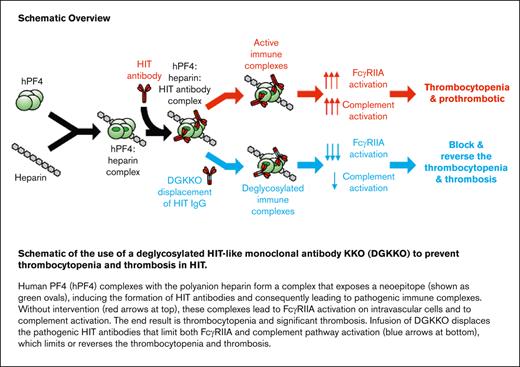
Key Points
DGKKO can reverse thrombocytopenia in a HIT murine model.
DGKKO can prevent/reverse thrombosis in vitro and in a HIT murine model.
Abstract
Heparin-induced thrombocytopenia (HIT) is characterized by thrombocytopenia associated with a highly prothrombotic state due to the development of pathogenic antibodies that recognize human platelet factor 4 (hPF4) complexed with various polyanions. Although nonheparin anticoagulants are the mainstay of care in HIT, subsequent bleeding may develop, and the risk of developing new thromboembolic events remain. We previously described a mouse immunoglobulin G2bκ (IgG2bκ) antibody KKO that mimics the sentinel features of pathogenic HIT antibodies, including binding to the same neoepitope on hPF4–polyanion complexes. KKO, like HIT IgGs, activates platelets through FcγRIIA and induces complement activation. We then questioned whether Fc-modified KKO could be used as a novel therapeutic to prevent or treat HIT. Using the endoglycosidase EndoS, we created deglycosylated KKO (DGKKO). Although DGKKO retained binding to PF4–polyanion complexes, it inhibited FcγRIIA-dependent activation of PF4-treated platelets triggered by unmodified KKO, 5B9 (another HIT-like monoclonal antibody), and IgGs isolated from patients with HIT. DGKKO also decreased complement activation and deposition of C3c on platelets. Unlike the anticoagulant fondaparinux, injection of DGKKO into HIT mice lacking mouse PF4, but transgenic for hPF4 and FcγRIIA, prevented and reversed thrombocytopenia when injected before or after unmodified KKO, 5B9, or HIT IgG. DGKKO also reversed antibody-induced thrombus growth in HIT mice. In contrast, DGKKO was ineffective in preventing thrombosis induced by IgG from patients with the HIT-related anti-PF4 prothrombotic disorder, vaccine-induced immune thrombotic thrombocytopenia. Thus, DGKKO may represent a new class of therapeutics for targeted treatment of patients with HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is an autoimmune disorder1 mediated by platelet-activating antibodies that recognize the chemokine, platelet factor 4 (PF4; CXCL4), complexed to heparin or other polyanions.2,3 Patients with HIT generally develop thrombocytopenia accompanied by an unusually intense hypercoagulable state, leading to venous and arterial thrombi.4 Present-day therapeutics for HIT center around nonheparin anticoagulants,5 which have limited efficacy at preventing new thrombi and carry a risk of untoward bleeding.6
KKO is a HIT-like monoclonal antibody that selectively binds to human PF4 (hPF4) complexed to polyanions, such as heparin,7 and binds to an epitope shared among most HIT immunoglobulin Gs (IgGs).8 KKO activates human platelets and neutrophils via both FcγRIIA9,10 and complement.11,12 Infusing KKO or HIT IgGs into transgenic mice that express both FcγRIIA and hPF4 recapitulates both the thrombocytopenia and prothrombotic states13,14 that characterize the human disease.
We postulated that if KKO’s FcγRIIA- and/or complement-activation properties were removed by Fc deglycosylation,15 the modified antibody could be therapeutic by competitively blocking the known HIT antigenic site on PF4 within PF4–polyanion complexes. Because KKO is an IgG2bκ monoclonal antibody,7 removal of the Fc domain by pepsin digestion proved not to be feasible.16 Instead, we modified KKO by deglycosylating its Fc domain at N297 to create deglycosylated KKO (DGKKO). We now show that DGKKO has abrogation of Fc-mediated signaling through FcγRIIA and reduction in complement activation while blocking the binding of HIT IgGs to PF4–heparin complexes. DGKKO not only prevented in vitro platelet activation and thrombosis in a photochemically injured, endothelial-lined microfluidic device but prevented and even reversed the thrombocytopenia in a murine model of HIT induced by KKO, 5B9 (another HIT-like monoclonal antibody17), or IgG isolated from patients with HIT, which was not seen using the anticoagulant fondaparinux.
In the same model, DGKKO also prevented and reversed the prothrombotic state. Moreover, in microfluidic studies, DGKKO was ineffective in preventing thrombus formation caused by IgG isolated from patients with vaccine-induced immune thrombotic thrombocytopenia (VITT), a post-coronavirus 2 adenoviral vaccine-induced prothrombotic disorder that also involves antibodies directed at PF4.18 Our data show that DGKKO is a potential HIT-specific therapy that may complement present-day therapeutics.
Material and methods
Human donor samples and mice studied
Human blood from healthy donors (10 mL each) were drawn for in vitro studies using a 19-gauge butterfly needle into 0.38% final concentration sodium citrate from a superficial, upper-extremity vein. The blood samples were stored at room temperature and were used within 30 to 60 minutes of being drawn. Deidentified plasma samples were obtained from patients diagnosed with HIT19 or VITT.20 IgG was isolated using protein G agarose (Thermo Fisher)
Double-transgenic mice, 6 to 12 weeks old, expressing both hPF4 and FcγRIIA and lacking mouse PF4 (hPF4+/FcγRIIA+/cxcl4−/−), termed as HIT mice, have been previously described.21 There was an equal sex distribution for the thrombocytopenia studies, but in the animal model, only male mice could be studied in the cremaster arteriole laser injury thrombosis model; however, we did not note any prior sex difference in thrombosis in this passive immunization HIT murine model after photochemical carotid injury.14
Antibodies and other reagents
KKO, a mouse IgG2bκ anti-hPF4/heparin monoclonal antibody and TRA, an isotype-control monoclonal IgG antibody,7 were purified from hybridoma supernatants, and deglycosylated using the endoglycosidase, EndoS (Genovis), as previously described.22 Monoclonal antibody 5B9, is a chimeric anti-PF4/heparin antibody with a human IgG1 Fc fragment.17 F(ab′)2 fragments of the monoclonal antimouse CD41 antibody MWReg30 (BD Biosciences) and antifibrin 59D823 monoclonal antibody (provided by Hartmut Weiler of the Blood Research Institute Versiti, Milwaukee) were labeled with Alexa Fluor 488 and 647 (Thermo Fisher), respectively, as previous described.22
Recombinant hPF4 was expressed in Drosophila Expression System (Invitrogen) S2 cells and purified as previously described.24 Proteins were analyzed for size distribution and purity via gel electrophoresis (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and were confirmed for low endotoxin using ToxinSensor Chromogenic LAL Endotoxin Assay Kit (Genscript). BD PosiFlush unfractionated heparin (UFH; 500 USP units/5 mL) was from Becton Dickinson and fondaparinux sodium was from Reddy's Laboratories Ltd.
Characterization of DGKKO
Binding of DGKKO to PF4 and complexes of PF4 with UFH complexes was evaluated using an in-house enzyme-linked immunosorbent assay. Briefly, 96-well microtiter plates (Nunc Maxisorp; Nunc International) were coated overnight with 100 μL hPF4 (10 μg/mL) with or without UFH (0.2 U/mL), followed by the blocking of unreactive sites with 1% bovine serum albumin (Sigma-Aldrich) in phosphate-buffered saline (Invitrogen). KKO or DGKKO was added in serial dilutions (1-1000 ng/mL) for 1 hour at room temperature. After washing out unbound antibody, horseradish peroxidase–labeled goat antimouse IgG (1:10 000 dilution; Jackson ImmunoResearch) was added followed by tetramethylbenzidine peroxidase substrate (KPL), as previously described.7 Color development was measured at 450 nm using a Spectramax 384 PLUS (Molecular Devices), and the results were analyzed using SoftMax PRO software (Molecular Devices).
Competition enzyme-linked immunosorbent assay
To study the ability of DGKKO to inhibit the binding of patient HIT IgG, plasma samples of 10 patients with HIT and 3 patients with VITT at 50% maximal binding concentration were mixed with DGKKO (0-5 μg/mL) and incubated for 1 hour at room temperature in PF4-heparin–coated, blocked wells. The binding of human IgG was detected using horseradish peroxidase–labeled goat antihuman IgG (Jackson ImmunoResearch).
Analysis of hPF4-containing complexes in solution using DLS
Complexes were formed by incubating hPF4 (10 μg/mL) with either KKO or DGKKO (35 μg/mL) and UFH (0.2U/mL) in Hanks balanced salt solution (HBSS; Gibco). Dynamic light scattering (DLS) studies were performed on a fixed scattering angle Zetasizer Nano-ZS system (Malvern Instruments) in disposable cuvettes, as previously described.3 The Z-average size distribution (ie, hydrodynamic diameters) of particles based on volume, were measured in HBSS at 25°C, with a light backscattering angle of 173. Up to 25 repetitive measurements were made at the indicated time points. Data analysis was performed using the Zetasizer software, version 7.03 (Malvern Instruments).
Platelet activation studies
Platelet activation studies were conducted using whole-blood samples collected in citrate (final concentration of 0.32%) for 45 minutes at room temperature in the presence of the following: Ca++/Mg++ HBSS (Gibco) 1/50 (volume-to-volume ratio [v/v]), 10 mM of Gly-Pro-Arg-Pro-NH2 peptide (Bachem H-1998), allophycocyanin-labeled anti-hCD41, phycoerythrin-labeled anti–P-selectin (both BD Biosciences), and FITC-labeled anti-C3c (Abcam) with hPF4 (10 μg/mL), and either KKO or DGKKO (0-200 μg/mL). Cellfix (BD Biosciences) was added 1/1 (v/v) for 15 minutes at room temperature, samples were diluted with 2 volumes HBSS, stored at 4°C, and analyzed by flow cytometry (CytoFLEX LX Beckman Coulter) within 24 hours. Platelets were gated based on the forward scatter and CD41 fluorescence parameters, and the binding of anti-C3c, and anti–P-selectin antibodies was quantified as geometric mean fluorescent intensity using FlowJo 10.8 software.
In vitro thrombosis studies under flow
Microfluidic studies in 48-well BioFlux plates (Fluxion biosciences) with channels coated with near-confluent human umbilical vein endothelial cells (Lifeline Cell Technology) were performed, as previously described.21,25 The human umbilical vein endothelial cells were injured using an HXP120C light source with a 475-nm excitation and 530-nm emission filter for 20 seconds while the channel was perfused with hematoporphyrin (final concentration of 50 μg/mL; Sigma-Aldrich; Supplement Figure 1A). Whole blood collected in citrate (final concentration of 0.32%) was labeled with 2 mM calcein AM green (Thermo Fisher) and supplemented with PF4 (25 μg/mL) and either KKO (50 μg/mL), 5B9 (50 μg/mL), or HIT or VITT IgGs (1 mg/mL) (supplement Figure 1B). After 15 minutes, DGKKO or a deglycosylated isotype-control IgG (DGTRA), each at 50 μg/mL, was added, and the sample was infused into the channel at 10 dyne/cm2. Images were taken immediately and at 5, 10, and 15 minutes after perfusion was started. Platelet accumulation on the injured endothelium field was captured using a Zeiss Axio Observer Z1 inverted microscope using Montage Fluxion software and analyzed using ImageJ, as previously described.25 Confluent, uninjured endothelial-lined channels, exposed to blood that had not had hPF4 or KKO added were used as the background, to be subtracted from values determined in injured vessels exposed to blood that had been exposed to various experimental conditions.
The effect of DGKKO on thrombocytopenia in the HIT murine model
Thrombocytopenia was induced in the HIT murine model using an intraperitoneal (IP) injection of 200 μg per mouse of either KKO or 5B9 or 1 mg per mouse of human IgG purified from plasma of patients with VITT or HIT. DGKKO (200 μg per mouse [IP] and 20 μg per mouse [IV]) or fondaparinux (20 ng per mouse [IV]) were then injected IP or IV at the indicated time points. Platelet counts were measured at baseline and then from 3 to 120 hours after the induction of thrombocytopenia.
Effect of DGKKO on thrombosis in the HIT murine model
Cremaster arteriole laser injury was performed, as previously described.21 Alexa Fluor 488–labeled antimouse CD41 F(ab′)2 fragments and Alexa Fluor 647–labeled antifibrin 59D8 were injected via the jugular vein to label platelets and fibrin, respectively, in thrombi induced with an SRS NL100 pulsed nitrogen dye laser (440 nm) in cremaster arterioles (supplement Figure 1C). Brightfield and fluorescence snapshots of each injury were taken 5 minutes after the initial injury. KKO (20 μg per mouse), 5B9 (20 μg per mouse), or human HIT IgGs (100 μg per mouse) were then injected through a jugular vein to induce a HIT prothrombotic state, and the same clots were reimaged 15 minutes later, as we have previously described.21 Fondaparinux was injected immediately after KKO, whereas DGKKO or DGTRA (20 μg per mouse) was injected 20 minutes after the HIT antibodies, and the injuries were monitored over the ensuing 30 minutes. Brightfield and fluorescence snapshots of the same injuries were then taken to compare platelet deposition before and after the induction of a HIT-like lesion and to assess the capacity of DGKKO and DGTRA interfering with thrombus growth. Fluorescence intensity analysis was done using ImageJ 1.53k and expressed as postinjury fold change.
Statistical analysis
Differences between 2 groups were compared using a 2-sided Student t test. Differences between >2 groups were determined using a two-way analysis of variance with the Geisser-Greenhouse correction and Dunnett multiple comparison test. All analyses were performed on GraphPad Prism 9.0 (GraphPad Software). Differences were considered significant if P ≤ 0.05.
Study ethics approval
Using a 19-gauge butterfly needle, human blood for platelet studies was collected into 129 mM sodium citrate (10:1, v/v) after informed consent from healthy volunteers who were not receiving aspirin, under a protocol approved by the Children’s Hospital of Philadelphia institutional review board for studies involving humans and were in accordance with the Helsinki Principles. Animal procedures were approved by the institutional animal care and use committee at the Children’s Hospital of Philadelphia in accordance with National Institute of Health guidelines and the Animal Welfare Act.
Results
Deglycosylation removes FcγRIIA-mediated KKO activity and reduces complement activation
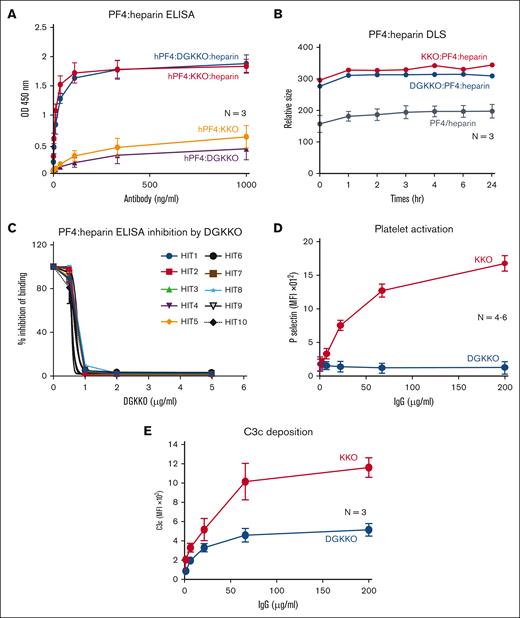
As we have reported previously, EndoS effectively deglycosylates KKO.22 Here, we show that DGKKO, like KKO, retained its capacity to bind preferentially to hPF4-UFH complexes as compared with to hPF4 alone (Figure 1A), and it formed ultralarge immune complexes similar in size to that of PF4-UFH-KKO complexes assessed using DLS (Figure 1B). DGKKO could effectively block the binding of 10 HIT IgG samples to PF4-UFH (Figure 1C) but not of 3 VITT IgG samples (supplement Figure 2). Deglycosylation also abrogated KKO’s ability to activate platelets in the presence of hPF4, as measured by P-selectin expression (Figure 1D), and partially inhibited complement activation and deposition of C3c on platelets (Figure 1E).
Biological characterization of DGKKO. (A) Binding of KKO and DGKKO to microtiter wells coated with either hPF4 or hPF4 + UFH at different antibody concentrations. (B) The size of hPF4-UFH–antibody complexes determined using DLS over time in hours (hr). (C) Binding of 10 distinct HIT IgGs to microtiter wells coated with hPF4 + UFH in the presence of DGKKO (concentration, 0-5 μg/mL); n = 2; in duplicates. (D) Platelet activation via KKO vs DGKKO over a range of antibody concentrations assessed using surface P-selectin expression shown as geometric MFI. (E) Complement activation as indicated by C3c deposition on the platelet surface in the presence of KKO vs DGKKO. In panels A through E, the mean ± 1 SEM are shown. The number of independent experiments in each study is indicated in the figure. In panels C and D, P < .0001 using 2-way analysis of variance. MFI, mean fluorescent intensity; SEM, standard error of mean.
Biological characterization of DGKKO. (A) Binding of KKO and DGKKO to microtiter wells coated with either hPF4 or hPF4 + UFH at different antibody concentrations. (B) The size of hPF4-UFH–antibody complexes determined using DLS over time in hours (hr). (C) Binding of 10 distinct HIT IgGs to microtiter wells coated with hPF4 + UFH in the presence of DGKKO (concentration, 0-5 μg/mL); n = 2; in duplicates. (D) Platelet activation via KKO vs DGKKO over a range of antibody concentrations assessed using surface P-selectin expression shown as geometric MFI. (E) Complement activation as indicated by C3c deposition on the platelet surface in the presence of KKO vs DGKKO. In panels A through E, the mean ± 1 SEM are shown. The number of independent experiments in each study is indicated in the figure. In panels C and D, P < .0001 using 2-way analysis of variance. MFI, mean fluorescent intensity; SEM, standard error of mean.
DGKKO prevents thrombosis in a photochemical endothelial injury microfluidic HIT model
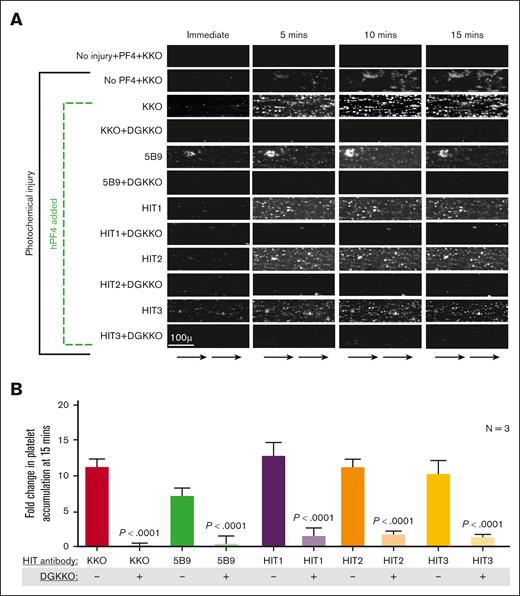
To test the ability of DGKKO to prevent thrombosis in vitro, we used a previously described endothelialized microfluidic model (supplemental Figures 1A and 2B).21,25 In this model, thrombi develop when the endothelium has been subject to a photochemical injury and the whole-blood samples has been preactivated by adding hPF4 and HIT antibodies. Adding DGKKO to the human blood 15 minutes after PF4 + KKO activation markedly reduced platelet accumulation on the injured endothelium (Figures 2A-B). Because DGKKO is derived from KKO, we tested another HIT-like monoclonal antibody, 5B9,17 and also polyclonal HIT IgG isolated from plasma of patients with HIT1 through HIT3. Similar to KKO, 5B9-induced thrombosis was abrogated by DGKKO. Significant inhibition of platelet adhesion was also noted when thrombosis had been induced by HIT1 through HIT3’s polyclonal IgGs. No significant inhibition of platelet adhesion was observed with the isotype-control antibody DGTRA (supplemental Figure 3A-B). In contrast, DGKKO did not block VITT IgG–mediated thrombosis in the photochemical injury microfluidic system (Figures 3; supplemental Figure 4), consistent with the report that distinct hPF4 sites were recognized by VITT IgG compared with HIT IgG.26
Effect of DGKKO on HIT thrombosis in a microfluidic system. (A) Representative images over a 15-minute window of flow through the channels from 1 of 3 independent studies showing platelet accumulation (white aggregates) on the endothelial lining. Scale bar is included, and arrows at the bottom indicate direction of flow. In this assay, optimal platelet adhesion occurs using human umbilical vein endothelial cell–lined channels subjected to photochemical injury by hematoporphyrin, followed by perfusion of whole blood supplemented with PF4 and HIT monoclonal (KKO and 5B9) or polyclonal (patient-derived) antibodies. The effect of DGKKO added 15 minutes after HIT antibody–induced activation is shown for each monoclonal or polyclonal antibody used. Note that KKO by itself led to platelet accumulation on injured endothelium, as previously noted,21 likely because of the presence of hPF4 released from the platelets. (B) Quantitative analysis of platelet adhesion showing mean ± 1 SEM of 3 independent experiments. P values were determined using 2-way Student t test comparing platelet accumulation in the absence of DGKKO with that in the presence of DGKKO.
