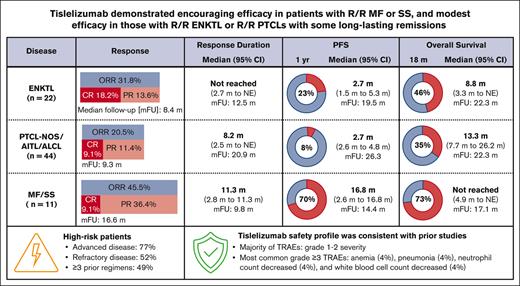
Key Points
Tislelizumab monotherapy showed modest efficacy in R/R mature T- and NK-cell neoplasms with some long-lasting remissions.
Tislelizumab was well tolerated and demonstrated promising efficacy in R/R mycosis fungoides and Sézary syndrome.
Abstract
Patients with relapsed/refractory (R/R) mature T- and natural killer (NK)–cell neoplasms lack effective treatments after failure of standard therapies. This phase 2 study evaluated the efficacy and safety of the programmed cell death protein 1 inhibitor tislelizumab in these patients. Seventy-seven patients were treated with 200 mg tislelizumab every 3 weeks. Twenty-two patients with extranodal NK-/T-cell lymphomas were enrolled in cohort 1; 44 patients with peripheral T-cell lymphoma (PTCL) were enrolled in cohort 2 (21 patients had PTCL not otherwise specified, 11 patients had angioimmunoblastic T-cell lymphoma, and 12 patients had anaplastic large-cell lymphoma). Cohort 3 comprised 11 patients with cutaneous T-cell lymphoma, of which 8 patients had mycosis fungoides (MF) and 3 had Sézary syndrome. Of the 77 patients, 76.6% had advanced-stage disease, 51.9% had refractory disease, and 49.4% received ≥3 prior systemic regimens. Promising efficacy was observed in cohort 3 (median follow-up [FU], 16.6 months; overall response rate [ORR], 45.5%; complete response [CR], 9.1%; median duration of response [DOR], 11.3 months; median progression-free survival, 16.8 months; median overall survival, not reached). Modest efficacy was observed in cohort 1 (median FU, 8.4 months; ORR, 31.8%; CR, 18.2%; median DOR, not reached) and cohort 2 (median FU, 9.3 months; ORR, 20.5%; CR, 9.1%; median DOR, 8.2 months). Most treatment-related adverse events were grade 1 or 2, and the safety profile was consistent with the known safety profile of tislelizumab. In conclusion, tislelizumab was well tolerated, achieving modest efficacy in R/R mature T- and NK-cell neoplasms, with some long-lasting remissions. This trial was registered at www.clinicaltrials.gov as #NCT03493451.
Introduction
Mature T-cell and natural killer (NK)-cell neoplasms are aggressive lymphoid malignancies. Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large-cell lymphoma (ALCL), and cutaneous T-cell lymphomas (CTCLs) are common T-cell lymphomas, occurring most frequently in Western populations; extranodal NK-/T-cell lymphomas (ENKTLs) are much more prevalent in Asian countries.1,2 Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common CTCL variants.3 Chemotherapy regimens designed for aggressive B-cell lymphomas are generally less effective in mature T- and NK-cell lymphomas. ENKTLs are resistant to anthracycline regimens,4-6 so primary treatment comprises nonanthracycline regimens, including L-asparaginase–based therapy.1 Patients with relapsed or refractory (R/R) ENKTLs have a poor prognosis and lack effective salvage treatment options after failure of L-asparaginase–based regimens.5-7 Similarly, R/R PTCL has a poor prognosis.8,9 Apart from brentuximab vedotin in R/R ALCL, the overall response rate (ORR) is typically <30% with other approved agents for R/R PTCL, such as antifolate (pralatrexate) or histone deacetylase inhibitors (eg, belinostat and chidamide).10,11 CTCLs are largely incurable, and ORRs range from 28% to 45% with US-approved therapies for MF and SS, including denileukin diftitox,12 bexarotene,13 vorinostat,14 romidepsin,15 and mogamulizumab,16 but remissions are typically not durable. Given the poor prognosis, there is an unmet treatment need for mature T- and NK-cell lymphomas.
The immune checkpoint inhibitory receptor, programmed cell death 1 (PD-1) is mainly expressed on activated T cells.17 PD-1 blockade is effective in multiple cancer types, but there are limited data available on the efficacy of PD-1 inhibitors in mature T- and NK-cell lymphomas. Recent studies show that ENKTL are susceptible to PD-1 blockade, likely related to the abundant expression of PD-1 ligand (PD-L1) driven by Epstein-Barr virus (EBV).18-21 A recent study of pembrolizumab also showed promising efficacy in R/R advanced MF or SS,22 but data in PTCLs are limited.
Tislelizumab (also known as BGB-A317) is a humanized immunoglobulin G4 variant monoclonal antibody that binds the extracellular domain of human PD-1 with high specificity and affinity. Unlike other anti–PD-1 antibodies, tislelizumab does not bind to macrophage Fc gamma receptors and the complement C1q. This unique feature of tislelizumab helps PD-1+ effector T cells avoid being eliminated by antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity, which are potential resistance mechanisms for other PD-1 inhibitors.23 In R/R classical Hodgkin lymphoma, tislelizumab showed a favorable safety profile with a high ORR (87.1%) and prolonged median progression-free survival (PFS) of 31.5 months (median follow-up of 33.8 months).24,25 Currently, tislelizumab is approved in China as a treatment option for R/R classical Hodgkin lymphoma as well as some solid tumors (urothelial carcinoma, non–small cell lung cancer, and hepatocellular carcinoma).
The aim of this study was to evaluate the efficacy and safety of tislelizumab in patients with R/R mature T- and NK-cell neoplasms.
Methods
Study design and patients
This was an international, multicenter, prospective, nonrandomized, open-label, phase 2 clinical study (NCT03493451) evaluating the safety and efficacy of tislelizumab in patients with R/R mature T- and NK-cell neoplasms. Adult patients with R/R mature T- and NK-cell neoplasms were allocated to 1 of 3 cohorts. Cohort 1 included patients with R/R ENKTL (nasal or nonnasal type); patients with aggressive NK leukemia were excluded. Cohort 2 included patients with R/R PTCL limited to PTCL-NOS (cohort 2a), AITL (cohort 2b), and ALCL (cohort 2c). Cohort 3 included patients with R/R CTCLs (stage IB or higher MF or SS). All patients had previously received at least 1 systemic therapy. Relapse was defined as disease progression during or after completion of the most recent therapy. Refractory disease was defined as failure to achieve a complete response (CR) or a partial response (PR) to the most recent therapy per investigator assessment, provided that the most recent therapy was an appropriate systemic therapy for mature T-cell or NK-cell lymphoma. Further details on patient selection are provided in supplemental Methods.
The study protocol, amendments, and informed consent forms were approved by the independent ethics committee or Institutional Review Board at participating centers, in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable regulatory requirements. Written informed consent was obtained from all patients. The study steering committee and the sponsor jointly made decisions regarding the study design and oversaw the conduct of the study. An independent data monitoring committee (IDMC) monitored the safety data and oversaw study conduct.
Treatments and assessments
Tislelizumab, 200 mg, was administered IV every 3 weeks until disease progression, intolerable toxicity, withdrawal of informed consent, or loss to follow-up, whichever occurred first.
In cohorts 1 and 2, investigators assessed responses per the Lugano criteria,26 with Lymphoma Response to Immunomodulatory Therapy criteria modification for immunomodulatory drugs.27 Response parameters included disease-related constitutional symptoms; physical examination of lymph nodes, liver, and spleen; bone marrow examination; and imaging, including positron emission tomography (PET), computed tomography (CT), and CT-based assessments. Tumor assessments, including imaging, were performed at screening, at week 12 from day 1 of cycle 1, every 12 weeks for 96 weeks, followed by every 24 weeks for an additional 96 weeks, and then yearly until disease progression. In the event of a treatment delay, disease assessments were to continue per the schedule of assessments.
