Key Points
Severe4 was generated with machine learning and was associated with inferior PFS and OS in DLBCL indicated for CART in both an LC and VC.
Severe4 was associated with severe cytokine release syndrome, and high comorbidity burden was associated with higher rates of neurotoxicity.
Abstract
Chimeric antigen receptor T-cell therapy (CART) has extended survival of patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). However, limited durability of response and prevalent toxicities remain problematic. Identifying patients who are at high risk of disease progression, toxicity, and death would inform treatment decisions. Although the cumulative illness rating scale (CIRS) has been shown to correlate with survival in B-cell malignancies, no prognostic score has been independently validated in CART recipients. We retrospectively identified 577 patients with relapsed/refractory DLBCL indicated for CART at 9 academic centers to form a learning cohort (LC). Random survival forest modeling of overall survival (OS) and progression-free survival (PFS) was performed to determine the most influential CIRS organ systems and severity grades. The presence of a severe comorbidity (CIRS score ≥ 3) in the respiratory, upper gastrointestinal, hepatic, or renal system, herein termed “Severe4,” had the greatest impact on post-CART survival. Controlling for other prognostic factors (number of prior therapies, Eastern Cooperative Oncology Group performance status, BCL6 translocation, and molecular subtype), Severe4 was strongly associated with shorter PFS and OS in the LC and in an independent single-center validation cohort (VC). Severe4 was also a significant predictor of grade ≥3 cytokine release syndrome in the LC, while maintaining this trend in the VC. Thus, our results indicate that adverse outcomes for patients with DLBCL meant to receive CART can be predicted using a simplified CIRS-derived comorbidity index.
Introduction
Chimeric antigen receptor T-cell therapy (CART) has revolutionized the treatment of relapsed and/or refractory (R/R) B-cell malignancies. To date, the US Food and Drug Administration (FDA) has approved the use of 6 CART products for 10 indications. In diffuse large B-cell lymphoma (DLBCL) alone, 3 products have been approved in the third-line setting. Tisagenlecleucel (tisa-cel) was approved based on the JULIET trial with a 52% objective response rate (ObRR) and a 40% complete response (CR) rate; axicabtagene ciloleucel (axi-cel) was approved after the ZUMA-1 trial findings of an 82% ObRR and a 54% CR; and lisocabtagene maraleucel (liso-cel) was approved after the TRANSCEND trial reported a 73% ObRR and a 53% CR.1-3 These response rates are historically high in this previously difficult-to-treat disease.4 The longest follow-up data are available for axi-cel, with patients having median event-free survival of 5.7 months and median overall survival (OS) of 25.8 months.5 In addition, axi-cel and liso-cel are FDA approved in second-line high-risk DLBCL based on the ZUMA-7 and TRANSFORM trials, respectively. In both trials an improvement in event-free survival was demonstrated over standard-of-care (SOC) salvage chemotherapy and autologous stem cell transplant (ASCT).6-8 Liso-cel is also FDA-approved in second-line DLBCL for patients not eligible for ASCT because of age or comorbidity, based on the PILOT trial.9 Despite these practice-changing response and survival results, the vast majority of patients will experience morbidity and mortality from progressive disease as well as the unique and sometimes fatal toxicities associated with this novel therapy, namely, cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).10 Up to 93% of patients face CRS or ICANS, whereas rates of severe (ie, grade ≥3) CRS and ICANS range from 2% to 22% and 10% to 28%, respectively, in landmark trials (TRANSCEND and ZUMA-1).1-3
CART has been widely adopted, with thousands of patients treated worldwide thus far, making it critically important to balance the potential benefit of this therapy with its associated morbidity and mortality risk, which can be significant, as shown by reported nonrelapse mortality (NRM) of between 4.4% and 6% in 2 large real-world evidence (RWE) studies.11,12
Outcomes of patients with hematologic malignancies are governed by disease-specific and patient-centric factors. Importantly, age is not predictive of short-term CART outcomes13 whereas indices that quantify frailty and comorbidities have not been verified in this setting. To date, only limited, unvalidated predictive scores have been applied to patients with DLBCL receiving CART.14-16 The modified cumulative illness rating scale (CIRS), a comorbidity score originally intended to predict mortality in elderly hospitalized patients, has recently been found to predict outcomes in patients with chronic lymphocytic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma (NHL).17-23 In a recent retrospective multicenter study, we found that high CIRS scores (≥7) were associated with worse OS in patients with DLBCL treated with CART.14 However, the relative importance of comorbidities was not explored in this smaller study that lacked a validation data set. In this study we used a machine-learning algorithm to assess the relative prognostic impact of specific CIRS-defined comorbidities in patients with DLBCL intended to receive CART in a multicenter learning cohort (LC) that was then tested in a separate single-institution validation cohort (VC).
Methods
Patient eligibility
For the LC, patient data were obtained from 9 academic medical centers in the United States after approval by the respective institutional review boards. Patients with R/R DLBCL or high-grade B-cell lymphoma per 2018 World Health Organization criteria, including those with transformed NHL or Richter transformation (RT), indicated for CART, between December 2015 and February 2021, were included for analysis. Patients who underwent leukapheresis and for whom CART products were successfully manufactured were included, regardless of whether they received the product (because we sought an intent-to-treat population). Additional details are included in the supplemental Methods section. Cell of origin was determined by Hans algorithm.24 CRS and ICANS were graded based on the American Society for Transplantation and Cellular Therapy guidelines.10 Progression of disease was defined retrospectively based on the Lugano criteria.25
A single-institution VC was subsequently obtained (identified retrospectively under the same inclusion criteria as the LC) that comprised patients from the MD Anderson Cancer Center indicated for CART between January 2017 and February 2021 (a time period overlapping with the LC leukapheresis dates). Several patient and disease attributes were either not available (eg, year of DLBCL diagnosis) or frequently missing (eg, molecular subtype) in this cohort compared with the LC (Table 1). Only severe comorbidity information for the 4 CIRS organ systems identified by our LC machine learning–based variable selection procedure were collected for patients within the VC. Thus, CIRS scores of ≥7 was not available for the VC. Approval was obtained from the institutional review boards of all institutions participating in the study. The study was conducted in accordance with the Declaration of Helsinki.
