TO THE EDITOR:
Despite the demonstrated efficacy and safety of liquid hydroxyurea for treating sickle cell anemia in children,1-3 the lack of a licensed liquid formulation presents challenges for patients, caregivers, and health care professionals. Treatment algorithms recommend early initiation of treatment and titrating weight-adjusted doses toward the maximum tolerated dose (MTD) with mild myelosuppression. With only capsule and tablet formulations of hydroxyurea approved globally, doses may need to be varied daily and rounded up or down. In some centers, to facilitate dose individualization and improve palatability, children are provided unlicensed liquid formulations compounded in pharmacies. Although such formulations meet a clinical need, they potentially expose children to medication errors, further compliance challenges due to unpalatability, and the consequential higher risk of adverse reactions or inadequate efficacy due to quality issues. For many patients, there are also significant challenges in accessing the compounded liquid hydroxyurea.4,5
A new, child-friendly, multidose, ready-to-use (no requirement for reconstitution) 100 mg/mL strawberry-flavored oral solution with a stable 2-year shelf life has been developed for licensing. This study aimed to determine population pharmacokinetics, safety, and efficacy in infants and older children with sickle cell anemia (SCA) (NCT03763656).
This multicenter, open-label, prospective study was conducted in accordance with the principles of the International Conference on Harmonization–Good Clinical Practice guidelines and was approved by the UK Medicines Healthcare products and Regulatory Agency and the Ministry of Health and Wellness in Jamaica. Ethical approval was obtained from the North West Liverpool Central Research Ethics Committee, United Kingdom and the Mona Campus Research Ethics Committee, Kingston, Jamaica. This study was conducted in accordance with the principles of the Declaration of Helsinki.
All children between 6 months and 18 years of age with sickle cell anemia (HbSS or HbS/β0), who were previously hydroxyurea naïve, were eligible for recruitment. The exclusion criteria included renal or hepatic insufficiency. All study participants started liquid hydroxyurea at 15 mg/kg once daily, with dose escalation every 8 to 12 weeks until MTD was achieved (absolute neutrophil count 1× 109/L to 3 × 109/L or a maximum dose of 35 mg/kg per day). The treatment was continued for 12 to 15 months.
Venous blood samples for pharmacokinetics were collected on day 1 and at 6 months with additional single samples at interim clinical visits. Pharmacokinetic analysis using the modeling/simulation methodology is detailed in the supplemental File. Full blood counts, fetal hemoglobin (HbF), safety, and review of clinical symptoms were assessed throughout the study. In addition, an exploratory transcranial Doppler (TCD) assessment was performed at screening and at the end of the study.
A total of 32 patients were recruited, and all participants were Black (African American, Caribbean, or Black British). The patient characteristics are shown in Table 1. The MTD with target myelosuppression was achieved by 20 (62.5%) study participants overall, with the highest proportion achieving MTD in the 6 months–1.99 years age group (5/6 participants) and the lowest in the 2 to 5.99 years age group (9/16 participants).
Participant demographics and key baseline characteristics
| . | 6 mo-1.99 y (N = 6) . | 2-5.99 y (N = 16) . | 6-17.99 y (N = 10) . | Overall (N = 32) . |
|---|---|---|---|---|
| Age (y), mean (SD) | 1.0 (0.1) | 3.1 (0.9) | 10.7 (3.5) | 5.0 (4.3) |
| Female sex, n (%) | 5 (83) | 5 (31) | 6 (60) | 16 (50) |
| SCA type, n (%) | ||||
| HbSS | 6 (100) | 15 (94) | 10 (100) | 32 (97) |
| HbS/ß0 | 0 | 1 (6) | 0 | 1 (3) |
| Height (cm), mean (SD) | 79.4 (5.2) | 98.1 (6.9) | 142.1 (15.9) | 109.3 (26.0) |
| Weight (kg), mean (SD) | 11.2 (1.0) | 14.7 (2.1) | 32.5 (11.4) | 19.6 (11.0) |
| Total number of days on liquid hydroxyurea, median (range) | 287 (112-435) | 397 (150-433) | 388 (257-432) | 373 (112-435) |
| Dose at study exit (mg/kg), mean (SD) | 23.0 (6.8) | 26.5 (5.5) | 26.5 (5.3) | 25.8 (5.7) |
| . | 6 mo-1.99 y (N = 6) . | 2-5.99 y (N = 16) . | 6-17.99 y (N = 10) . | Overall (N = 32) . |
|---|---|---|---|---|
| Age (y), mean (SD) | 1.0 (0.1) | 3.1 (0.9) | 10.7 (3.5) | 5.0 (4.3) |
| Female sex, n (%) | 5 (83) | 5 (31) | 6 (60) | 16 (50) |
| SCA type, n (%) | ||||
| HbSS | 6 (100) | 15 (94) | 10 (100) | 32 (97) |
| HbS/ß0 | 0 | 1 (6) | 0 | 1 (3) |
| Height (cm), mean (SD) | 79.4 (5.2) | 98.1 (6.9) | 142.1 (15.9) | 109.3 (26.0) |
| Weight (kg), mean (SD) | 11.2 (1.0) | 14.7 (2.1) | 32.5 (11.4) | 19.6 (11.0) |
| Total number of days on liquid hydroxyurea, median (range) | 287 (112-435) | 397 (150-433) | 388 (257-432) | 373 (112-435) |
| Dose at study exit (mg/kg), mean (SD) | 23.0 (6.8) | 26.5 (5.5) | 26.5 (5.3) | 25.8 (5.7) |
For age, 0.99 denotes the day before the study participant’s birthday.