For patients with MF or SS (cohort 3), investigators assessed responses per International Society for Cutaneous Lymphomas/European Organization of Research and Treatment of Cancer guidelines.28 Response parameters included skin assessment using the modified severity weight assessment tool (mSWAT), lymph node or visceral involvement via PET and/or CT, and peripheral blood analysis via flow cytometry. Not all response assessments were used depending on the presence or absence of measurable disease on CT or circulating Sézary cells. ORR was determined based on the global response score per ISCL/EORTC guidelines. Tumor assessments, including imaging for patients with measurable disease using CT, were performed starting at week 12 from day 1 of cycle 1, then every 12 weeks for 96 weeks, followed by every 24 weeks for an additional 96 weeks, and then yearly thereafter. If disease progression was suspected at week 12, an additional response assessment was performed at week 16 to rule in or rule out tumor flare. Patients with suspected disease progression after week 12 were required to have all response assessments performed regardless of whether it was a scheduled response assessment time point to confirm disease progression.
Safety assessments included adverse events (AEs), treatment-emergent AEs (TEAEs), treatment-related AEs (TRAEs), serious AEs, infusion-related reactions (IRRs), and immune-related AEs (irAEs). Investigators assessed AEs per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. The AE verbatim descriptions were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0. A patient with multiple occurrences of the same coded event was counted only once for that event, at the most severe grade.
End points
The primary end point was investigator-assessed ORR (defined as the proportion of patients achieving a best overall response of either a CR or PR). Subgroup analyses were also performed on the primary end point as per the sex, age (<60 years and ≥60 years), region (Asia Pacific [APAC]/non–APAC), Eastern Cooperative Oncology Group (ECOG) performance status (0 and ≥1), number of prior regimens of systemic therapy (<3 and ≥3), baseline lactate dehydrogenase (LDH; normal/high/low), bone marrow involvement (yes/no), disease stage (I/II and III/IV), and baseline circulating EBV DNA status (under detection limit/detected/missing; cohort 1 only).
Secondary end points (investigator assessed) included duration of response (DOR) (time from first determination of an objective response until disease progression or death, whichever occurs first), PFS (time from first study drug administration to the date of disease progression or death, whichever occurs first), CR or complete metabolic response rate, time to response (TTR; the time from first dose to the time when criteria for a CR or PR was first met), and for cohorts 1 and 2, overall survival (OS) (time from first dose to date of death owing to any reason). Safety and tolerability were secondary end points. OS was an exploratory end point for cohort 3.
Statistical analysis
Sample size was determined based on the precision of the estimation of the primary ORR end point and the feasibility of recruitment for each patient population. It was planned as ≤70 patients for cohort 1, ≤50 patients for cohort 2 (20 for cohort 2a, 10 for cohort 2b, and 20 for cohort 2c), and ≤10 patients for cohort 3. The final enrollment number was determined based on the calculated sample size together with the IDMC’s decision to end enrollment in cohorts 1 and 2 based on emerging data. The primary analyses of efficacy and safety were conducted on the Safety Analysis Set, which included all patients receiving at least 1 tislelizumab dose. The primary efficacy analysis for each cohort was conducted when mature ORR data were obtained.
Response rates were summarized as point estimates with Clopper-Pearson 95% confidence intervals (CIs). The Kaplan-Meier method was used for time-to-event end points, including PFS, DOR, and OS, with medians and other quantiles calculated; 2-sided 95% CIs were calculated per the generalized Brookmeyer and Crowley method. Selected landmark event-free rates were provided, with the corresponding 95% CI based on Greenwood’s formula. TTR was summarized descriptively. Frequencies and proportions of patients with AEs were reported.
Results
Patient characteristics
Overall, 146 patients were screened, and 77 patients were enrolled from 24 centers in 5 countries/regions (Canada; Mainland China; France; Italy; and Taiwan, China) from April 2018 to October 2019. In agreement with the IDMC and the sponsor, enrollment for cohort 1 and cohort 2 was stopped early in April 2019 and September 2019, respectively, owing to modest efficacy, resulting in fewer than the planned number of patients in these cohorts. All enrolled patients were evaluable for efficacy and safety analyses. The most common reasons for treatment discontinuation were progressive disease (57%), AEs of any type (ie, not just TEAEs; 26.0%), study termination (7.8%), and patient decision to withdraw (5.2%). The most common reasons for study discontinuation were death (51.9%) and study termination (33.8%) (supplemental Figure 1).
Baseline characteristics are shown in Table 1. Twenty-two patients (median age, 47.5 years) were enrolled in cohort 1. Forty-four patients (median age, 58.0 years) were enrolled in cohort 2, including 21 patients with PTCL-NOS, 11 with AITL, and 12 with ALCL. Among patients with ALCL, 2 had anaplastic lymphoma kinase (ALK)–positive ALCL, 9 had ALK-negative ALCL, and 1 had unknown ALK status. Eleven patients (median age, 62.0 years) were enrolled in cohort 3, including 8 patients with MF and 3 with SS. Most patients in cohort 1 were Asian (86.4%), whereas most patients in cohort 3 were White (72.7%). The ECOG performance status was grade 0 for 30 of 77 patients (39.0%), grade 1 for 42 of 77 (54.5%) patients, and grade 2 for 5 of 77 (6.5%) patients. In cohort 3, the median baseline mSWAT score was 89.0 (range, 14.0-100.0).