Characteristics of patients indicated for CART (LC and VC)
| Patient feature . | Categories or statistics . | LC: 577 patients, n (%) . | VC: 218 patients, n (%) . | P value∗ . |
|---|---|---|---|---|
| Number of centers | Count | 9 | 1 | N/A |
| IPI risk category† | Low | 174 (30.2) | N/A | |
| Low-intermed | 187 (32.4) | |||
| High-intermed | 151 (26.2) | |||
| High | 65 (11.3) | |||
| Elevated serum LDH (>ULN)‡ | No | 274 (47.5) | 81 (37.2) | .010 |
| Yes | 303 (52.5) | 137 (62.8) | ||
| Concurrent indolent lymphoma (any type of transformed disease) | No | 394 (68.3) | 170 (78.0) | .102 |
| Yes | 154 (26.7) | 48 (22.0) | ||
| NA | 29 (5.0) | 0 | ||
| Transformed follicular lymphoma | No | 428 (74.2) | 178 (81.7) | .373 |
| Yes | 118 (20.5) | 40 (18.3) | ||
| NA | 31 (5.4) | 0 | ||
| Richter transformation | No | 528 (91.5) | 215 (98.6) | .219 |
| Yes | 18 (3.1) | 3 (1.4) | ||
| NA | 31 (5.4) | 0 | ||
| Dx to T-cell collection (y) | median (IQR) | 1 (1-3) | N/A | |
| range | 0 -37 | |||
| NA | n=48 | |||
| Age at T-cell collection | median (IQR): | 63 (55-70) | 60.5 (51-68) | .049 |
| range: | 19-90 | 18-89 | ||
| ECOG at T-cell collection | 0 | 195 (33.8) | 55 (25.2) | <.001 |
| 1 | 325 (56.3) | 107 (49.1) | ||
| 2/3/4 | 56 (9.7) | 56 (25.7) | ||
| NA | 1 (0.2) | 0 | ||
| Number of previous treatments | median (IQR): | 3 (2-4) | 3 (2-4) | .002 |
| range: | 1-11 | 1-11 | ||
| Previous transplant (autologous or allogeneic) | No | 432 (74.9) | 173 (79.4) | .193 |
| Yes | 145 (25.1) | 45 (20.6) | ||
| Number of medications (excluding PRN and vit.) | median (IQR) | 5 (3-7) | N/A | |
| range | 0-17 | |||
| Complex karyotype | No | 427 (74.0) | N/A | |
| Yes | 42 (7.3) | |||
| NA | 108 (18.7) | |||
| Double hit (MYC translocation with BCL2 or BCL6 translocation) | No | 431 (74.7) | 91 (41.7) | .508 |
| Yes | 95 (16.5) | 24 (11.0) | ||
| NA | 51 (8.8) | 103 (47.2) | ||
| Molecular subtype | GCB | 312 (54.1) | 87 (39.9) | .924 |
| non-GCB | 218 (37.8) | 59 (27.1) | ||
| NA | 47 (8.1) | 72 (33.0) | ||
| Respiratory (CIRS) | 0/1/2 (≤moderate) | 553 (95.8) | 211 (96.8) | .682 |
| 3/4 (severe) | 24 (4.2) | 7 (3.2) | ||
| Upper GI (CIRS) | 0/1/2 (≤moderate) | 561 (97.2) | 217 (99.5) | .052 |
| 3/4 (severe) | 16 (2.8) | 1 (0.5) | ||
| Hepatic (CIRS) | 0/1/2 (≤moderate) | 568 (98.4) | 207 (95.0) | .009 |
| 3/4 (severe) | 9 (1.6) | 11 (5.0) | ||
| Renal (CIRS) | 0/1/2 (≤moderate) | 566 (98.1) | 197 (90.4) | <.001 |
| 3/4 (severe) | 11 (1.9) | 21 (9.6) | ||
| Severe4 | No | 523 (90.6) | 183 (83.9) | .011 |
| Yes | 54 (9.4) | 35 (16.1) | ||
| Bridging therapy (to CART infusion) | No | 279 (48.4) | N/A | |
| Yes | 298 (51.6) | |||
| Bendamustine included in bridging therapy | No | 515 (89.3) | N/A | |
| Yes | 62 (10.7) | |||
| Nonbendamustine chemotherapy bridging | No | 465 (80.6) | N/A | |
| Yes | 112 (19.4) | |||
| Steroids as bridging therapy | No | 504 (87.3) | N/A | |
| Yes | 73 (12.7) | |||
| Radiation as bridging therapy | No | 543 (94.1) | N/A | |
| Yes | 34 (5.9) | |||
| Other bridging therapy | No | 365 (63.3) | N/A | |
| Yes | 212 (36.7) | |||
| CART product § | Axi-cel (Yescarta) | 393 (71.5) | 213 (97.7) | <.001 |
| Tisa-cel (Kymriah) | 120 (21.8) | 5 (2.3) | ||
| Liso-cel (Breyanzi) | 37 (6.7) | 0 | ||
| NA | 27 (4.7) | 0 | ||
| Response to CART § | CR | 290 (52.7) | 59 (27.1) | .417 |
| PR | 130 (23.6) | 36 (16.5) | ||
| SD | 13 (2.4) | 4 (1.8) | ||
| PD | 93 (16.9) | 17 (7.8) | ||
| NA | 24 (4.4) | 102 (46.8) | ||
| CRS grade § | 0 | 115 (19.9) | 17 (7.8) | <.001 |
| 1 | 227 (39.3) | 91 (41.7) | ||
| 2 | 166 (28.8) | 73 (33.5) | ||
| 3 | 27 (4.7) | 24 (11.0) | ||
| 4 | 11 (1.9) | 13 (6.0) | ||
| NA | 31 (5.4) | 0 | ||
| ICANS grade § | 0 | 289 (50.1) | 83 (38.1) | <.001 |
| 1 | 76 (13.2) | 29 (13.3) | ||
| 2 | 64 (11.1) | 22 (10.1) | ||
| 3 | 86 (14.9) | 51 (23.4) | ||
| 4/5 | 23 (4.0) | 33 (15.1) | ||
| NA | 39 (6.8) | 0 |
| Patient feature . | Categories or statistics . | LC: 577 patients, n (%) . | VC: 218 patients, n (%) . | P value∗ . |
|---|---|---|---|---|
| Number of centers | Count | 9 | 1 | N/A |
| IPI risk category† | Low | 174 (30.2) | N/A | |
| Low-intermed | 187 (32.4) | |||
| High-intermed | 151 (26.2) | |||
| High | 65 (11.3) | |||
| Elevated serum LDH (>ULN)‡ | No | 274 (47.5) | 81 (37.2) | .010 |
| Yes | 303 (52.5) | 137 (62.8) | ||
| Concurrent indolent lymphoma (any type of transformed disease) | No | 394 (68.3) | 170 (78.0) | .102 |
| Yes | 154 (26.7) | 48 (22.0) | ||
| NA | 29 (5.0) | 0 | ||
| Transformed follicular lymphoma | No | 428 (74.2) | 178 (81.7) | .373 |
| Yes | 118 (20.5) | 40 (18.3) | ||
| NA | 31 (5.4) | 0 | ||
| Richter transformation | No | 528 (91.5) | 215 (98.6) | .219 |
| Yes | 18 (3.1) | 3 (1.4) | ||
| NA | 31 (5.4) | 0 | ||
| Dx to T-cell collection (y) | median (IQR) | 1 (1-3) | N/A | |
| range | 0 -37 | |||
| NA | n=48 | |||
| Age at T-cell collection | median (IQR): | 63 (55-70) | 60.5 (51-68) | .049 |
| range: | 19-90 | 18-89 | ||
| ECOG at T-cell collection | 0 | 195 (33.8) | 55 (25.2) | <.001 |
| 1 | 325 (56.3) | 107 (49.1) | ||
| 2/3/4 | 56 (9.7) | 56 (25.7) | ||
| NA | 1 (0.2) | 0 | ||
| Number of previous treatments | median (IQR): | 3 (2-4) | 3 (2-4) | .002 |
| range: | 1-11 | 1-11 | ||
| Previous transplant (autologous or allogeneic) | No | 432 (74.9) | 173 (79.4) | .193 |
| Yes | 145 (25.1) | 45 (20.6) | ||
| Number of medications (excluding PRN and vit.) | median (IQR) | 5 (3-7) | N/A | |
| range | 0-17 | |||
| Complex karyotype | No | 427 (74.0) | N/A | |
| Yes | 42 (7.3) | |||
| NA | 108 (18.7) | |||
| Double hit (MYC translocation with BCL2 or BCL6 translocation) | No | 431 (74.7) | 91 (41.7) | .508 |
| Yes | 95 (16.5) | 24 (11.0) | ||
| NA | 51 (8.8) | 103 (47.2) | ||
| Molecular subtype | GCB | 312 (54.1) | 87 (39.9) | .924 |
| non-GCB | 218 (37.8) | 59 (27.1) | ||
| NA | 47 (8.1) | 72 (33.0) | ||
| Respiratory (CIRS) | 0/1/2 (≤moderate) | 553 (95.8) | 211 (96.8) | .682 |
| 3/4 (severe) | 24 (4.2) | 7 (3.2) | ||
| Upper GI (CIRS) | 0/1/2 (≤moderate) | 561 (97.2) | 217 (99.5) | .052 |
| 3/4 (severe) | 16 (2.8) | 1 (0.5) | ||
| Hepatic (CIRS) | 0/1/2 (≤moderate) | 568 (98.4) | 207 (95.0) | .009 |
| 3/4 (severe) | 9 (1.6) | 11 (5.0) | ||
| Renal (CIRS) | 0/1/2 (≤moderate) | 566 (98.1) | 197 (90.4) | <.001 |
| 3/4 (severe) | 11 (1.9) | 21 (9.6) | ||