N, number of subjects in the population; SCA, sickle cell anemia.
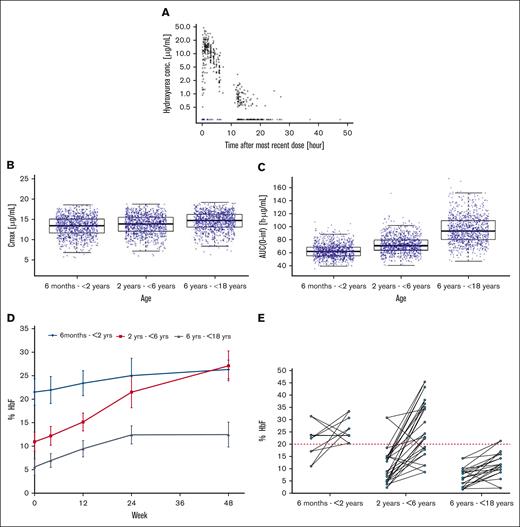
A total of 464 plasma hydroxyurea concentrations were available for pharmacokinetic modeling (Figure 1A). The model predicted that the hydroxyurea area under the curve (AUC)(0-inf) increased with age (and body weight), although the distribution of exposures overlapped considerably across age categories. Age did not significantly influence the Cmax (Figure 1B,C).
Pharmacokinetic and pharmacodynamic investigations. (A) Scatterplot of observed hydroxyurea plasma concentration vs time after the most recent dose. Circles represent quantified observations and triangles represent the Below Limit of Quantification (BLQ) observations positioned at half the lower limit of quantitation. Black shapes depict observations included in the final population PK model development, whereas blue shapes depict BLQ observations that were excluded owing to model instability. The data are plotted using a log10 Y-axis scale. (B) Boxplot of simulated hydroxyurea Cmax (C) and AUC(0-inf) after 8 weeks of 15 mg/kg per day stratified by age group. Box-and-whisker plots are stratified by age group. Each box covers the IQR, with the median as the vertical black line inside the box, and with the whiskers extending to the last data point within 1.5 times the IQR. A total of 1000 virtual patients were simulated for each age group. Blue circles depict the simulated values within each age group. (D) Mean %HbF over the course of the study. Error bars represent the standard error of the mean. (E) Individual subject changes in %HbF from baseline to the end of the study stratified by age group. The red dotted line represents the 20% target threshold associated with improved clinical outcomes.6 IQR, interquartile range.
Pharmacokinetic and pharmacodynamic investigations. (A) Scatterplot of observed hydroxyurea plasma concentration vs time after the most recent dose. Circles represent quantified observations and triangles represent the Below Limit of Quantification (BLQ) observations positioned at half the lower limit of quantitation. Black shapes depict observations included in the final population PK model development, whereas blue shapes depict BLQ observations that were excluded owing to model instability. The data are plotted using a log10 Y-axis scale. (B) Boxplot of simulated hydroxyurea Cmax (C) and AUC(0-inf) after 8 weeks of 15 mg/kg per day stratified by age group. Box-and-whisker plots are stratified by age group. Each box covers the IQR, with the median as the vertical black line inside the box, and with the whiskers extending to the last data point within 1.5 times the IQR. A total of 1000 virtual patients were simulated for each age group. Blue circles depict the simulated values within each age group. (D) Mean %HbF over the course of the study. Error bars represent the standard error of the mean. (E) Individual subject changes in %HbF from baseline to the end of the study stratified by age group. The red dotted line represents the 20% target threshold associated with improved clinical outcomes.6 IQR, interquartile range.
The pharmacokinetics of oral hydroxyurea are well defined and show remarkable consistency in Cmax and AUC estimates between studies and across the age range, irrespective of the formulation.6-9 The dose normalized AUC at the steady state estimated in this study was 4.3 μg.h/mL per mg, comparable with the previously reported values of 4.6 and 4.9 μg.h/mL per mg.6,9 Moreover, our results show that hydroxyurea pharmacokinetics in infants are similar to those in older children and adults, with body weight alone accounting for much of the interpatient variability across the population. Accordingly, no age-related dosage adjustments are necessary, and weight-based dosing results in similar systemic exposures, even for infants aged <2 years.