Patient demographics and baseline disease characteristics
| . | Cohort 1 (ENKTL) (n = 22) . | Cohort 2 (PTCLs) (n = 44) . | Cohort 3 (MF and SS) (n = 11) . | Total (N = 77) . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 47.5 | 58.0 | 62.0 | 56.0 |
| Min, max | 24, 76 | 21, 84 | 35, 76 | 21, 84 |
| Age group | ||||
| <60 y | 13 (59.1) | 24 (54.5) | 5 (45.5) | 42 (54.5) |
| ≥60 y | 9 (40.9) | 20 (45.5) | 6 (54.5) | 35 (45.5) |
| Sex | ||||
| Male | 14 (63.6) | 29 (65.9) | 8 (72.7) | 51 (66.2) |
| Female | 8 (36.4) | 15 (34.1) | 3 (27.3) | 26 (33.8) |
| Race | ||||
| Asian | 19 (86.4) | 23 (52.3) | 2 (18.2) | 44 (57.1) |
| White | 2 (9.1) | 18 (40.9) | 8 (72.7) | 28 (36.4) |
| Not reported | 1 (4.5) | 3 (6.8) | 1 (9.1) | 5 (6.5) |
| ECOG performance status at baseline | ||||
| 0 | 3 (13.6) | 21 (47.7) | 6 (54.5) | 30 (39.0) |
| 1 | 17 (77.3) | 21 (47.7) | 4 (36.4) | 42 (54.5) |
| 2 | 2 (9.1) | 2 (4.5) | 1 (9.1) | 5 (6.5) |
| Disease status | ||||
| Relapsed disease | 12 (54.5) | 21 (47.7) | 4 (36.4) | 37 (48.1) |
| Refractory disease | 10 (45.5) | 23 (52.3) | 7 (63.6) | 40 (51.9) |
| Advanced-stage disease∗,† | 14 (63.6) | 36 (81.8) | 9 (81.8) | 59 (76.6) |
| Bone marrow involvement† | 2 (9.1) | 9 (20.5) | 3 (27.3) | 14 (18.2) |
| B-symptoms† | 6 (27.3) | 11 (25.0) | 1 (9.1) | 18 (23.4) |
| High LDH at baseline‡ | 13 (59.1) | 16 (36.4) | 3 (27.3) | 32 (41.6) |
| Low lymphocyte count at baseline†,‡ | 9 (40.9) | 23 (52.3) | 3 (27.3) | 35 (45.5) |
| Number of prior regimens | ||||
| Median | 2.0 | 2.0 | 4.0 | 2.0 |
| Min, max | 1, 5 | 1, 8 | 2, 6 | 1, 8 |
| Number of prior regimens | ||||
| <3 | 13 (59.1) | 24 (54.5) | 2 (18.2) | 39 (50.6) |
| ≥3 | 9 (40.9) | 20 (45.5) | 9 (81.8) | 38 (49.4) |
| Prior autologous stem cell transplant | 0 (0.0) | 6 (13.6) | 0 (0.0) | 6 (7.8) |
| Prior allogenic stem cell transplant§ | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| Prior radiation therapy | 16 (72.7) | 8 (18.2) | 4 (36.4) | 28 (36.4) |
| . | Cohort 1 (ENKTL) (n = 22) . | Cohort 2 (PTCLs) (n = 44) . | Cohort 3 (MF and SS) (n = 11) . | Total (N = 77) . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 47.5 | 58.0 | 62.0 | 56.0 |
| Min, max | 24, 76 | 21, 84 | 35, 76 | 21, 84 |
| Age group | ||||
| <60 y | 13 (59.1) | 24 (54.5) | 5 (45.5) | 42 (54.5) |
| ≥60 y | 9 (40.9) | 20 (45.5) | 6 (54.5) | 35 (45.5) |
| Sex | ||||
| Male | 14 (63.6) | 29 (65.9) | 8 (72.7) | 51 (66.2) |
| Female | 8 (36.4) | 15 (34.1) | 3 (27.3) | 26 (33.8) |
| Race | ||||
| Asian | 19 (86.4) | 23 (52.3) | 2 (18.2) | 44 (57.1) |
| White | 2 (9.1) | 18 (40.9) | 8 (72.7) | 28 (36.4) |
| Not reported | 1 (4.5) | 3 (6.8) | 1 (9.1) | 5 (6.5) |
| ECOG performance status at baseline | ||||
| 0 | 3 (13.6) | 21 (47.7) | 6 (54.5) | 30 (39.0) |
| 1 | 17 (77.3) | 21 (47.7) | 4 (36.4) | 42 (54.5) |
| 2 | 2 (9.1) | 2 (4.5) | 1 (9.1) | 5 (6.5) |
| Disease status | ||||
| Relapsed disease | 12 (54.5) | 21 (47.7) | 4 (36.4) | 37 (48.1) |
| Refractory disease | 10 (45.5) | 23 (52.3) | 7 (63.6) | 40 (51.9) |
| Advanced-stage disease∗,† | 14 (63.6) | 36 (81.8) | 9 (81.8) | 59 (76.6) |
| Bone marrow involvement† | 2 (9.1) | 9 (20.5) | 3 (27.3) | 14 (18.2) |
| B-symptoms† | 6 (27.3) | 11 (25.0) | 1 (9.1) | 18 (23.4) |
| High LDH at baseline‡ | 13 (59.1) | 16 (36.4) | 3 (27.3) | 32 (41.6) |
| Low lymphocyte count at baseline†,‡ | 9 (40.9) | 23 (52.3) | 3 (27.3) | 35 (45.5) |
| Number of prior regimens | ||||
| Median | 2.0 | 2.0 | 4.0 | 2.0 |
| Min, max | 1, 5 | 1, 8 | 2, 6 | 1, 8 |
| Number of prior regimens | ||||
| <3 | 13 (59.1) | 24 (54.5) | 2 (18.2) | 39 (50.6) |
| ≥3 | 9 (40.9) | 20 (45.5) | 9 (81.8) | 38 (49.4) |
| Prior autologous stem cell transplant | 0 (0.0) | 6 (13.6) | 0 (0.0) | 6 (7.8) |
| Prior allogenic stem cell transplant§ | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| Prior radiation therapy | 16 (72.7) | 8 (18.2) | 4 (36.4) | 28 (36.4) |
Data are expressed as number of patients (%), unless otherwise stated.
Cohort 1: patients with R/R ENKTL; cohort 2: patients with R/R PRCL-NOS, R/R AITL, or R/R ALCL; and cohort 3: patients with R/R MF or R/R SS.
ECOG, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; Max, maximum; Min, minimum.
Stages III to IV for cohort 1 and 2; stage IIB or higher for cohort 3.
Disease stage at study entry was missing for 1 patient in cohort 1. Bone marrow involvement at study entry was unknown for 3 patients in cohort 2 and 2 patients in cohort 3. B-symptom data at study entry was missing for 1 patient in cohort 2 and 2 patients in cohort 3. Lymphocytes count at study entry was missing for 1 patient in cohort 2.
Higher/lower than normal range.
One patient with prior allogenic stem cell transplant was enrolled as a protocol deviation. The deviation was considered unlikely to have had a substantive impact on the outcomes or conclusions of this study.
More than half of the patients had refractory disease to their most recent treatment (51.9% overall; 52.3% in cohort 2; and 63.6% in cohort 3). Advanced disease was present in 63.6% of cohort 1 patients (stage III, 9.1% and stage IV, 54.5%), 81.8% of cohort 2 patients (stage III, 27.3% and stage IV, 54.5%), and 81.8% of cohort 3 patients (stage IIB, 9.1%; stage IIIA, 9.1%; stage IIIB, 18.2%; stage IVA1, 9.1%; and stage IVA2, 36.4%). Overall, 49.4% of patients had received ≥3 prior systemic regimens (81.8% in cohort 3). In cohort 1, 95.5% had received prior L-asparaginase, and 72.7% received prior radiation. In cohort 2, 100% had received prior cyclophosphamide, 95.5% received vinca alkaloids and analogues, and 93.2% received anthracyclines. In cohort 3, 72.7% had received prior brentuximab vedotin (6 patients with MF and 2 patients with SS). The most common prior systemic therapies are shown in supplemental Table 1. Eight patients in cohort 3 (7 patients with MF and 1 with SS) had prior nonsystemic therapy (radiation in 4 patients and topical or transdermal treatment in 5).
Treatment exposure
All 77 patients received ≥1 dose of tislelizumab. The median duration of exposure was 18.0 weeks (range, 2.3-131.0 weeks), with a median of 5.0 treatment cycles (range, 1-38 cycles). Cohorts 1, 2, and 3 received a median of 5.0 (range, 1-37), 4.5 (range, 1-38), and 17.0 (range, 3-25) treatment cycles, respectively.
Efficacy
Response rates
In cohort 1, ORR was 31.8% (95% CI, 13.9-54.9), including CR in 18.2% and PR in 13.6% (Table 2). In cohort 2, ORR was 20.5% (95% CI, 9.8-35.3), including CR in 9.1% and PR in 11.4%. PTCL-NOS, AITL, and ALCL subtypes had ORRs of 23.8% (95% CI, 8.2-47.2; CR in 14.3%), 18.2% (95% CI, 2.3-51.8; CR in 9.1%), and 16.7% (95% CI, 2.1-48.4; no CR), respectively. ORR in cohort 3 was 45.5% (95% CI, 16.7-76.6), with CR in 9.1% and PR in 36.4% (Table 2). Among 3 patients with SS, best overall responses of CR, PR, and stable disease were observed in 1 patient each. Among 8 patients in cohort 3 with at least 1 postbaseline mSWAT score, the median reduction from baseline to nadir of mSWAT score was −67.6% (range, from −95.8% to 0.0%). Subgroup analyses for cohorts 1 to 3 are presented in supplemental Figure 2. No significant differences in ORR were identified within any subgroups. However, no firm conclusions can be made, given the limited number of patients in many of the subgroups. No significant correlation was observed between PD-L1 expression and ORR (supplemental Figure 3), but no firm conclusions can be made, given the limited PD-L1 expression data available in responders. There appeared to be a trend toward greater benefit in patients who had undetectable baseline circulating EBV DNA than in those who had detectable circulating EBV DNA values at baseline (cohorts 1 and 2; supplemental Figures 2A and 4). Notably, in cohort 1, responders with detectable baseline circulating EBV DNA showed decreases in EBV concentration after 4 cycles of treatment, although increases in later cycles were observed in 2 of the 3 responders. In cohort 2, no postbaseline increases in circulating EBV DNA were observed in responders (supplemental Figure 4). However, as with the PD-L1 data, there are too few data points to draw clear conclusions from these data.
Analysis of disease response per investigator assessment
| . | Cohort 1 (ENKTL) (N = 22) . | Cohort 2a PTCL-NOS (n = 21) . | Cohort 2b AITL (n = 11) . | Cohort 2c ALCL (n = 12) . | Cohort 2 Total (N = 44) . | Cohort 3 (MF and SS) (N = 11) . |
|---|---|---|---|---|---|---|
| BOR, n (%) | ||||||
| CR | 4 (18.2) | 3 (14.3) | 1 (9.1) | 0 (0.0) | 4 (9.1) | 1 (9.1) |
| PR | 3 (13.6) | 2 (9.5) | 1 (9.1) | 2 (16.7) | 5 (11.4) | 4 (36.4) |
| Stable disease | 1 (4.5) | 3 (14.3) | 3 (27.3) | 0 (0.0) | 6 (13.6) | 3 (27.3) |
| Indeterminate response | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (2.3) | 0 (0.0) |
| Progressive disease | 11 (50.0) | 11 (52.4) | 4 (36.4) | 6 (50.0) | 21 (47.7) | 1 (9.1) |
| Discontinued prior to first assessment | 3 (13.6) | 2 (9.5) | 2 (18.2) | 3 (25.0) | 7 (15.9) | 2 (18.2) |
| ORR,∗n (%) | 7 (31.8) | 5 (23.8) | 2 (18.2) | 2 (16.7) | 9 (20.5) | 5 (45.5) |
| 95% CI† | (13.9-54.9) | (8.2-47.2) | (2.3-51.8) | (2.1-48.4) | (9.8-35.3) | (16.7-76.6) |
| TTR, mo‡ | ||||||
| n | 7 | 5 | 2 | 2 | 9 | 5 |
| Median | 5.8 | 4.6 | 2.5 | 2.7 | 2.9 | 6.8 |
| Min, max | 2.1, 13.9 | 2.8, 5.5 | 2.1, 2.9 | 2.7, 2.7 | 2.1, 5.5 | 2.6, 11.1 |
| Time to CR, mo | ||||||
| n | 4 | 3 | 1 | 0 | 4 | 1 |
| Median | 8.6 | 4.6 | 2.1 | NA | 3.7 | 14.3 |
| Min, max | 2.1, 13.9 | 2.8, 5.5 | 2.1, 2.1 | NA | 2.1, 5.5 | 14.3, 14.3 |
| . | Cohort 1 (ENKTL) (N = 22) . | Cohort 2a PTCL-NOS (n = 21) . | Cohort 2b AITL (n = 11) . | Cohort 2c ALCL (n = 12) . | Cohort 2 Total (N = 44) . | Cohort 3 (MF and SS) (N = 11) . |
|---|---|---|---|---|---|---|
| BOR, n (%) | ||||||
| CR | 4 (18.2) | 3 (14.3) | 1 (9.1) | 0 (0.0) | 4 (9.1) | 1 (9.1) |
| PR | 3 (13.6) | 2 (9.5) | 1 (9.1) | 2 (16.7) | 5 (11.4) | 4 (36.4) |
| Stable disease | 1 (4.5) | 3 (14.3) | 3 (27.3) | 0 (0.0) | 6 (13.6) | 3 (27.3) |
| Indeterminate response | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (2.3) | 0 (0.0) |
| Progressive disease | 11 (50.0) | 11 (52.4) | 4 (36.4) | 6 (50.0) | 21 (47.7) | 1 (9.1) |
| Discontinued prior to first assessment | 3 (13.6) | 2 (9.5) | 2 (18.2) | 3 (25.0) | 7 (15.9) | 2 (18.2) |
| ORR,∗n (%) | 7 (31.8) | 5 (23.8) | 2 (18.2) | 2 (16.7) | 9 (20.5) | 5 (45.5) |
| 95% CI† | (13.9-54.9) | (8.2-47.2) | (2.3-51.8) | (2.1-48.4) | (9.8-35.3) | (16.7-76.6) |
| TTR, mo‡ | ||||||
| n | 7 | 5 | 2 | 2 | 9 | 5 |
| Median | 5.8 | 4.6 | 2.5 | 2.7 | 2.9 | 6.8 |
| Min, max | 2.1, 13.9 | 2.8, 5.5 | 2.1, 2.9 | 2.7, 2.7 | 2.1, 5.5 | 2.6, 11.1 |
| Time to CR, mo | ||||||
| n | 4 | 3 | 1 | 0 | 4 | 1 |
| Median | 8.6 | 4.6 | 2.1 | NA | 3.7 | 14.3 |
| Min, max | 2.1, 13.9 | 2.8, 5.5 | 2.1, 2.1 | NA | 2.1, 5.5 | 14.3, 14.3 |
BOR, best overall response; Max, maximum; Min, minimum.
ORR was defined as the proportion of patients achieving a BOR of either CR or PR.
Two-sided Clopper-Pearson 95% CI.
TTR was defined as time from the first dose date to the date of earliest qualifying response (PR or CR).
DOR
In cohort 1, the median DOR was not reached (95% CI, 2.7 months to not estimable [NE]; median follow-up, 12.5 months). In cohort 2, at a median follow-up of 20.9 months, median DOR was 8.2 months (95% CI, 2.5 months to NE) and not reached in cohort 2a (95% CI, 2.7 months to NE). In cohort 3, median DOR was 11.3 months (95% CI, 2.8-11.3 months; median follow-up, 9.8 months; Table 3).
Analysis of DOR by the investigator
| . | Cohort 1 ENKTL (N = 22) . | Cohort 2a PTCL-NOS (n = 21) . | Cohort 2b AITL (n = 11) . | Cohort 2c ALCL (n = 12) . | Cohort 2 Total (N = 44) . | Cohort 3 MF or SS (N = 11) . |
|---|---|---|---|---|---|---|
| Number of responders, n (%) | 7 (31.8) | 5 (23.8) | 2 (18.2) | 2 (16.7) | 9 (20.5) | 5 (45.5) |
| Median follow-up, mo (95% CI)∗ | 12.5 (8.3-13.9) | 20.9 (9.1-20.9) | NE (NE-NE) | NE (NE-NE) | 20.9 (9.1-20.9) | 9.8 (2.8-NE) |
| Median DOR, mo (95% CI)† | NE (2.7-NE) | NE (2.7-NE) | 2.9 (2.5-3.2) | 8.3 (8.2-8.4) | 8.2 (2.5-NE) | 11.3 (2.8-11.3) |
| Event-free rate, % (95% CI)‡ | ||||||
| At 3 mo | 85.7 (33.4-97.9) | 80.0 (20.4-96.9) | 50.0 (0.6-91.0) | 100.0 (NE-NE) | 77.8 (36.5-93.9) | 53.3 (6.8-86.3) |
| At 6 mo | 71.4 (25.8-92.0) | 80.0 (20.4-96.9) | 0.0 (NE-NE) | 100.0 (NE-NE) | 66.7 (28.2-87.8) | 53.3 (6.8-86.3) |
| At 9 mo | 57.1 (17.2-83.7) | 60.0 (12.6-88.2) | 0.0 (NE-NE) | 0.0 (NE-NE) | 33.3 (7.8-62.3) | 53.3 (6.8-86.3) |
| At 12 mo | 57.1 (17.2-83.7) | 60.0 (12.6-88.2) | 0.0 (NE-NE) | 0.0 (NE-NE) | 33.3 (7.8-62.3) | 0.0 (NE-NE) |
| At 18 mo | NE (NE-NE) | 60.0 (12.6-88.2) | 0.0 (NE-NE) | 0.0 (NE-NE) | 33.3 (7.8-62.3) | 0.0 (NE-NE) |
| . | Cohort 1 ENKTL (N = 22) . | Cohort 2a PTCL-NOS (n = 21) . | Cohort 2b AITL (n = 11) . | Cohort 2c ALCL (n = 12) . | Cohort 2 Total (N = 44) . | Cohort 3 MF or SS (N = 11) . |
|---|---|---|---|---|---|---|
| Number of responders, n (%) | 7 (31.8) | 5 (23.8) | 2 (18.2) | 2 (16.7) | 9 (20.5) | 5 (45.5) |
| Median follow-up, mo (95% CI)∗ | 12.5 (8.3-13.9) | 20.9 (9.1-20.9) | NE (NE-NE) | NE (NE-NE) | 20.9 (9.1-20.9) | 9.8 (2.8-NE) |
| Median DOR, mo (95% CI)† | NE (2.7-NE) | NE (2.7-NE) | 2.9 (2.5-3.2) | 8.3 (8.2-8.4) | 8.2 (2.5-NE) | 11.3 (2.8-11.3) |
| Event-free rate, % (95% CI)‡ | ||||||
| At 3 mo | 85.7 (33.4-97.9) | 80.0 (20.4-96.9) | 50.0 (0.6-91.0) | 100.0 (NE-NE) | 77.8 (36.5-93.9) | 53.3 (6.8-86.3) |
| At 6 mo | 71.4 (25.8-92.0) | 80.0 (20.4-96.9) | 0.0 (NE-NE) | 100.0 (NE-NE) | 66.7 (28.2-87.8) | 53.3 (6.8-86.3) |
| At 9 mo | 57.1 (17.2-83.7) | 60.0 (12.6-88.2) | 0.0 (NE-NE) | 0.0 (NE-NE) | 33.3 (7.8-62.3) | 53.3 (6.8-86.3) |
| At 12 mo | 57.1 (17.2-83.7) | 60.0 (12.6-88.2) | 0.0 (NE-NE) | 0.0 (NE-NE) | 33.3 (7.8-62.3) | 0.0 (NE-NE) |
| At 18 mo | NE (NE-NE) | 60.0 (12.6-88.2) | 0.0 (NE-NE) | 0.0 (NE-NE) | 33.3 (7.8-62.3) | 0.0 (NE-NE) |
Only responders are included in the analysis. Percentages are based on patients with BOR of at least PR, except for number of responders.
PD, progressive disease.
Median follow-up was estimated using the reverse Kaplan-Meier method.
DOR for responders (CR or PR) was defined as the time from the date of the earliest qualifying response (PR or better) to the date of PD or death for any cause, whichever occurred earlier. Medians were estimated with the Kaplan-Meier method and 95% CIs were estimated using the method of Brookmeyer and Crowley.
Event-free rates were estimated with the Kaplan-Meier method and 95% CIs were estimated using Greenwood’s formula.
PFS
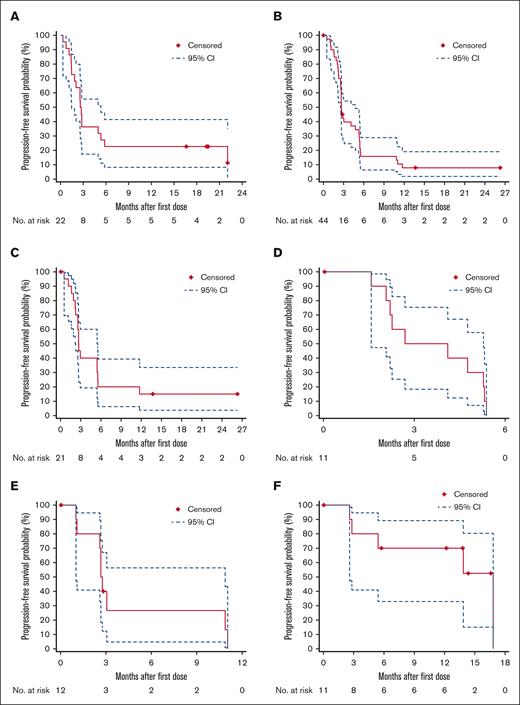
In cohort 1, the median PFS (median follow-up, 19.5 months) was 2.7 months (95% CI, 1.5-5.3 months), with a 1-year PFS rate of 22.7% (95% CI, 8.3-41.5; Figure 1A). In cohort 2, the median PFS (median follow-up, 26.3 months) was 2.7 months (95% CI, 2.6-4.8), with a 1-year PFS rate of 7.9% (95% CI, 2.1-19.2; Figure 1B). In the PTCL-NOS, AICL, and ALCL subtypes, the median PFS was 2.7 months (95% CI, 2.2-5.5), 3.4 months (95% CI, 1.6-5.3), and 2.7 months (95% CI, 1.0-10.9), respectively (Figure 1C-E). In cohort 3, the median PFS (median follow-up, 14.4 months) was 16.8 months (95% CI, 2.6-16.8), with a 1-year PFS rate of 70.0% (95% CI, 32.9-89.2; Figure 1F).
Kaplan-Meier plots of PFS per the investigator assessment. (A) Cohort 1 (ENKTL); (B) cohort 2 (PTCL-NOS, AITL, and ALCL); (C) cohort 2a (PTCL-NOS); (D) cohort 2b (AITL); (E) cohort 2c (ALCL); and (F) cohort 3 (MF or SS). CIs were calculated using a generalized Brookmeyer and Crowley method.
Kaplan-Meier plots of PFS per the investigator assessment. (A) Cohort 1 (ENKTL); (B) cohort 2 (PTCL-NOS, AITL, and ALCL); (C) cohort 2a (PTCL-NOS); (D) cohort 2b (AITL); (E) cohort 2c (ALCL); and (F) cohort 3 (MF or SS). CIs were calculated using a generalized Brookmeyer and Crowley method.
OS
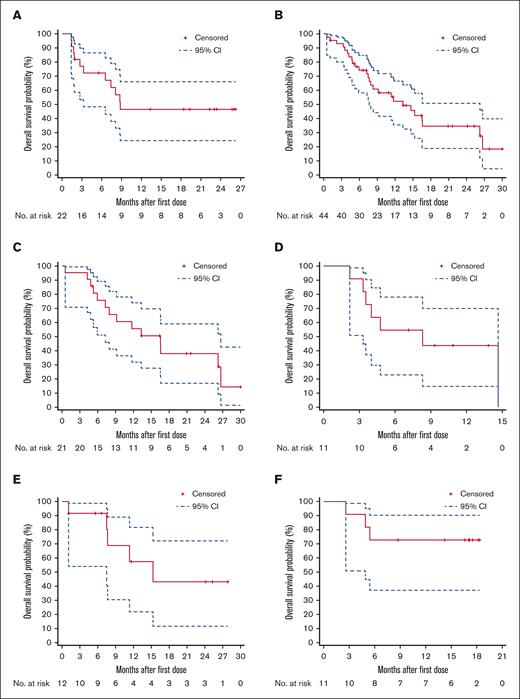
In cohort 1, the median OS (median follow-up, 22.3 months) was 8.8 months (95% CI, 3.3 to NE), with an 18-month OS rate of 46.4% (95% CI, 24.3-66.0; Figure 2A). For cohort 2, the median OS (median follow-up, 21.6 months) was 13.3 months (95% CI, 7.7-26.2), with an 18-month OS rate of 34.6% (95% CI, 18.9-50.8; Figure 2B). The median OS in cohort 2 PTCL-NOS, AITL, and ALCL subtypes was 16.5 months (95% CI, 7.3-26.7), 8.3 months (95% CI, 3.3-14.7), and 15.3 months (95% CI, 7.5 to NE), respectively (Figure 2C-E). For cohort 3, the median OS was not reached (95% CI, 4.9 months to NE), with an 18-month OS rate of 72.7% (95% CI, 37.1-90.3) at a median follow-up of 17.1 months (Figure 2F).
Kaplan-Meier plots of OS. (A) Cohort 1 (ENKTL); (B) cohort 2 (PTCL-NOS, AITL, and ALCL); (C) cohort 2a (PTCL-NOS); (D) cohort 2b (AITL); (E) cohort 2c (ALCL); and (F) cohort 3 (MF or SS). CIs were calculated using a generalized Brookmeyer and Crowley method.
Kaplan-Meier plots of OS. (A) Cohort 1 (ENKTL); (B) cohort 2 (PTCL-NOS, AITL, and ALCL); (C) cohort 2a (PTCL-NOS); (D) cohort 2b (AITL); (E) cohort 2c (ALCL); and (F) cohort 3 (MF or SS). CIs were calculated using a generalized Brookmeyer and Crowley method.
TTR
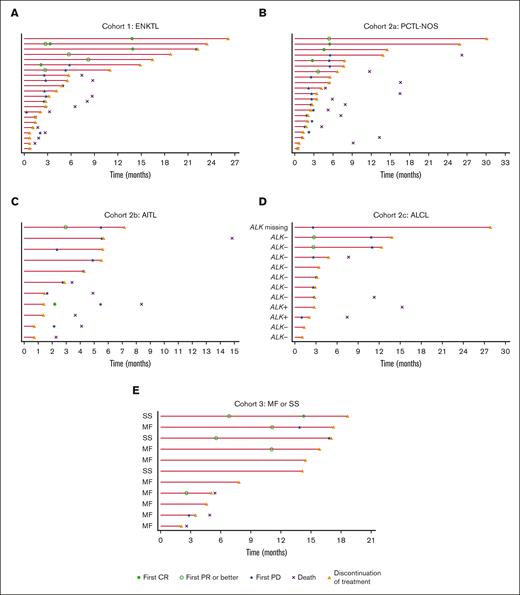
Among 7 responders in cohort 1, the median TTR was 5.8 months (range, 2.1-13.9 months). Among 9 responders in cohort 2, the median TTR was 2.9 months (range, 2.1-5.5 months). For PTCL-NOS, AITL, and ALCL subtypes, the median TTR was 4.6 months (range, 2.8-5.5 months), 2.5 months (range, 2.1-2.9 months), and 2.7 months (range, 2.7-2.7 months), respectively. Among 5 responders in cohort 3, the median TTR was 6.8 months (range, 2.6-11.1 months; Table 2; Figure 3).
Duration of treatment and TTR. (A) Cohort 1 (ENKTL); (B) cohort 2a (PTCL-NOS); (C) cohort 2b (AITL); (D) cohort 2c (ALCL); and (E) cohort 3 (MF or SS). Each lane represents 1 patient. PD, progressive disease.
Duration of treatment and TTR. (A) Cohort 1 (ENKTL); (B) cohort 2a (PTCL-NOS); (C) cohort 2b (AITL); (D) cohort 2c (ALCL); and (E) cohort 3 (MF or SS). Each lane represents 1 patient. PD, progressive disease.
Safety
Most patients (94.8%) experienced ≥1 TEAE, with grade ≥3 TEAEs in 59.7% (grade 3, 42.9%; grade 4, 10.4%; and grade 5 6.5%; Table 4). The most common TEAEs (≥15% patients) were pyrexia (32.5%), anemia (18.2%), arthralgia (18.2%), and diarrhea (15.6%). The most frequently reported grade ≥3 AEs (in ≥3 patients) were anemia (7.8%), pneumonia (6.5%), neutropenia (5.2%), decreased neutrophil count, thrombocytopenia, and decreased white blood cell count (3.9% each). Serious TEAEs occurred in 35 patients (45.5%). Five patients (6.5%) experienced a TEAE leading to death. Fourteen patients (18.2%) experienced TEAEs leading to treatment discontinuation, and 27 patients (35.1%) experienced TEAEs leading to dose modification.
Summary of TEAEs
| . | Cohort 1 (ENKTL) (n = 22) n (%) . | Cohort 2 (PTCLs) (n = 44) n (%) . | Cohort 3 (MF or SS) (n = 11) n (%) . | Total (N = 77) n (%) . |
|---|---|---|---|---|
| Patients with ≥1 TEAE | 22 (100.0) | 41 (93.2) | 10 (90.9) | 73 (94.8) |
| Grade ≥3 TEAE | 13 (59.1) | 25 (56.8) | 8 (72.7) | 46 (59.7) |
| Grade ≥3 TEAE in ≥3 patients based on preferred term | ||||
| Anemia | 3 (13.6) | 3 (6.8) | 0 (0.0) | 6 (7.8) |
| Pneumonia | 2 (9.1) | 3 (6.8) | 0 (0.0) | 5 (6.5) |
| Neutropenia | 0 (0.0) | 4 (9.1) | 0 (0.0) | 4 (5.2) |
| Neutrophil count decreased | 3 (13.6) | 0 (0.0) | 0 (0.0) | 3 (3.9) |
| Thrombocytopenia | 0 (0.0) | 3 (6.8) | 0 (0.0) | 3 (3.9) |
| White blood cell count decreased | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Patients with ≥1 TRAE | 17 (77.3) | 33 (75.0) | 7 (63.6) | 57 (74.0) |
| Grade ≥3 TRAE | 7 (31.8) | 10 (22.7) | 0 (0.0) | 17 (22.1) |
| Grade ≥3 TRAE in ≥2 patients by preferred term | ||||
| Anemia | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Pneumonia | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Neutrophil count decreased | 3 (13.6) | 0 (0.0) | 0 (0.0) | 3 (3.9) |
| White blood cell count decreased | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Platelet count decreased | 1 (4.5) | 1 (2.3) | 0 (0.0) | 2 (2.6) |
| Pyrexia | 0 (0.0) | 2 (4.5) | 0 (0.0) | 2 (2.6) |
| irAE | 6 (27.3) | 13 (29.5) | 3 (27.3) | 22 (28.6) |
| Grade ≥3 irAE | 2 (9.1) | 2 (4.5) | 0 (0.0) | 4 (5.2) |
| Grade ≥3 irAE based on preferred term | ||||
| Hepatitis | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| Hypothyroidism | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| Blood creatine phosphokinase increased | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| Rash | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| Urticaria | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| IRR | 5 (22.7) | 7 (15.9) | 2 (18.2) | 14 (18.2) |
| Grade ≥3 IRR | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| . | Cohort 1 (ENKTL) (n = 22) n (%) . | Cohort 2 (PTCLs) (n = 44) n (%) . | Cohort 3 (MF or SS) (n = 11) n (%) . | Total (N = 77) n (%) . |
|---|---|---|---|---|
| Patients with ≥1 TEAE | 22 (100.0) | 41 (93.2) | 10 (90.9) | 73 (94.8) |
| Grade ≥3 TEAE | 13 (59.1) | 25 (56.8) | 8 (72.7) | 46 (59.7) |
| Grade ≥3 TEAE in ≥3 patients based on preferred term | ||||
| Anemia | 3 (13.6) | 3 (6.8) | 0 (0.0) | 6 (7.8) |
| Pneumonia | 2 (9.1) | 3 (6.8) | 0 (0.0) | 5 (6.5) |
| Neutropenia | 0 (0.0) | 4 (9.1) | 0 (0.0) | 4 (5.2) |
| Neutrophil count decreased | 3 (13.6) | 0 (0.0) | 0 (0.0) | 3 (3.9) |
| Thrombocytopenia | 0 (0.0) | 3 (6.8) | 0 (0.0) | 3 (3.9) |
| White blood cell count decreased | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Patients with ≥1 TRAE | 17 (77.3) | 33 (75.0) | 7 (63.6) | 57 (74.0) |
| Grade ≥3 TRAE | 7 (31.8) | 10 (22.7) | 0 (0.0) | 17 (22.1) |
| Grade ≥3 TRAE in ≥2 patients by preferred term | ||||
| Anemia | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Pneumonia | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Neutrophil count decreased | 3 (13.6) | 0 (0.0) | 0 (0.0) | 3 (3.9) |
| White blood cell count decreased | 2 (9.1) | 1 (2.3) | 0 (0.0) | 3 (3.9) |
| Platelet count decreased | 1 (4.5) | 1 (2.3) | 0 (0.0) | 2 (2.6) |
| Pyrexia | 0 (0.0) | 2 (4.5) | 0 (0.0) | 2 (2.6) |
| irAE | 6 (27.3) | 13 (29.5) | 3 (27.3) | 22 (28.6) |
| Grade ≥3 irAE | 2 (9.1) | 2 (4.5) | 0 (0.0) | 4 (5.2) |
| Grade ≥3 irAE based on preferred term | ||||
| Hepatitis | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| Hypothyroidism | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| Blood creatine phosphokinase increased | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| Rash | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| Urticaria | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
| IRR | 5 (22.7) | 7 (15.9) | 2 (18.2) | 14 (18.2) |
| Grade ≥3 IRR | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (1.3) |
TEAE was defined as an AE that had an onset date or a worsening in severity from baseline (pretreatment) or on or after the first dose of study drug up to 30 days after study drug discontinuation or initiation of a new anticancer therapy.
TEAEs also included all irAEs and drug-related serious AEs recorded up to 90 days after the last dose of study drug, regardless of whether or not the patient started a new anticancer therapy.
The worsening of an AE to grade 5 beyond day 30 after the last dose of study treatment was also considered a TEAE (if it occurred before the start of a new anticancer therapy). TRAEs included those events considered by the investigator to be related to drug treatment or with missing assessment of the causal relationship.
Patients with multiple events for a given preferred term are counted only once for each preferred term.
TRAEs were reported in 74.0% with grade ≥3 TRAEs in 22.1% (grade 3, 16.9%; grade 4, 5.2%; no grade 5). Most frequent grade ≥3 TRAEs are shown in Table 4. Sixteen patients (20.8%) had serious TRAEs. No TRAEs led to death. Five patients (6.5%) experienced TRAEs leading to treatment discontinuation and 18 (23.4%) experienced TRAEs leading to dose modification.
Twenty-two (28.6%) patients experienced irAEs, including 4 (5.2%) with grade ≥3 (grade 3, 3.9%; grade 4, 1.3%; and no grade 5). The most frequent (≥5% patients) irAEs were hypothyroidism (10.4%) and hyperglycemia (5.2%). Grade ≥3 irAEs included increased blood creatine phosphokinase, hepatitis, hypothyroidism, rash, and urticaria (1 patient [1.3%] each). Two patients (2.6%) experienced irAEs leading to treatment discontinuation. No patients required treatment interruption for irAEs; and dose delays were required in 2 patients (2.6%). No irAEs led to death.
Fourteen patients (18.2%) experienced IRRs, with grade ≥3 in only 1 patient (grade 3 pyrexia). The most frequent IRRs (≥5% patients) were pyrexia (11.7%) and pruritus (5.2%).
Tumor flares (all grade 2 and in the first treatment cycle) occurred in 3 patients (3.9%) with PTCL-NOS, AITL, or SS (1 patient each subtype). The patient with PTCL-NOS was treated with systemic corticosteroids plus antihistamines and recovered in 7 days; the patient with AITL received analgesics only and recovered in 18 days; and the patient with SS received systemic corticosteroids and recovered in 4 days. No patients had dose modifications or discontinued treatment due to tumor flare.
Forty patients (51.9%) died during the study, including 20 patients who died because of the disease under study. Sixteen patients (20.8%) died of AEs, including 5 who died of TEAEs not considered treatment related, 6 of non-TEAEs that were not irAEs, and 5 of non-TEAEs occurring after the patient had started the next line of anticancer therapy. No deaths were caused by TRAEs. Three deaths were of indeterminate cause; and 1 death was COVID-19–related.
Subsequent therapies
After discontinuation of tislelizumab treatment, 51.9% of patients received subsequent systemic therapy, and 6.5% received subsequent local treatment. The most frequent subsequent systemic therapies were corticosteroids (27.3%), alkylating agents and related substances (26.0%), and antimetabolites (19.5%). Subsequent treatments are summarized in supplemental Table 2.
Discussion
This study evaluated the efficacy and safety of tislelizumab in patients with R/R mature T- and NK-cell neoplasms. Although the study population showed several high-risk features, including advanced disease in 76.6%, refractory disease in 51.9%, and ≥3 prior systemic regimens in 49.4%, tislelizumab monotherapy had modest antitumor activity in patients with R/R ENKTLs and R/R PTCLs, and promising activity in patients with R/R MF or SS. Tislelizumab was generally well tolerated. There were no unexpected or new safety findings compared with prior experience in hematological malignancies and solid tumors, and the profile of irAEs was consistent with the known profile of tislelizumab.24,29,30
Notably, encouraging efficacy with tislelizumab monotherapy was observed in patients in cohort 3 despite the high percentages of advanced disease, heavy pretreatment, and refractory disease. Compared with other PD-1 inhibitors as therapy for CTCLs, the ORR in this study was numerically higher than that observed with nivolumab (ORR, 15% [2 of 13] without any CRs)31 and comparable with that observed with pembrolizumab (ORR, 38% [9 of 24] with 8.3.% CR);22 however, the total patient number is small, and patient characteristics vary across studies.22,31 Beyond checkpoint inhibitors, ORRs with standard therapies for MF and SS are similar. These include denileukin diftitox (ORR, 30%),12 bexarotene (ORR, 45%),13 vorinostat (ORR, 29.7%),14 romidepsin (ORR, 34%),15 and mogamulizumab (ORR, 28%).16 One exception is that brentuximab vedotin monotherapy showed an outstanding ORR of 65% (31 of 64), with 10% CRs in patients with CD30+ MF with less advanced disease stage (31% IA–IIA, 40% IIB, and 27% IIIA or higher), which is different from that of our study population.32 Results with investigational therapies for R/R MF/SS, including lenalidomide (ORR, 28%),33 lacutamab (ORR, 36.4%),34 and duvelisib (ORR, 31.6%)35 show the difficulty of achieving high ORRs in this setting. The ORR with tislelizumab in this study seems promising compared with other single agents for R/R MF/SS, although differences in study designs and baseline characteristics limit comparisons with other trials. Tumor flare was relatively infrequent and manageable with tislelizumab (3 patients [3.9%]; 1 in each of the PTCL-NOS, AITL, and SS subtypes). Tumor flare was noted in 1 patient with SS, an observation also made in a previous study with pembrolizumab in which more than half of the patients with SS (53%) had a tumor flare after pembrolizumab administration.22 Current findings indicate that tislelizumab is an effective and tolerable treatment for patients with R/R MF or SS, and further studies are warranted.
The efficacy of tislelizumab in ENKTL was more modest in comparison with other PD-1 inhibitors in ENKTL, but responses to tislelizumab were durable. Studies of other PD-1 inhibitors in a limited number of patients with R/R ENKTLs have shown promising responses (ORRs, 33%-100%), but median DOR was short (∼1.3-4.1 months).18-21 The pembrolizumab and nivolumab studies included ∼3 to 7 patients, and both studies evaluating pembrolizumab were retrospective, making it difficult to draw conclusions about the efficacy.18-20 The ORIENT-4 study evaluated sintilimab in 28 patients with R/R ENKTL.21 Patients in the ORIENT-4 study were younger (median age, 37 years), and enrollment was permitted if no CR was achieved after 6 cycles of a prior regimen. These differences may partially explain the relatively higher ORR observed in that study (ORR 75.0%, taking pseudoprogression into account). Tumor assessment schedules also differed. In ORIENT-4, 28% of the responders reached PR at ∼6 weeks (first assessment) and then progressed at ∼15 weeks (second assessment). However, responders with this temporal pattern would have been missed in our study because the first tumor assessment was at 12 weeks. Also, most patients in ORIENT-4 continued sintilimab treatment after progression, at the investigator’s discretion. Interestingly, these patients shifted back and forth between response and progression many times during a long-term period, and these were not cases of commonly defined pseudoprogressions. This means that a response might be achieved after confirmed progression with the long-term use of anti-PD-1. In our study, half of the patients with ENKTL discontinued treatment before or at the first tumor assessment, meaning that a potential later response might have been missed. Another reason for the modest ORR with tislelizumab might be that patients with ENKTL in our study generally had high tumor burden at baseline. This unfavorable feature can be reflected indirectly by the rapid progression occurring in 11 patients from cohort 1 whose disease progressed within the first 3 treatment cycles. In general, conditions of patients with R/R ENKTL progress rapidly, and they have a very poor prognosis. The modest ORR in patients with ENKTL in this study and the short DOR reported in patients with ENKTL receiving other PD-1 inhibitors may reflect the aggressive nature of this tumor and resistance to prior therapies.
In cohort 2, tislelizumab monotherapy showed modest efficacy. Similar results have been observed in other limited studies of PD-1 inhibitors in nodal PTCLs. In the phase 1 nivolumab study, 2 of 5 (40%) patients with PTCL responded, and in the phase 2 pembrolizumab study, the ORR was 1 of 7 (14%) in patients with PTCL-NOS.31,36 In both studies, disease progression was rapid (median PFS ranging from 14 weeks to 3.2 months).31,36 Different PTCL subtypes may have different clinical courses and outcomes; even the same subtype can be heterogeneous (eg, PTCL-NOS is a diverse group of diseases that may respond differentially to therapies). Similarly, without DUSP22 and P63 status of patients with ALCL, the interpretation of the efficacy in this group is difficult, especially because DUSP22-rearranged ALCL is PD-L1 negative.37,38 Further understanding of the characteristics of PTCLs and specific characteristics associated with benefits from PD-1 blockade should be the focus of future studies.
For these aggressive diseases, future studies should explore anti-PD-(L)1 combined with another therapeutic modality. Currently, several ongoing studies in mature T- and NK-cell neoplasms are testing PD-(L)1 antibodies combined with various agents, including chidamide, copanlisib, romidepsin, brentuximab vedotin, decitabine, and pralatrexate (data from clinicaltrial.gov). In addition, preliminary results from the ongoing NIVEAU study of nivolumab in combination with gemcitabine and oxaliplatin (± rituximab) show promising efficacy and safety profiles in patients with R/R PTCL.39 The final results of these studies and optimized combinations are anticipated in the future and will aid treatment decisions and hopefully improve patient outcomes in R/R T-cell lymphomas.
Our study has several limitations. The cohort sizes are small, particularly for some subgroups, with information missing on biologic and clinical features that may inform patients’ baseline risk, such as nasal type and extranasal type for patients with ENKTL. Similarly, the encouraging efficacy with tislelizumab monotherapy in cohort 3 was based on a sample size of 11 patients; therefore, further study in a larger patient population is necessary to confirm these findings. Given disease heterogeneity, a deeper understanding of the effects of PD-1 blockade in mature T- and NK-cell neoplasms is also warranted to identify patients who will benefit most from anti–PD-1 immunotherapy. Another limitation is that in the cases of rapid progression observed in our study, we cannot differentiate hyperprogression due to immunotherapy from rapid progression due to the disease itself because tumor growth rate data during prior regimens were not collected. Previous research has raised concerns regarding a potential association with PD-1 inhibition and rapid progression in some T-cell lymphomas.40,41 In animal models, PD-1 inhibition was shown to accelerate and/or reactivate T-cell clones and lead to unrestricted T-cell growth after oncogenic signals, causing the rapid development of aggressive tumors.40 In humans, rapid disease progression was observed after nivolumab treatment in 3 patients with adult T-cell leukemia-lymphoma.41 Although it is more likely that disease severity and high tumor burden at baseline led to rapid progression in some patients from cohort 1 and cohort 2, the contribution of PD-1 inhibition to rapid disease progression cannot be completely discounted. Further studies to understand the possibility of hyperprogression would be worthwhile.
In conclusion, tislelizumab demonstrated encouraging efficacy in patients with R/R MF or SS and modest efficacy in patients with R/R ENKTL and those with R/R PTCLs. Safety and tolerability were acceptable. Further studies are warranted to determine the biologic features associated with response and to identify optimal combination therapies.
Acknowledgments
The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers.
This study was supported by research funding from BeiGene Co., Ltd, who confirmed the accuracy of the data and compiled the data for analysis. Medical editing support was funded by BeiGene and provided by Twist Medical, LLC.
Authorship
Contribution: E.B., K.J.S., H.H., Y.L.K., P.L.Z., J.P., and W.N. were involved in concept and desing formulation; S.H. drafted the manuscript; W.Z. analyzed the data; and all authors had access to the data, carried out data acquisition, analysis, or interpretation, and gave approval of final manuscript for submission.
Conflict-of-interest disclosure: E.B. reports serving on advisory boards for Roche, Takeda, Bristol Myers Squibb (BMS), and Incyte, and honoraria from Novartis, Kite/Gilead, and Roche. K.J.S. reports honoraria/consulting from Seattle Genetics, Merck, BMS, Janssen, Kyowa, AstraZeneca, Novartis, and Incyte, and serves on steering committees for BeiGene, and Data and Safety Monitoring Committees for Regeneron. Y.L.K. reports consultancy for Amgen, Astellas, Bayer, BeiGene, BMS, Celgene, Janssen, Merck, Novartis, Roche, and Takeda, and receives educational funds from Novartis. G.G. reports consulting/advisory fees from Takeda, Gilead Sciences, IQVIA, Clinigen Group, Roche, and Italfarmaco; receives travel expenses from Janssen and Gilead Sciences; and serves on speakers’ bureaus for Amgen and Roche. A.M.L. reports sponsored research support related to this publication from BeiGene to her institution; sponsored research to her institution from Takeda, Servier, Roche, Celgene, AbbVie, Incyte, Janssen, Sanofi, Verastem, Novartis, MorphoSys, GlaxoSmithKline, Oncopeptides, Karyopharm, Onconova, Archigen, Pfizer, and Fibrogen; honoraria from IQVIA, Servier, Celgene, AbbVie, BMS, and Janssen; travel expenses from Takeda, Roche, Janssen, Celgene, BMS, AbbVie, Novartis, