecific CD19/CD22 CAR T-cell therapy has been published.64 Although some indication of antitumor activity of the anti-CD38 CAR T cells was observed, the patient suffered major toxicities, possibly because of on target/off tumor effects. Preclinical data suggest the possible role of other targets for clinical CAR T-cell therapy in B-ALL in the future, such as BAFF-R,65 CRLF2,66 and CSPG4 (for MLL-rearrangement ALL67).
Allogeneic CARs
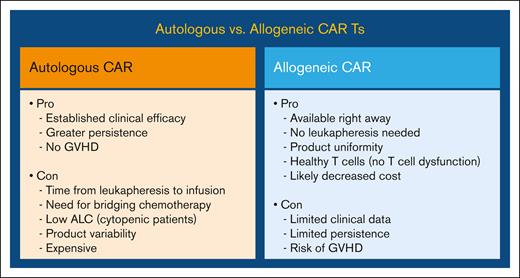
One of the appealing options to circumvent some of the shortcomings of the autologous CAR T cell, such as the need for a personalized CAR as well as the time-consuming manufacturing process, has been “off-the-shelf” allogeneic CAR T cells. However, allogeneic CARs have the potential risk of triggering graft-versus-host disease (GVHD) in the recipient. The pros and cons of autologous vs allogeneic CAR T-cell are shown in Figure 2.
Advantages and disadvantages of autologous vs allogeneic CAR T-cell therapy.
Two phase 1 trials with donor-derived allogeneic CD19-directed CAR T cells in children and adults have reported preliminary results. Participants received UCART19, a genetically engineered CAR construct, with knockout of T-cell receptor (TCR)α and CD52 using transcription activator–like effector nucleases. This is aimed to reduce risk of GVHD by reducing the number of TCRαβ+ T cells as well as enabling alemtuzumab (a CD52-directed monoclonal antibody) administration as part of lymphodepletion to deplete host T cells without affecting infused CAR T cells. The majority of the patients received fludarabine, cyclophosphamide, and alemtuzumab as the LD regimen. A total of 67% of the 21 patients achieved CR/CRi; 10 of 14 responders proceeded to receive allo-SCT.68 In an updated analysis, the investigators reported long-term outcomes of 25 adult patients treated with UCART19.69 The median patient age was 37 years, and patients had received a median of 4 prior lines of therapy, with 72% of patients having had a prior allo-SCT. The CR/CRi rate was 48% (12 of 25) and 9 of 12 responders had a subsequent allo-SCT as a consolidation strategy. The median OS of the entire cohort was 13.4 months.
Preliminary results with another allogeneic CAR T-cell construct, PBCAR0191, were presented at the 2021 American Society of Hematology meeting.70 This CD19-directed product includes a novel N6 costimulatory domain and TCR disruption via direct CAR insertion into the TRAC locus for GVHD prevention using the ARCUS gene-editing platform. The 15 patients included in the reported study were heavily pretreated, with 70% having received ≥4 previous treatment lines. No patient developed GVHD or grade ≥3 CRS, and 1 patient developed grade 3 ICANS. Higher cell dose and enhanced lymphodepletion with higher doses of fludarabine and cyclophosphamide, were associated with higher CR rates.
Preliminary results of a phase 1 trial with UCART22, an allogeneic anti-CD22 CAR T-cell construct, manufactured from non-HLA–matched healthy donor cells, were presented at the 2021 American Society of Hematology meeting.71 The 11 adult participants had a median of 3 prior treatment lines. The fludarabine, cyclophosphamide, and alemtuzumab LD regimen was associated with improved host lymphocyte suppression and UCART22 expansion, which in turn correlated with antileukemic activity.
Incorporating CAR T cells in adult B-ALL management
We are only beginning to appreciate the role of CAR T-cell therapy in the management of B-ALL. Before CAR T-cell approval, blinatumomab and inotuzumab ozogamicin were targeted therapies approved for R/R disease, after the aforementioned pivotal phase 3 TOWER and INO-VATE trials, respectively. In the TOWER study, approximately three-fourth of the patients in the blinatumomab arm were at salvage 1 (S1) or S2 stage of the disease,6 and the INO-VATE trial evaluated inotuzumab only in patients with S1 or S2 disease.5 Analyses of outcomes of both these trials has demonstrated improved EFS when these drugs are administered as an earlier salvage treatment.5,6 In comparison, in the ZUMA-3 trial, the patients were more heavily pretreated with a median of 2 prior therapies, and almost half of the patients received at least 3 prior treatment lines. Although cross-trial comparisons are difficult, we can appreciate the potency of CAR T-cell therapy in inducing remission in patients with advanced disease, and thus, it may be prudent to advance CAR T-cell therapies to earlier treatment lines in B-ALL. Autologous CAR T cells have been used mostly in heavily pretreated patients (Table 1). Fewer lines of therapy have been shown to correlate with higher response rates in both pediatric15 and adult20 patients.
There is also emerging data that the CAR T-cell strategy is more effective in the setting of low tumor burden, such as in patients with MRD-positive disease. The impact of tumor burden on efficacy outcomes of CAR T-cell therapy has been observed in both pediatric15 and adult18 patients. In a recent pooled analysis of 200 children and young adults who received tisa-cel, EFS and OS were higher for those with undetectable/low disease burden vs high disease burden.14 Notably, the rates of CRS and ICANS were lower in undetectable/low disease burden vs high disease burden.
One approach that could be used to further improve CAR T-cell use in B-ALL is as consolidation therapy after induction therapy. It seems rational to design a future treatment schema that includes a first phase of chemotherapy in order to provide tumor debulking, and then proceed with CAR T-cell infusion as consolidation. This would be in line with recent developments in clinical trials of CAR T-cell treatment for lymphoma; promising results from the ZUMA-12 trial,72 using axi-cel in frontline patients with high-risk large B-cell lymphoma after 2 cycles of chemoimmunotherapy, challenge the paradigm of chemoimmunotherapy in the upfront setting for high grade B-cell lymphoma. Similar advancement of CAR T cells into earlier treatment lines should be investigated in B-ALL.
The role of allo-SCT after CAR T-cell therapy continues to evolve. For adult patients who did not have an allo-SCT before CAR T-cell infusion, an allo-SCT after CAR T-cell therapy during remission can be considered, with the caveat that the data in this regard are conflicting. This practice may change if we see durable remissions in ongoing CAR T-cell therapy trials without consolidation allo-SCT; this strategy may be particularly important for patients achieving negative MRD via next-generation sequencing after CAR T-cell infusion. For patients who already had an allo-SCT before CAR T-cell infusion, the decision for a second allo-SCT will depend on the duration of remission after first transplant, posttransplant complications, and donor characteristics.
Conclusions
The introduction of CD19 CAR T-cell therapy has revolutionized the field of B-ALL and other CD19+ B-cell malignancies. With the current generation of approved CAR products, we are seeing very encouraging clinical activity in multiply R/R B-ALL. The toxicities associated with CAR T cells are manageable and most institutions have developed clinical practice algorithms to manage these toxicities. The field of CAR T-cell therapy continues to evolve with several novel constructs, novel targets, and combination targets in clinical development, including trials with allogeneic CARs. In the next few years, we expect to see trials incorporating CAR T cells in earlier lines of therapy with the eventual goal of providing long-term disease control for the majority of the patients with B-ALL.
Authorship
Contribution: O.P. and N.J. provided conceptualization and manuscript preparation; and P.K., B.D.S., and E.J. critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: P.K. provided consultancy for Kite, Pfizer, and Jazz. B.D.S. provides consulting and education for Amgen, Pfizer, Novartis, Bristol Myers Squibb (BMS), Kite, Precision Biosciences, Jazz, BeiGene, Adaptive, Century Therapeutics, Autolus, Deciphera, and Lilly; receives grants and investigator-initiated trials for Kite/Gilead, Jazz, and Servier; and is a member of the steering committee for PeproMene Bio. E.J. receives research grants from AbbVie, Amgen, Adaptive Biotechnologies, Takeda, Pfizer, and Ascentage, and receives consultancy fees from AbbVie, Amgen, Adaptive Biotechnologies, Takeda, Pfizer, Ascentage, Genentech, BMS, Novartis, and Kite. N.J. receives research funding from Pharmacyclics, AbbVie, Genentech, AstraZeneca, BMS, Pfizer, Servier, ADC Therapeutics, Cellectis, Adaptive Biotechnologies, Incyte, Precision Biosciences, Aprea Therapeutics, Fate Therapeutics, Kite/Gilead, Mingsight, Takeda, Medisix, Loxo Oncology, Novalgen, Dialectic Therapeutics, Newave, TransThera Sciences, Novartis, and CarnaBio Sciences, and is a member of the advisory board/receives honoraria from Pharmacyclics, Janssen, AbbVie, Genentech, AstraZeneca, BMS, Adaptive Biotechnologies, Kite/Gilead, Precision Biosciences, BeiGene, Cellectis, TG Therapeutics, MEI Pharma, Ipsen, and CareDX. O.P. declares no competing financial interests.
Correspondence: Nitin Jain, Department of Leukemia, The University of Texas MD Anderson Cancer Center 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: njain@mdanderson.org.
References
Author notes
Data are available on request from the corresponding author, Nitin Jain (njain@mdanderson.org).