| Severe4 | No | 523 (90.6) | 183 (83.9) | .011 |
| Yes | 54 (9.4) | 35 (16.1) | ||
| Bridging therapy (to CART infusion) | No | 279 (48.4) | N/A | |
| Yes | 298 (51.6) | |||
| Bendamustine included in bridging therapy | No | 515 (89.3) | N/A | |
| Yes | 62 (10.7) | |||
| Nonbendamustine chemotherapy bridging | No | 465 (80.6) | N/A | |
| Yes | 112 (19.4) | |||
| Steroids as bridging therapy | No | 504 (87.3) | N/A | |
| Yes | 73 (12.7) | |||
| Radiation as bridging therapy | No | 543 (94.1) | N/A | |
| Yes | 34 (5.9) | |||
| Other bridging therapy | No | 365 (63.3) | N/A | |
| Yes | 212 (36.7) | |||
| CART product § | Axi-cel (Yescarta) | 393 (71.5) | 213 (97.7) | <.001 |
| Tisa-cel (Kymriah) | 120 (21.8) | 5 (2.3) | ||
| Liso-cel (Breyanzi) | 37 (6.7) | 0 | ||
| NA | 27 (4.7) | 0 | ||
| Response to CART § | CR | 290 (52.7) | 59 (27.1) | .417 |
| PR | 130 (23.6) | 36 (16.5) | ||
| SD | 13 (2.4) | 4 (1.8) | ||
| PD | 93 (16.9) | 17 (7.8) | ||
| NA | 24 (4.4) | 102 (46.8) | ||
| CRS grade § | 0 | 115 (19.9) | 17 (7.8) | <.001 |
| 1 | 227 (39.3) | 91 (41.7) | ||
| 2 | 166 (28.8) | 73 (33.5) | ||
| 3 | 27 (4.7) | 24 (11.0) | ||
| 4 | 11 (1.9) | 13 (6.0) | ||
| NA | 31 (5.4) | 0 | ||
| ICANS grade § | 0 | 289 (50.1) | 83 (38.1) | <.001 |
| 1 | 76 (13.2) | 29 (13.3) | ||
| 2 | 64 (11.1) | 22 (10.1) | ||
| 3 | 86 (14.9) | 51 (23.4) | ||
| 4/5 | 23 (4.0) | 33 (15.1) | ||
| NA | 39 (6.8) | 0 |
Dx, diagnosis; intermed., intermediate; IQR, interquartile range; NA, not available (ie, missing data); N/A, not applicable; num., number; PD, progressive disease; PR, partial response; PRN, as needed; SD, stable disease; ULN, upper limit of normal; vit., vitamins.
P values from Wilcoxon rank-sum test for continuous features and from Fisher exact test or Pearson chi-square test for categorical features.
Determined at DLBCL diagnosis for LC; missing for VC because the IPI component risk factors were determined at CART infusion not at diagnosis.
Determined at DLBCL diagnosis for LC, and at CART infusion for VC.
Data from the 27 patients in the LC who died before receiving CART cells will automatically be missing (ie, NA) for this feature.
Assessment of comorbidities by CIRS
Comorbidities were evaluated based on documentation of medical problems and are detailed in the supplemental Methods, with CIRS scores collected as described by Salvi et al.17 In keeping with previous studies that have applied CIRS to NHL, patients were deemed to have a high comorbidity burden if the total CIRS score, summed over the 14 organ systems, was at least 7 (CIRS score ≥7).14,26,27
Statistical methods
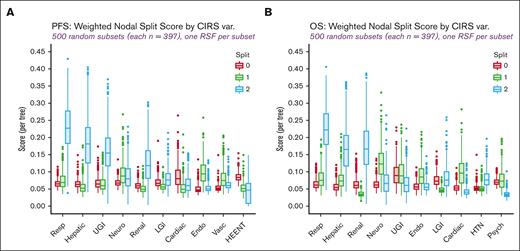
Progression-free survival (PFS) and OS were measured from T-cell collection. Random survival forest (RSF) models of PFS and OS, each comprising 10 000 bootstrap sample-specific survival trees, were fit to identify the CIRS organ system variables with the strongest relative influence on the respective time-to-event measure. The strength of influence was evaluated by variable importance, minimal depth, and weighted nodal split score (WSS)23 RSF predictors included each of the 14 CIRS variables (coded from 0 to 3 in order of increasing comorbidity or organ dysfunction, with 3 representing raw CIRS scores of 3 or 4) and the following known prognostic factors: International Prognostic Index (IPI) risk category, concurrent indolent lymphoma, time between DLBCL diagnosis and T-cell collection, patient age, Eastern Cooperative Oncology Group performance status, number of prior treatments, transplant history, MYC translocation, “double-hit” cytogenetics (ie, MYC rearrangement plus BCL6 or BCL2 rearrangement), and germinal center B-cell (GCB) vs non-GCB molecular subtype. Repeated random subsampling of the LC was performed to create influence measure distributions that aid in the selection of a reduced set of CIRS variables most likely to affect postleukapheresis outcomes. This repeated subsampling procedure led to the formation of 500 subsets, each consisting of ∼70% of the patients in the LC. A separate RSF with identical hyperparameters (specified in the supplemental Methods section) was fit to each LC subset so that variable importance and minimal depth could be computed and ranked for each predictor (ie, CIRS variable or known prognostic factor), and WSS calculated for each CIRS variable and potential cutoff or split point (ie, ≤0, ≤1, ≤2). Although all RSF-derived influence metrics were initially considered, we ultimately chose CIRS variable split points with median WSS values (computed across the 500 random LC subsets) of >0.15 per tree (for PFS or OS as the outcome) for constructing our CIRS-based comorbidity index.
Median follow-up time was estimated by reverse Kaplan-Meier (KM) and the standard KM methods, and log-rank test were used to estimate and compare PFS and OS curves. Cox proportional hazards models of PFS and OS were fit to adjust for patient and disease features (listed in Table 1) when estimating hazard ratios (HRs) of CIRS-based indices. Predictive accuracy (specifically, Cox model discrimination) was assessed by estimating the area under the incident/dynamic-defined receiver operating characteristic (ROC) curve.28 The length of time between leukapheresis and CART infusion (for those patients surviving to infusion) was considered as left truncation (ie, delayed entry) time for models that included predictors, such as CART product type, that were not known at T-cell collection. The binary outcomes of grade ≥3 CRS, grade ≥3 ICANS, objective response (≥partial response), and complete response (≥CR) were each modeled with logistic regression. Regardless of outcome type, multivariable models were built by first considering patient or disease features that met specific model assumptions (eg, constant HR and predictor linearity in the log-hazard for Cox regression) and that had univariable P values <.20, and then applying Akaike Information Criterion (AIC)-based backward elimination. To minimize sample size attrition in multivariable models, not available (NA) categories were created for molecular and cytogenetic predictors with non-negligible missingness (>5% of patients having missing values) as long as this had little impact on the non-NA HRs estimated from the complete case analysis.
Statistical significance was ascribed to effects with P values < .05, and no multiplicity adjustments were made. All analyses were performed and plots generated using R version 4.2.1.
Results
Baseline patient characteristics: LC
The LC comprised 577 patients with baseline characteristics that are provided in Table 1. The median patient age at T-cell collection was 63 years (range, 19-90 years) and 90% had an ECOG performance status score of 0 to 1. The median number of prior therapies was 3 (range, 1-11); 25% of patients (n = 143) underwent prior ASCT. GCB subtype was found in 54% (n = 312) of patients, and non-GCB in 38% (n = 218) by Hans algorithm,24 with 8% (n = 47) being unclassified. In total, 27% (n = 154) of patients were transformed from a low-grade lymphoma (n = 118 [20%] with transformed follicular lymphoma; n = 18 [3%] RT; n = 13 [2%] marginal zone lymphoma; n = 2 [<1%] lymphoplasmacytic lymphoma; and n = 3 [1%] unknown). MYC gene rearrangement was detected in 21% (n = 123), with 16% of patients (n = 95) having a MYC and an additional translocation in either BLC2 or BCL6 (double hit). Forty-two patients (7%) had a complex karyotype, although 19% had inadequate data needed to make this distinction. Twenty-seven patients (4.7%) died before CART infusion (24 had progressive disease and 3 developed an infection). Of the 550 patients infused with CART, 71% (n = 393) received axi-cel, 22% (n = 120) tisa-cel, and 7% (n = 37) liso-cel.
CART outcomes and CIRS comorbidities
Among patients in the LC who received CART (n = 550), the overall response rate (ORR) was 76% (n = 420), with a CR rate of 53% (n = 290) and data missing from 4% (n = 24) (Table 1). Factors associated with CR in the LC were: >1 year elapsed time between DLBCL diagnosis and T-cell collection (odds ratio [OR] = 1.95; P < .001), >60 years of age at T-cell collection (OR = 1.48; P = .028), ECOG status (ECOG 1 vs 0, OR = 0.55; P = .002; 2/3 vs 0, OR = 0.26; P < .001), ≥3 previous therapies (OR = 0.58; P = .002), and CART product type (axi-cel vs tisa-cel. OR = 2.42; P < .001; liso-cel vs tisa-cel, OR = 1.78; P = .135; axi-cel vs liso-cel, OR = 1.36; P = .376).
In the LC, the median follow-up time was 21 months, 41% (n = 238) of patients died during follow-up, and 56% had a PFS event. The median PFS was 11 months (95% confidence interval [CI], 8-15) and the median OS was 30 months (95% CI, 23–NA). Factors evaluated for association with survival outcomes are summarized in supplemental Table 1. Multivariable analysis showed that elevated serum lactate dehydrogenase (LDH) at DLBCL diagnosis (HR = 1.23; P = .075), impaired functional status at T-cell collection (ECOG 1 vs 0, HR = 1.38; P = .015; ECOG 2/3 vs 0, HR = 2.46; P < .001), ≥3 prior lines of treatment (HR = 1.39; P = .005), BCL6 translocation (HR = 1.33; P = .051), and non-GCB subtype (HR = 1.32; P = .019) were independent predictors of shorter PFS. When limiting analysis to patients who received CART (n = 550), steroid bridging therapy (HR = 2.12; P < .001) and receiving tisa-cel rather than axi-cel (HR = 1.42; P = .011), were associated with increased risk of disease progression or death.
In the multivariable model for OS, elevated LDH (HR = 1.30; P = .049), ECOG status (1 vs 0, HR = 1.83; P < .001; 2/3 vs 0, HR = 3.17; P < .001), ≥3 previous lines of therapy (HR = 1.32; P = .042) and BCL6 translocation (HR = 1.32; P = .100) were risk factors for any-cause death. Although CART product type was not significantly related to OS when controlling for other factors, patients exposed to steroids as bridging therapy had an elevated risk of post-CART death (HR = 2.30; P ≤ .001). Surprisingly, double-hit cytogenetics did not significantly correlate with either survival outcome.
In the LC, the most frequently encountered comorbidities of any degree by CIRS organ system were vascular (n = 297, 51%) and endocrine (n = 257, 45%) related, and hypertension (n = 248, 43%). Severe (ie, CIRS grade 3-4) comorbidities were most prevalent in the vascular (n = 99, 17%), psychiatric (n = 54, 9%), and endocrine (n = 53, 9%) categories (complete list in supplemental Table 2). The median CIRS score was 7 (range, 0-25) with 54% of patients (n = 309) having a CIRS score of ≥7. Consistent with our earlier reports, CIRS scores of ≥7 were significantly associated with inferior PFS (HR = 1.26; P = .040) and OS (HR = 1.35; P = .022) in univariable analysis (supplemental Figure 1). However, the prognostic significance of a CIRS score of ≥7 was lost when accounting for other factors in the multivariable setting.
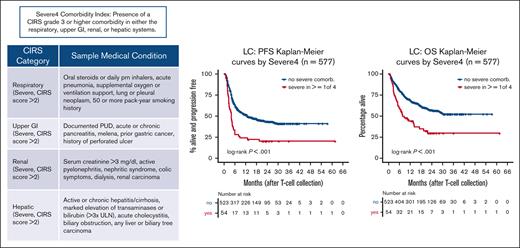
‘Severe4’ strongly correlates with post-CART survival
We sought to identify the CIRS categories most highly associated with survival. RSF nodal split scores, minimal depth ranks, and variable importance ranks (Figure 1; supplemental Figures 2 and 3) were calculated to evaluate the relative impact of specific CIRS categories on PFS and OS. Finding that severe CIRS scores (>2) in the respiratory, upper gastrointestinal (GI), renal, and hepatic systems had the strongest impact on PFS and OS, we defined an index, called “Severe4,” which represents the presence of a severe comorbidity in any of these 4 CIRS organ systems. Examples of medical conditions that would qualify as severe in these CIRS categories are listed in supplemental Table 3. Severe4, present in 9% of patients in the LC, was independently associated with inferior PFS (HR = 2.15; 95% CI, 1.54-2.99; P < .001) and OS (HR = 1.94; 95% CI, 1.35-2.78; P < .001; Figure 2A-B). As an alternative to our intent-to-treat approach of measuring survival from the date of leukapheresis, we limited our analysis to those 550 patients who received CART so that bridging therapy and CART product type could be considered as baseline predictors. Doing so did not change the independent prognostic effect of Severe4 (PFS HR = 2.18; 95% CI, 1.54-3.08; P < .001; OS HR = 2.08; 95% CI, 1.43-3.02; P < .001; Figure 2C-D). A summary of univariable and multivariable Cox modeling of both survival outcomes is presented in Table 2.
WSS distribution for 500 random subsets and RSF models of the LC. (A) PFS. (B) OS. Endo, endocrine; HEENT, head, ears, eyes, nose, throat; HTN, hypertension, LGI, lower gastrointestinal; Neuro, neurological; Psych, psychiatric; Resp, respiratory; UGI, upper gastrointestinal; var, variable; Vasc, vascular.