Effect of DGKKO on HIT thrombosis in a microfluidic system. (A) Representative images over a 15-minute window of flow through the channels from 1 of 3 independent studies showing platelet accumulation (white aggregates) on the endothelial lining. Scale bar is included, and arrows at the bottom indicate direction of flow. In this assay, optimal platelet adhesion occurs using human umbilical vein endothelial cell–lined channels subjected to photochemical injury by hematoporphyrin, followed by perfusion of whole blood supplemented with PF4 and HIT monoclonal (KKO and 5B9) or polyclonal (patient-derived) antibodies. The effect of DGKKO added 15 minutes after HIT antibody–induced activation is shown for each monoclonal or polyclonal antibody used. Note that KKO by itself led to platelet accumulation on injured endothelium, as previously noted,21 likely because of the presence of hPF4 released from the platelets. (B) Quantitative analysis of platelet adhesion showing mean ± 1 SEM of 3 independent experiments. P values were determined using 2-way Student t test comparing platelet accumulation in the absence of DGKKO with that in the presence of DGKKO.
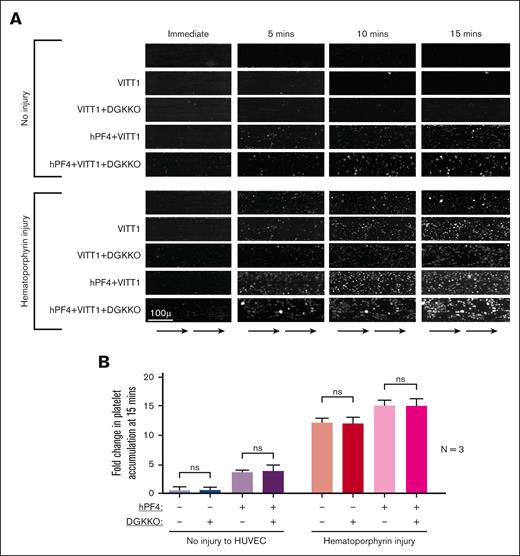
Effect of DGKKO on VITT thrombosis in a microfluidic system. Studies were performed as described in Figure 2 but in the presence of IgG isolated from 3 patients with VITT, in duplicates. (A) Representative studies from 1 patient with VITT (patient VITT1). (B) Cumulative data of studies with IgGs isolated from patients with VITT (VITT1-VITT3).
Effect of DGKKO on VITT thrombosis in a microfluidic system. Studies were performed as described in Figure 2 but in the presence of IgG isolated from 3 patients with VITT, in duplicates. (A) Representative studies from 1 patient with VITT (patient VITT1). (B) Cumulative data of studies with IgGs isolated from patients with VITT (VITT1-VITT3).
DGKKO prevents and reverses thrombocytopenia in vivo
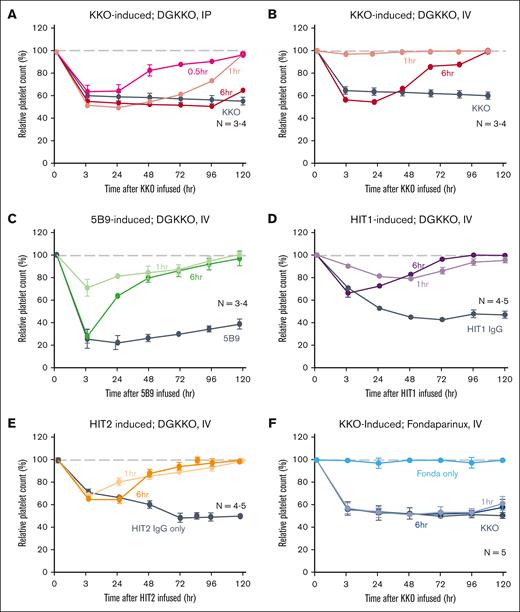
Thrombocytopenia is a core feature of HIT. hPF4+/FcγRIIA+/cxcl4−/− (HIT) mice21 develop thrombocytopenia within 3 hours after IP injection of KKO or 5B9 or HIT IgG (Figure 4). IP injection of an equal amount of DGKKO at 0.5, 1, and 6 hours after IP injection of KKO accelerated platelet recovery (Figure 4A), with a maximal effect noted when DGKKO was injected 0.5 hours after KKO. IV injection of DGKKO 1 hour after IP KKO prevented thrombocytopenia from developing and significantly (P < .05) accelerated platelet recovery. Even when DGKKO was injected IV 6 hours after IP KKO, accelerated recovery was still seen (Figure 4B). Similar effects by IV DGKKO were seen when thrombocytopenia was induced by 5B9 (Figure 4C) and by polyclonal HIT IgGs (Figures 4D-E). In contrast, fondaparinux at a dose that can inhibit thrombosis in HIT mice,27 was unable to prevent the development of thrombocytopenia after injection of KKO in the HIT murine model (Figure 4F).
Effects of infused DGKKO in HIT-induced thrombocytopenia in a mice model. Studies were performed in HIT mice after IP injection of HIT-like monoclonal antibody or HIT IgGs to induce thrombocytopenia and was followed by IP (A) or IV (B) injection of DGKKO at the indicated times. The number of independent experiments for each condition is indicated in the figures. Mean ± 1 SEM of relative platelet counts compared with the baseline are shown; N values are included in each study. Gray, dashed line refers to baseline platelet count before the study. (A) Relative platelet counts after an IP infusion of KKO followed by an IP injection of DGKKO. (B) Same as in panel A, but DGKKO was administered IV. (C) Same as in panel B, but 5B9 was administered instead of KKO. (D,E) Studies were performed as in panel B using IgG isolated from the plasma of 2 patients with HIT. (F) Studies were performed similarly but after fondaparinux (20 μg per mouse) was given IV either 1 or 6 hours after KKO induction of thrombocytopenia.
Effects of infused DGKKO in HIT-induced thrombocytopenia in a mice model. Studies were performed in HIT mice after IP injection of HIT-like monoclonal antibody or HIT IgGs to induce thrombocytopenia and was followed by IP (A) or IV (B) injection of DGKKO at the indicated times. The number of independent experiments for each condition is indicated in the figures. Mean ± 1 SEM of relative platelet counts compared with the baseline are shown; N values are included in each study. Gray, dashed line refers to baseline platelet count before the study. (A) Relative platelet counts after an IP infusion of KKO followed by an IP injection of DGKKO. (B) Same as in panel A, but DGKKO was administered IV. (C) Same as in panel B, but 5B9 was administered instead of KKO. (D,E) Studies were performed as in panel B using IgG isolated from the plasma of 2 patients with HIT. (F) Studies were performed similarly but after fondaparinux (20 μg per mouse) was given IV either 1 or 6 hours after KKO induction of thrombocytopenia.
DGKKO inhibits HIT-induced thrombosis in vivo
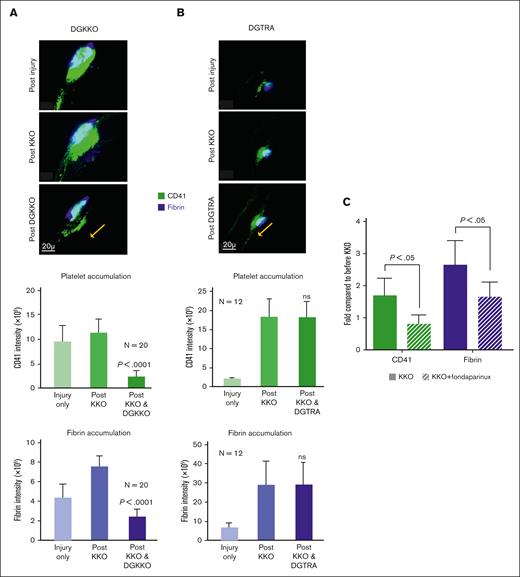
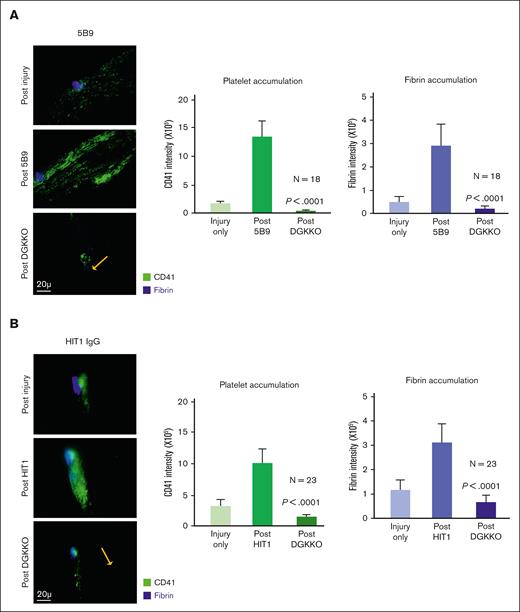
HIT is associated with a high risk of limb- or life-threatening thrombosis.6 Therefore, we then examined the effect of DGKKO on thrombosis using a cremaster arteriole laser injury model in which we had demonstrated HIT-induced secondary thrombus growth (schematic in supplement Figure 1C).21 Five minutes after an initial clot formed at a site of arteriole injury in a HIT mouse, the HIT-like antibody of interest was given IV, resulting in further thrombus growth, the size of which was measured after 20 minutes, just before DGKKO or DGTRA was infused IV. Thrombus size, as indicated by platelet and fibrin accumulation, was measured again after another 30 minutes (Figures 5A). The infusion of DGKKO after KKO caused a reversal in platelet and fibrin accumulation at the site of laser injury (Figure 5A) whereas DGTRA did not (Figure 5B). To determine whether the effect of DGKKO was restricted to injury caused by KKO, we tested its effect when 5B9 or an HIT IgG was infused. These studies showed similar or even greater efficacy in reversing thrombus growth at 30 minutes after DGKKO infusion based on platelet and fibrin accumulation for both 5B9 and HIT1 IgG (Figures 6A-B, respectively). To compare the efficacy of DGKKO in the treatment of HIT thrombosis, we injected fondaparinux immediately after KKO. Fondaparinux also prevented the KKO-induced increase in thrombus size21 (Figure 5C).
Effect of DGKKO and fondaparinux on thrombosis induced by KKO in a murine model of HIT. Cremaster arteriole injury followed by infused KKO. HIT prothrombotic studies done as outlined in supplemental Figure 2C. (A) (Top) Representative image of 5 minutes after injury vs 15 minutes after KKO infusion vs 30 minutes after DGKKO infusion on platelet and fibrin accumulation. Scale bar is included, as is the arrow indicating the direction of flow. (Middle) Quantitative analysis of platelet accumulation at the site of injury before and after KKO and then after DGKKO. Mean ± 1 SEM are shown. P values were determined using 2-way Student t test, comparing platelet accumulation after KKO vs after DGKKO. (Bottom) Same as the middle graph but for fibrin accumulation. (B) Same as panel A but DGTRA-infused after KKO rather than after DGKKO. (C) Cremaster arteriole injury followed by KKO or KKO + fondaparinux. Data are expressed as the fold change of platelet accumulation and fibrin deposition after KKO (1 mg/kg) or KKO + fondaparinux injection (1 μg/kg) compared with that from before the therapy. Values greater than 1 are consistent with a prothrombotic effect. n = 3 studies per arm; P values were determined using Student t test.
Effect of DGKKO and fondaparinux on thrombosis induced by KKO in a murine model of HIT. Cremaster arteriole injury followed by infused KKO. HIT prothrombotic studies done as outlined in supplemental Figure 2C. (A) (Top) Representative image of 5 minutes after injury vs 15 minutes after KKO infusion vs 30 minutes after DGKKO infusion on platelet and fibrin accumulation. Scale bar is included, as is the arrow indicating the direction of flow. (Middle) Quantitative analysis of platelet accumulation at the site of injury before and after KKO and then after DGKKO. Mean ± 1 SEM are shown. P values were determined using 2-way Student t test, comparing platelet accumulation after KKO vs after DGKKO. (Bottom) Same as the middle graph but for fibrin accumulation. (B) Same as panel A but DGTRA-infused after KKO rather than after DGKKO. (C) Cremaster arteriole injury followed by KKO or KKO + fondaparinux. Data are expressed as the fold change of platelet accumulation and fibrin deposition after KKO (1 mg/kg) or KKO + fondaparinux injection (1 μg/kg) compared with that from before the therapy. Values greater than 1 are consistent with a prothrombotic effect. n = 3 studies per arm; P values were determined using Student t test.
Effect of DGKKO on thrombosis, as induced by 5B9 and HIT IgG in a murine model of HIT. Thrombus formation in HIT mice was examined as described in Figure 5 but substituting 5B9 (A) and HIT1 IgG (B).
Effect of DGKKO on thrombosis, as induced by 5B9 and HIT IgG in a murine model of HIT. Thrombus formation in HIT mice was examined as described in Figure 5 but substituting 5B9 (A) and HIT1 IgG (B).
Discussion
Contemporary therapy of HIT is neither entirely effective in terms of preventing new thromboembolic events nor safe in terms of risk of bleeding.6,28,29 The critical event for propagation of the intensely prothrombotic state typical in HIT is engagement and crosslinking of Fcγ receptors, predominantly FcγRIIA on platelets and other vascular cells,9,10,30 by an array consisting of the Fc ends of antibodies that are approximated on ultralarge hPF4–polyanion complexes.31 The finding that most HIT antibodies and the monoclonal HIT-like antibody KKO compete for binding to hPF48,32 led us to hypothesize that a KKO antibody analog that does not bind FcγRIIA, required for platelet activation in vitro9 and the development of thrombosis and thrombocytopenia in murine HIT,13,14 and that has limited ability to activate complement, which amplifies the autoantibody response,12,33 might provide a rational, disease-specific intervention.
We had previously shown that deglycosylation of KKO created a novel monoclonal antibody, DGKKO.22 In this study, we show that DGKKO does not affect its binding to hPF4-UFH complexes while blocking FcγRIIA activation of platelets and reducing complement activation. DGKKO maintains the ability to compete with pathogenic human HIT IgG, including the 10 randomly selected HIT samples studied. DGKKO inhibits KKO-induced platelet adhesion to the endothelium in flow in a microfluidic system and mitigates the severity of thrombocytopenia and prevents thrombotic vascular occlusion in murine HIT. DGKKO also blocked the effector function of 5B9, an IgG1 monoclonal antibody shown previously to recognize an epitope that overlaps with KKO and competes with KKO for binding to hPF4.17 Surprisingly, DGKKO administered within 20 minutes after KKO reversed platelet and fibrin deposition in the microvasculature, suggesting that the clots are initially unstable and subject to endogenous dissolution if not organized by accretion of additional platelets and fibrin as a result of unopposed thrombin production.
The finding that DGKKO did not prevent clot formation by all 3 VITT20 IgGs that we studied is consistent with recent findings that the binding site for VITT and HIT IgG do not overlap.26 It has also been proposed that the rare cases of autoimmune HIT involve the same antigenic target as that in VITT.34,35 Clinical studies have shown that almost all HIT antibodies compete with KKO for binding, based on HemosIL, a rapid diagnostic tests for HIT.32 Therefore, although antibodies from a larger number of patients with HIT need to be tested, these preliminary studies suggest that DGKKO would be effective for most patients with HIT, especially those with persistent antibodies directed to the same antigenic site. Because patients with HIT usually require anticoagulation for their underlying disease and 50% of patients have developed overt thrombosis at the time of diagnosis, DGKKO most probably will not obviate the need for a nonheparin anticoagulant but its inclusion in addition may reduce the dosing and duration of therapy and improve patient outcome.
Alternative approaches to mitigating the effect of PF4-anti-PF4 immune complexes have been described. IVIG blocks platelet activation by HIT antibodies and is effective in vivo, at least in some patients.36-38 We still do not know whether IVIG can reverse thrombosis and thrombocytopenia, as we observed with DGKKO. In vivo infusion of IdeS, a bacterial protease that cleaves the hinge region of heavy-chain IgG, abrogates its ability to bind to FcγR, preventing thrombus formation and thrombocytopenia in a mouse HIT model induced by 5B9 monoclonal antibody39; however, the ability of IdeS to block thrombocytopenia or thrombosis induced by patient IgG was not tested. Moreover, IdeS cleaves all IgG and can cause hypogammaglobulinemia40 and compromise immune defense against bacterial infections. Moreover, anti-IdeS antibodies are frequent in the general population41 and their titer increases after exposure to IdeS.40 Thus, whether these alternative approaches would be advantageous in vivo is currently not clear. Of note, the IdeS approach may be effective in VITT and autoimmune HIT, whereas DGKKO would not.
Our study should be placed in the context of other approaches to create disease-specific prothrombotic interventions. The FcγRIIA-blocking monoclonal antibody IV.3 has been proposed as a therapeutic agent for HIT42 by blocking downstream platelet activation via FcγRIIA; whether it is able to reverse thrombocytopenia and thrombosis, as we have shown with DGKKO, and its relative efficacy compared with an Fc-modified HIT-like KKO antibody still needs to be examined. Another Fc-modified anti-PF4 monoclonal antibody, designated 1E12, which binds to the heparin-binding domain of PF4 and blocks binding to VITT antibodies has been proposed as a therapeutic in VITT,35 although its ability to prevent/reverse thrombocytopenia and thrombosis in vivo is yet to be reported.
In summary, a deglycosylated version of the HIT-like monoclonal antibody KKO, DGKKO, appears to be a rational, disease-specific intervention because it blocks HIT IgG binding to platelets and likely to monocytes and neutrophils based on the in vivo results reported herein. Thrombocytopenia and thrombosis by tested HIT-like monoclonal antibodies and by HIT IgG preparations were both prophylactically prevented and reversed by DGKKO. The VITT IgGs tested could not be blocked by DGKKO. Further preclinical studies of additional HIT and VITT IgGs and comparative studies against additional anticoagulants and alternative therapeutics are needed before the clinical potential of DGKKO can be estimated.
Acknowledgments
The authors thank Khalil Bdeir, Sergey V. Zaytsev, and Sergi V. Yarovoi at the Univeristy of Pennsylvania for their help and guidance for the DLS experiments.
This work was supported by the NIH/NHLBI R01HL151730 (G.M.A., D.B.C., and L.R.) and R35HL150698 (L.R. and M.P.).
Authorship
Contribution: A.S. carried out and evaluated these studies, and prepared the manuscript; S.K. helped in complement studies; H.K. and G.T.K. helped in some mice studies; J.R and Y.G. developed 5B9 and edited the manuscript; G.D.W. and F.P. provided VITT plasmapheresis samples and edited the manuscript; G.M.A. developed KKO, provided HIT IgG samples, helped in complement study, provided research guidance, and edited the manuscript; D.B.C. provided input in DLS studies, overall research guidance for the manuscript, and edited the manuscript; L.R. helped with characterization of DGKKO, provided research guidance, interpreted the data, and edited the manuscript; and M.P. provided overall conceptual development and direction, data interpretation, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lubica Rauova, The Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, ARC Room 316F, Philadelphia, PA 19104; e-mail: lubica@chop.edu.
References
Author notes
∗L.R. and M.P. contributed equally to this study.
Data are available on request from the corresponding author, Amrita Sarkar (sarkara1@chop.edu).
The full-text version of this article contains a data supplement.