The pharmacodynamic response is typical in children with SCA treated with hydroxyurea.10 The percentage HbF increased in all age groups during the treatment (Figure 1D). At study enrollment, the overall mean (standard deviation [SD]) baseline HbF was 11.9% (8.7). At the end of the study, the overall mean (SD) HbF increased to 23.5% (11.2). Induction of HbF was more successful in infants and younger children than in older children when pushing the HbF over the 20% threshold, a putative target associated with reduced hospitalization and without significant toxicity (Figure 1E).11,12
Total Hb, mean corpuscular volume, and mean corpuscular hemoglobin all increased with time whereas platelets, ARC, absolute neutrophil count, WBC, LDH, and bilirubin levels all decreased; mean absolute neutrophil count and platelet at the end of the study were comfortably above the toxicity thresholds of 1.0 × 109/L and 80 × 109/L, respectively (supplemental Table 2).
Eight patients (25%) were hospitalized on 12 occasions during the study; acute chest syndrome in 4 patients and vaso-occlusive/painful crisis in 6 participants. The mean rate of hospitalization before hydroxyurea was 1.7 events/year, decreasing to 0.3 events/year after initiation of hydroxyurea. The risk of hospitalization was reduced by 85% after initiation of hydroxyurea treatment (odds ratio 0.15 [95% confidence interval, 0.05-0.45]; P < .05). This study was not designed or powered for a comparative analysis; however, a similar decline in hospitalization has been reported in multiple studies.2,13-15
There was an overall mean (SD) decline in the TCD-measured maximum time-averaged mean velocity (TAMV) of 12 (21) cm/sec. All changes in the TAMV transcranial Doppler category were lowered to the normal range; either abnormal TCD velocities into the conditional range or conditional into the normal range. These changes are clinically relevant and represent the salutary effects of hydroxyurea with primary stroke protection16,17 (supplemental Table 3).
All except 1 of the 32 study participants experienced at least 1 adverse effect (AE), the most common being a vaso-occlusive crisis. There were 28 related AEs in 9 participants, the most frequent of which were isolated and transient occurrences of hematological toxicity, with no serious infections and resulting in temporary dose interruption, dose reduction, or no change in dose. Cytopenias were typically associated with a recent and concurrent respiratory or viral illness. All serious AEs (7 in total) and most of the Common Terminology Criteria for Adverse Events grade ≥3 AEs were unrelated to hydroxyurea and indicative of the typical complications of SCA. Indeed, the results of this study support a dose escalation approach. Dose escalation to MTD is more efficacious than fixed dosing without increasing the risk of infections or toxicities and is routinely recommended in treatment algorithms.18-20
After 8 weeks of treatment, the palatability and acceptability of the new liquid formulation were assessed in a face-to-face interview using a previously reported visual (hedonic) and verbal response tool.21 Palatability and acceptability scores for all respondents (patients and parents/caregivers combined and across all age cohorts) were positive (supplemental Table 4). A cornerstone of successful drug therapy in children, particularly for chronic conditions, is that the medicine formulation is palatable and acceptable. Adherence is further improved if the medicines are accessible, convenient (without multiple preparation steps), and easy to administer.22
In conclusion, adopting a strategy of early initiation of hydroxyurea with dose escalation toward MTD and a target percentage HbF necessitates weight-based dosing that is regularly adjusted as the child grows. Such personalized dosing requires flexible oral formulation. The availability of a licensed, readily available, child-friendly liquid hydroxyurea formulation facilitates early initiation and dose titration in infants, with the potential to delay or prevent disease progression.
Acknowledgments: The authors thank all the participants, parents/caregivers, and study teams (doctors, nurses, and pharmacists) at all participating sites. This study was funded by Nova Laboratories Limited.
Contribution: A.R.-M., R.K., S.C., B.I., P.T., and M.V. were involved in data collection and review of the manuscript; R.E.W. was involved in the study design, data interpretation, and manuscript review; J.J.M. and A.L.L. were involved in data analysis, interpretation, and review of the manuscript; and S.E. and H.M. were involved in the study design and management, data collection, analysis and interpretation, and drafting of the manuscript.
Conflict-of-interest: S.E. and H.M. are employees of Nova Laboratories Limited, who was the study sponsor. J.M. and A.L. are employees of BAST Inc. Ltd. and were contracted by Nova Laboratories Ltd. to conduct the population PK modeling and simulation. The remaining authors declare no competing financial interests.
Correspondence: Hussain Mulla, Nova Laboratories Limited, Leicester LE184YL, United Kingdom; e-mail: hussain.mulla@novalabs.co.uk.
References
Author notes
Data are available on request from contact the corresponding author, Hussain Mulla (hussain.mulla@novalabs.co.uk).
The full-text version of this article contains a data supplement.