WSS distribution for 500 random subsets and RSF models of the LC. (A) PFS. (B) OS. Endo, endocrine; HEENT, head, ears, eyes, nose, throat; HTN, hypertension, LGI, lower gastrointestinal; Neuro, neurological; Psych, psychiatric; Resp, respiratory; UGI, upper gastrointestinal; var, variable; Vasc, vascular.
Severe4 index predicts inferior outcomes in patients receiving CART. (A,B) All patients and (C,D) excluding patients who died before receiving CART (left truncation).
Severe4 index predicts inferior outcomes in patients receiving CART. (A,B) All patients and (C,D) excluding patients who died before receiving CART (left truncation).
Severe4 independently correlates with PFS and OS in both the LC and VC
| Model . | N . | Covariates‡ . | Main predictor . | HR (95% CI) . | P value . | iAUC . |
|---|---|---|---|---|---|---|
| PFS | ||||||
| LC | ||||||
| Univariate | 577 | N/A | Severe4 | 2.15 (1.56-2.97) | <.001 | 0.535 |
| CIRS score ≥7 | 1.26 (1.01-1.57) | .0399 | 0.529 | |||
| Multivariate | 576 | High LDH, ECOG, previous tx ≥3, GCB subtype | Severe4 | 2.06 (1.48-2.86) | <.001 | 0.620 |
| CIRS score ≥7 | 1.08 (0.86-1.36) | .498 | 0.605 | |||
| Multivariate, LT | 550 | High LDH, ECOG, previous tx ≥3, GCB subtype, steroids as bridging tx and its interaction with Severe4, stratify on CART product | Severe4∗ | 2.09 (1.42-3.09) | <.001 | 0.627 |
| Severe4† | 9.32 (4.26-20.4) | <.001 | 0.722 | |||
| CIRS score ≥7 | 1.16 (0.91-1.48) | .221 | 0.624 | |||
| TFL subgroup | 118 | High LDH, ECOG, previous tx ≥3, GCB subtype | Severe4 | 1.58 (0.69-3.59) | .280 | 0.670 |
| CIRS score ≥7 | 0.67 (0.38-1.20) | .180 | 0.672 | |||
| VC main predictor is always Severe4 because CIRS score of ≥7 was not available for VC. | ||||||
| Univariate | 218 | N/A | Severe4 | 1.71 (1.15-2.52) | .008 | 0.541 |
| Multivariate | 218 | Model not fit because IPI component variables were not measured until time of CART infusion (ie, after T-cell collection) in the VC. Moreover, unlike in the LC, all patients in the VC received CART. | ||||
| Multivariate, LT | 218 | Age, high LDH, >1 extranodal site, ECOG | Severe4 | 1.85 (1.24-2.76) | .003 | 0.659 |
| TFL subgroup | 40 | Age, ECOG, previous tx ≥3, high LDH, >1 extranodal site | Severe4 | 4.20 (1.25-14.11) | .020 | 0.701 |
| OS | ||||||
| LC | ||||||
| Univariate | 577 | N/A | Severe4 | 2.18 (1.53-3.12) | <.001 | 0.538 |
| CIRS score ≥7 | 1.35 (1.05-1.75) | .022 | 0.538 | |||
| Multivariate | 576 | High LDH, previous tx ≥3, stratify on ECOG | Severe4 | 1.89 (1.32-2.71) | <.001 | 0.574 |
| CIRS score ≥7 | 1.12 (0.85-1.46) | .421 | 0.557 | |||
| Multivariate, LT | 550 | High LDH, ECOG, previous tx ≥3, steroids as bridging therapy | Severe4 | 2.08 (1.43-3.02) | <.001 | 0.639 |
| CIRS score ≥7 | 1.14 (0.86-1.51) | .364 | 0.638 | |||
| TFL subgroup | 118 | High LDH, previous tx ≥3, ECOG | Severe4 | 1.48 (0.60-3.65) | .398 | 0.690 |
| CIRS score≥7 | 0.78 (0.40-1.51) | .462 | 0.688 | |||
| VC main predictor is always Severe4 because CIRS score of ≥7 was not available for VC. | ||||||
| Univariate | 218 | N/A | Severe4§ | 1.70 (1.11-2.61) | .015 | 0.542 |
| Multivariate | 218 | Model not fit because IPI component variables were not measured until time of CART infusion (ie, after T-cell collection). Unlike in the LC, all patients in the VC received CART. | ||||
| Multivariate, LT | 218 | Age, ECOG, previous tx ≥3, high LDH, stratify on >1 extranodal site | Severe4§ | 1.70 (1.09-2.66) | .019 | 0.665 |
| TFL subgroup | 40 | ECOG, high LDH, >1 extranodal site, CART type, stratify age > 60 | Severe4 | 2.51 (0.60-10.50) | .208 | 0.715 |
| Model . | N . | Covariates‡ . | Main predictor . | HR (95% CI) . | P value . | iAUC . |
|---|---|---|---|---|---|---|
| PFS | ||||||
| LC | ||||||
| Univariate | 577 | N/A | Severe4 | 2.15 (1.56-2.97) | <.001 | 0.535 |
| CIRS score ≥7 | 1.26 (1.01-1.57) | .0399 | 0.529 | |||
| Multivariate | 576 | High LDH, ECOG, previous tx ≥3, GCB subtype | Severe4 | 2.06 (1.48-2.86) | <.001 | 0.620 |
| CIRS score ≥7 | 1.08 (0.86-1.36) | .498 | 0.605 | |||
| Multivariate, LT | 550 | High LDH, ECOG, previous tx ≥3, GCB subtype, steroids as bridging tx and its interaction with Severe4, stratify on CART product | Severe4∗ | 2.09 (1.42-3.09) | <.001 | 0.627 |
| Severe4† | 9.32 (4.26-20.4) | <.001 | 0.722 | |||
| CIRS score ≥7 | 1.16 (0.91-1.48) | .221 | 0.624 | |||
| TFL subgroup | 118 | High LDH, ECOG, previous tx ≥3, GCB subtype | Severe4 | 1.58 (0.69-3.59) | .280 | 0.670 |
| CIRS score ≥7 | 0.67 (0.38-1.20) | .180 | 0.672 | |||
| VC main predictor is always Severe4 because CIRS score of ≥7 was not available for VC. | ||||||
| Univariate | 218 | N/A | Severe4 | 1.71 (1.15-2.52) | .008 | 0.541 |
| Multivariate | 218 | Model not fit because IPI component variables were not measured until time of CART infusion (ie, after T-cell collection) in the VC. Moreover, unlike in the LC, all patients in the VC received CART. | ||||
| Multivariate, LT | 218 | Age, high LDH, >1 extranodal site, ECOG | Severe4 | 1.85 (1.24-2.76) | .003 | 0.659 |
| TFL subgroup | 40 | Age, ECOG, previous tx ≥3, high LDH, >1 extranodal site | Severe4 | 4.20 (1.25-14.11) | .020 | 0.701 |
| OS | ||||||
| LC | ||||||
| Univariate | 577 | N/A | Severe4 | 2.18 (1.53-3.12) | <.001 | 0.538 |
| CIRS score ≥7 | 1.35 (1.05-1.75) | .022 | 0.538 | |||
| Multivariate | 576 | High LDH, previous tx ≥3, stratify on ECOG | Severe4 | 1.89 (1.32-2.71) | <.001 | 0.574 |
| CIRS score ≥7 | 1.12 (0.85-1.46) | .421 | 0.557 | |||
| Multivariate, LT | 550 | High LDH, ECOG, previous tx ≥3, steroids as bridging therapy | Severe4 | 2.08 (1.43-3.02) | <.001 | 0.639 |
| CIRS score ≥7 | 1.14 (0.86-1.51) | .364 | 0.638 | |||
| TFL subgroup | 118 | High LDH, previous tx ≥3, ECOG | Severe4 | 1.48 (0.60-3.65) | .398 | 0.690 |
| CIRS score≥7 | 0.78 (0.40-1.51) | .462 | 0.688 | |||
| VC main predictor is always Severe4 because CIRS score of ≥7 was not available for VC. | ||||||
| Univariate | 218 | N/A | Severe4§ | 1.70 (1.11-2.61) | .015 | 0.542 |
| Multivariate | 218 | Model not fit because IPI component variables were not measured until time of CART infusion (ie, after T-cell collection). Unlike in the LC, all patients in the VC received CART. | ||||
| Multivariate, LT | 218 | Age, ECOG, previous tx ≥3, high LDH, stratify on >1 extranodal site | Severe4§ | 1.70 (1.09-2.66) | .019 | 0.665 |
| TFL subgroup | 40 | ECOG, high LDH, >1 extranodal site, CART type, stratify age > 60 | Severe4 | 2.51 (0.60-10.50) | .208 | 0.715 |
ECOG performance status was included as a categorical variable, with categories: 0 vs 1 vs 2/3/4.
iAUC, integrated Area Under the incident/dynamic ROC curve (a measure of predictive accuracy or concordance); LT, left truncation time between T-cell collection and CART infusion (denoting delayed entry into the risk set) was accounted for in the model; N/A, not applicable; TFL, transformed follicular lymphoma; tx, treatment.
Patients without steroids as bridging therapy (n = 480).
Patients receiving a steroid for bridging therapy (n = 70).
Covariate set for each multivariable Cox model was chosen in the absence of any CIRS-based predictor and includes patient and disease features with univariable Cox model P value < .20 that satisfied model assumptions and was retained upon AIC-based backward elimination.
Cox model proportional hazards assumption was violated.
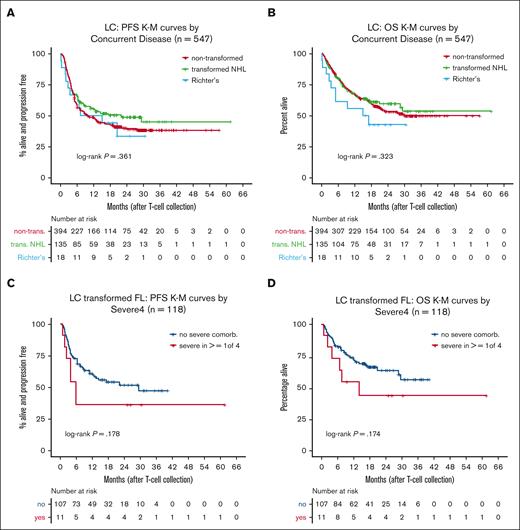
Because transformed lymphoma is associated with poor outcomes with standard therapies yet treatment with CART has shown promise in retrospective studies,1-3,29-31 we evaluated survival in transformed NHL and, specifically, RT patients. Patients with transformed NHL had modestly better outcomes than those with nontransformed disease (PFS: HR = 0.91; 95% CI, 0.60-1.37; OS: HR = 0.76; 95% CI, 0.47-1.23), whereas patients with RT fared worse (PFS: HR = 1.53; 95% CI, 0.49-4.83; OS: HR = 2.25; 95% CI, 0.71-7.12) (Figure 3A-B). In the transformed follicular lymphoma subgroup (n = 118), Severe4 was associated with a trend toward inferior PFS (HR = 1.92; 95% CI, 0.84-4.40) and OS (HR = 2.01; 95% CI, 0.82-4.94) (Figure 3C-D; Table 2). In summary, the Severe4 index was highly associated with outcomes among intended CART recipients with DLBCL in the LC.
Outcomes of patients with transformed NHL. (A) KM curves of PFS by concurrent disease from the LC. (B) KM curves of OS by concurrent disease from the LC. (C) KM curves of PFS by Severe4 in patients with transformed FL. (D) KM curves of OS by Severe4 in patients with transformed FL. FL, follicular lymphoma.
Outcomes of patients with transformed NHL. (A) KM curves of PFS by concurrent disease from the LC. (B) KM curves of OS by concurrent disease from the LC. (C) KM curves of PFS by Severe4 in patients with transformed FL. (D) KM curves of OS by Severe4 in patients with transformed FL. FL, follicular lymphoma.
Comorbidities correlate with CART toxicities
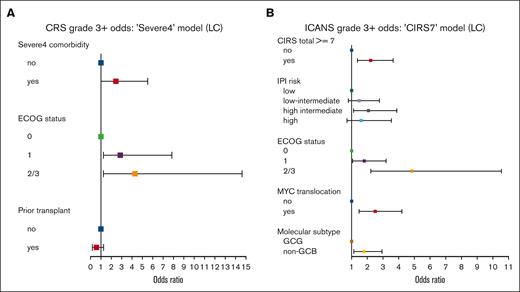
Among patients receiving CART in the LC , 78% (n = 431) developed CRS of any grade, whereas 7% (n = 38) had severe CRS, defined as grade ≥3 (Table 1). ICANS was identified in 45% (n = 249) of patients in the LC, with severe ICANS (grade ≥3) observed in 20% (n = 109). Importantly, Severe4 was associated with a higher risk of severe CRS when evaluated alone (OR = 2.88; 95% CI, 1.17-6.42; P =.013) and in the presence of other significant factors (OR = 2.43; 95% CI, 0.97-5.51; P = .042; Figure 4A). Further included in the multivariable model for grade ≥3 CRS, ECOG ≥1 was associated with a higher risk (ECOG 1 vs 0, OR = 2.87; P = .023; 2/3 vs 0, OR = 4.30; P = .017) and previous transplant with a lower risk (OR = 0.54; P = .181) of severe CRS. Although Severe4 did not significantly correlate with an increased risk of grade ≥3 ICANS (OR = 1.39; P = .331), CIRS score of ≥7 was (univariable OR = 2.60; P < .001; multivariable OR = 2.21; P = .001; Figure 4B). Other factors associated with severe ICANS included ECOG status (1 vs 0, OR = 1.82; P = .031; 2/3 vs 0, OR = 4.83; P < .001), MYC translocation (OR = 2.49; P = .001), and non-GCB subtype (OR = 1.81; P = .014).
Severe4 is associated with grade ≥3 CRS and CIRS score of ≥7 with grade ≥3 ICANS. (A) Forest plot of factors associated with development of grade ≥3 CRS. (B) Forest plot of factors associated with development of grade ≥3 ICANS. CIRS7, cumulative illness rating scale ≥ 7.
Severe4 is associated with grade ≥3 CRS and CIRS score of ≥7 with grade ≥3 ICANS. (A) Forest plot of factors associated with development of grade ≥3 CRS. (B) Forest plot of factors associated with development of grade ≥3 ICANS. CIRS7, cumulative illness rating scale ≥ 7.
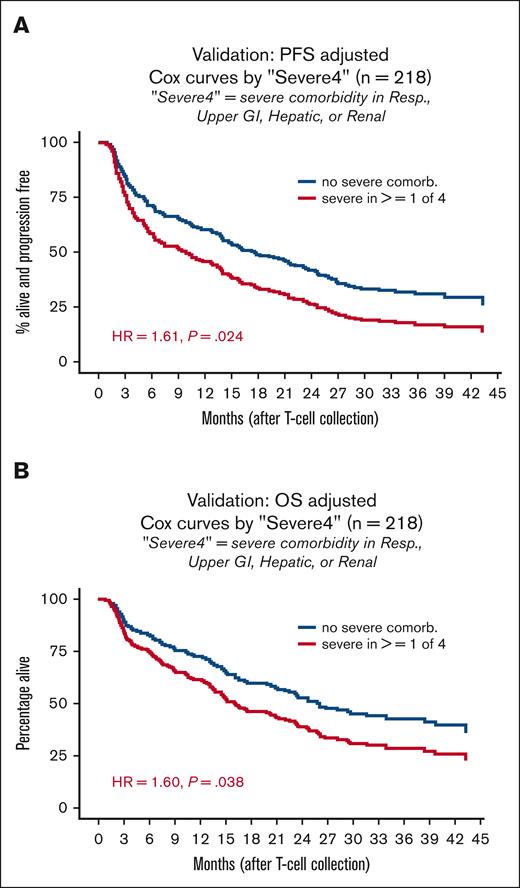
Severe4 validated as a predictor of post-CART survival
To corroborate these findings, we analyzed a separate, single-center VC (n = 218). The LC and VC were similar in most baseline characteristics (Table 1). Median age was 60.5 years (range, 51-68). The VC had relatively fewer patients with good performance status, 74% (n = 162) ECOG 0 to 1. The median number of prior lines of therapy was 3 (interquartile range, 2-4) and 20% of patients (n = 44) had previous ASCT. GCB subtype was present in 40% of patients (n = 87) whereas 33% (n = 72) had unknown cell-of-origin subtype per Hans algorithm. Nearly all patients in the VC received axi-cel (97.7%, n = 213) and the remainder received tisa-cel (2.3%, n = 5). Median follow-up was 35 months, 56% (n = 121) of the patients died, and 69% had a PFS event. Median PFS was 16 months (95% CI, 13-21) and median OS was 25 months (95% CI, 20-34). Severe CRS was documented in 17% (n = 37) and severe ICANS in 39% (n = 84) of patients in the VC.
At 16% (n = 35) of patients in the VC, Severe4 was identified in a higher percentage of patients in the VC than in the LC (9%). Importantly, Severe4 remained independently associated with inferior PFS (HR = 1.85; 95% CI, 1.24-2.76; P = .003) and OS (HR = 1.70; 95% CI, 1.09-2.66; P = .019) in this group of patients (Figure 5; supplemental Table 4). Although not statistically significant, Severe4 was again associated with development of grade ≥3 CRS (OR = 2.05; 95% CI, 0.81-4.90; P = .114).
Severe4 is associated with worse PFS and OS in the VC. (A) Cox curves showing PFS by presence of absence of Severe4 in the VC. (B) Cox curves showing OS by presence of absence of Severe4 in the VC. Resp, respiratory.
Severe4 is associated with worse PFS and OS in the VC. (A) Cox curves showing PFS by presence of absence of Severe4 in the VC. (B) Cox curves showing OS by presence of absence of Severe4 in the VC. Resp, respiratory.
Discussion
In this large multicenter retrospective RWE analysis, we used a machine learning technique to identify a simplified CIRS-based index capable of predicting survival outcomes in patients with DLBCL indicated for CART. This novel comorbidity index was validated in an independent cohort of patients with DLBCL from a separate institution. We found that a CIRS score of ≥3 in any of the 4 organ systems (respiratory, upper gastrointestinal, renal, and hepatic), which we denoted Severe4, was independently associated with inferior PFS and OS. Interestingly, Severe4 was also strongly associated with relapse-related mortality in both cohorts (supplemental Table 5).
Various objective measures have been used to predict CART outcomes. A small study of 31 patients reported that a comprehensive geriatric assessment predicted survival in older (aged > 65 years) CART recipients with R/R DLBCL.16 Rejeski et al proposed a score, CAR HEMATOTOX, generated by combining absolute neutrophil count, platelet count, hemoglobin, C-reactive protein, and ferritin prelymphodepletion, which was predictive of prolonged neutropenia, infections, and relapse after CART.32,33 Our study, to the best of our knowledge, is the largest study, to date, to validate a simple prognostic index in recipients of CART. Severe4 is a relatively simple comorbidity evaluation tool that could replace the more cumbersome haemopoietic cell–transplantation comorbidity index, which requires pulmonary function assessment that is not routine before to CART.
We found that ECOG performance status scores of ≥2 were independently associated with worse OS and PFS. This underscores the importance of assessing functional status in addition to comorbidities, because comorbidity scores poorly correlate with performance status in adult patients with cancer.34,35 In addition, we confirmed that elevated LDH was associated with poor outcomes, as previously described.1,12,36 Likely, LDH elevation is a reflection of tumor volume, however, information regarding the bulk of lymphoma burden was not available in our analysis. Furthermore, our data identified ≥3 prior lines of therapy as a strong predictor of inferior survival, suggesting that CART may be more effective when given earlier. Although comparative prospective analyses will need to confirm this finding, it may be explained by diminished CAR T-cell fitness in patients who are heavily pretreated, as previously suggested.37 Finally, we found an association between bridging therapy with steroids and poor survival. The influence of bridging therapy on outcomes is controversial and there has been suggestion that response to bridging therapy may be more predictive of outcomes than bridging itself, however, this information was not available for the patients treated in our study.
The median survival estimates for the LC (PFS = 11 months, OS = 30 months) and VC (PFS = 16 months, OS = 25 months) are similar to published landmark trials (ZUMA-1, JULIET, and TRANSCEND) and retrospective studies reported in the literature.1,3,5,11,12,38-41 Earlier publications on RWE report median PFS estimates ranging from 5.2 to 8.3 months and median OS from ranging from 11.8 months to not reached.11,12,39-41 We used date of leukapheresis as the time origin for measuring survival in an attempt to identify the intent-to-treat population and capture mortality events that occurred after leukapheresis but before CART infusion. In the LC, 27 patients (4.7%) died before CART (there were no such deaths in the VC). Comparatively, these patients were excluded from the landmark trials and prior RWE analyses. Despite including these pre-CART deaths, our postleukapheresis OS estimates were excellent for R/R DLBCL, underscoring the considerable benefit patients obtain from this therapy.
The underlying pathophysiologic link between high comorbidity scores (such as CIRS score ≥ 7 or Severe4) and poor survival is not yet understood. To address effect modification and confounding, we included other factors alongside Severe4 in our multivariable survival models. In addition, comorbidities that were likely consequences of the patient’s lymphoma were excluded from CIRS scoring. It is tempting to speculate on potential mechanisms whereby severe comorbidities in any of the Severe4 CIRS categories could predispose patients to poor survival. Upper GI comorbidities, for example, could lead to poor nutrition, weight loss, and muscle mass depletion (ie, sarcopenia), which has been associated with poor CART outcomes.42 Renal comorbidities could predispose to renal failure or impaired medication clearance, thus leading to additional toxicity. A recent RWE study found that recipients of CART who had compromised renal function at baseline had longer intensive care unit stays and higher rates of early nonrelapse mortality (19.5% vs 8.3% at 3 months).43 Without a more in-depth analysis beyond the scope of this study, however, these ideas are speculative. Surprisingly, severe comorbidity in the cardiac system was not predictive of survival or toxicity in our large data set. This may be a reflection of practice patterns unique to this subgroup. For example, it is standard practice to perform echocardiographic or equivalent evaluation of patients before CART, which identifies a significant portion of cardiac comorbidities. Once identified, patients may be monitored more closely and early intervention when CRS occurs may prevent more severe CRS in this subgroup.
We found that factors typically associated with poor outcomes after chemoimmunotherapy for DLBCL (such as MYC rearrangement, double-hit lymphoma, and transformation from low-grade lymphoma29,30,44-48) were not associated with inferior post-CART outcomes in our study. In landmark studies of axi-cel, tisa-cel, and liso-cel (and 1 RWE analysis), response rates in double-hit DLBCL and transformed follicular lymphoma were comparable with those of patients without these features.1-3,49 In our LC, non-GCB subtype was significantly associated with inferior PFS but not OS whereas BCL6 translocation (not collected in our VC) was a risk factor for death. The effect of BCL6 translocation on DLBCL outcomes has been a controversial topic. To our knowledge, an association between BCL6 translocation and worse post-CART outcomes has not been reported. However, a recent meta-analysis identified BCL6 translocation as an independent predictor of inferior OS in DLBCL treated with chemoimmunotherapy in the rituximab era.50 Meanwhile, although CART may improve outcomes in RT when compared with standard therapy, this group of patients do poorly compared with patients without RT, suggesting that innovative approaches are still needed to treat this deadly disease. Because of its rarity, extensive analysis of RT outcomes after CART are not readily available, and although we report outcomes on only 18 patients, this represents 1 of the largest cohorts of patients with RT described in this setting. Among patients with RT, 11 received axi-cel, 3 received tisa-cel, 1 received liso-cel, and 3 died before receiving their CART product. Eighty percent (n = 12) of patients had bridging therapy, reflecting the aggressiveness of this disease. Only 1 of 18 patients had allogeneic stem cell transplantation before CART, limiting the applicability of our data to patients with RT after allogeneic stem cell transplantation .
The rates of any-grade CRS (78% in LC, 92% in VC) and ICANS (45% in LC, 62% in VC) in our patient cohorts are similar to those reported in landmark trials. Importantly, we demonstrate that our composite comorbidity index, Severe4, correlates with severe CRS. Unfortunately, our data set did not include information on tumor volume, which has also been associated with CRS,36 thus, we are not able to account for this in multivariate modeling. In addition, we show that high general comorbidity burden (CIRS score ≥ 7) correlates with severe ICANS. Identifying a mechanism underlying these observations is an area of ongoing investigation; however, routine evaluation of CIRS and Severe4 in patients indicated for CART could help identify those at highest risk for adverse reactions to CART. Future studies could use a CIRS-based index to develop strategies to mitigate CART-associated toxicities. Such mitigation strategies have included prophylactic corticosteroids and tocilizumab, as in the ZUMA-1 trial (cohort 6)51 and anakinra in another small study of 31 patients.52 Toxicity risk–adapted approaches may help tailor these prophylactic strategies.
Our study has several limitations, the most significant of which is lack of prospective validation on a cohort with CIRS scores for all 14 organ systems. Our findings, however, clearly demonstrate that Severe4, alone or in the presence of prognostic factors, can predict PFS and OS in R/R patients with DLBCL who receive commercial CART. There were notable differences between the LC and VC, such as patients in the VC being younger yet having worse performance status. In addition, the VC lacked CART product variability (almost all patients got axi-cel). We posit that these differences between cohorts strengthen the applicability of our index. Other limitations include missing variables (eg, CIRS score of ≥7) and large numbers of missing values (eg, for clinical response) in the VC, and the fact that our RWE comes from 10 academic centers that are all experienced with CART.
With a median age at diagnosis of 60 years, our patients in the LC tended to be young for DLBCL, which mostly afflicts older individuals with a median age at diagnosis of 67 years. Although older patients are known to have worse outcomes with DLBCL,53 it appears that CART outcomes remain excellent for older adults.13 It is likely, however, that as such, the grading of comorbidities may be a more significant predictor of outcomes than age in patients with DLBCL treated with CART. In fact, our study included 174 patients (21.9%) aged >70 years and, importantly, we found that Severe4 was not found more frequently in older age groups (data not shown). Based on the results reported herein, we are implementing a comprehensive geriatric assessment–based approach to improve CART outcomes at City of Hope, including assessment of baseline comorbidities. Using Severe4 within this approach, we can identify patients at risk of poor outcomes and used interventions to reduce this risk.
In summary, our findings suggest that determining Severe4 should be part of standard of care when selecting patients with DLBCL for CART. In addition, Severe 4 and formal CIRS evaluation could be used when assessing risk of CART toxicity for those treated with this promising therapy.
Acknowledgments
A.S.K. is a recipient of the Conquer Cancer, American Society of Clinical Oncology Foundation Career Development Award. A.V.D. is a Leukemia and Lymphoma Society Scholar (#2319-19). The authors acknowledge the patients with DLBCL whose contributions to this study and others are immeasurable.
Authorship
Contribution: G. Shouse, A.K., and A.V.D. participated in concept development, data collection, data analysis, and manuscript preparation; A.K. performed statistical analysis; and M.J.G., A.A., D.Y., A.M.S., G. Smilnak., S.B., A.M., L.F., A.B., S.J., N.B., M.S., K.P., D.M.S., M.K., B.H., J.G., R.K., L.J.N., and A.S.K. performed data collection and data analysis, and prepared the manuscript.
Conflict-of-interest disclosure: A.S.K. receives research funding from AstraZeneca and has consulted for AbbVie, AstraZeneca, BeiGene, and Janssen. A.V.D. has received consulting fees from AbbVie, AstraZeneca, Bayer Oncology, BeiGene, Bristol Myers Squibb, Genentech, Incyte, Lilly Oncology, Morphosys, Nurix, Oncovalent, Pharmacyclics, and TG Therapeutics, and has ongoing research funding from AbbVie, AstraZeneca, Bayer Oncology, Bristol Myers Squibb, Cyclacel, MEI Pharma, Nurix, and Takeda Oncology. J.G. is working as an ad hoc consultant; has received honoraria from Sobi, Legend Biotech, Janssen, Kite Pharma, and MorphoSys; and has ongoing research funding from Sobi, Juno Therapeutics (a Bristol Myers Squibb company), Celgene (a Bristol Myers Squibb company), and Angiocrine Bioscience. G.S. receives honoraria from Kite and BeiGene, and consulting fees from AbbVie. K.P. consults for AbbVie, ADC, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou, Epizyme, Fate Therapeutics, Genentech, Janssen, Kite, Lilly Oncology, Morphosys, Pfizer, and Xencor, and received research funding (institutional) from AstraZeneca, Adaptive, Bristol Myers Squibb, CRISPR, Epizyme, Fate Therapeutics, Genentech, Janssen/Pharmacyclics, Kite, Lilly Oncology, MEI Pharma, Merck, Nurix, Pfizer, and Xencor. R.K. serves on the advisory board for Bristol Myers Squibb, Gilead Sciences/Kite Pharma, Janssen, Karyopharm, Pharmacyclics, Morphosys, Epizyme, Genentech/Roche, EUSA, Calithera, and Janssen; and received grants/research support from Bristol Myers Squibb, Takeda, BeiGene, and Gilead Sciences/Kite; and speakers' bureau from AstraZeneca, BeiGene, and Morphosys. S.J. serves on the advisory boards for Kite, Novartis, Bristol Myers Squibb, CRISPR, Takeda, and Miltenyi, and received research funding from Kite, Novartis, and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Alexey V. Danilov, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: adanilov@coh.org.
References
Author notes
∗G. Shouse, A.K., L.J.N., A.S.K., and A.V.D. contributed equally to this study.
Data are available on request from the corresponding author, Alexey V. Danilov (adanilov@coh.org).
The full-text version of this article contains a data supplement.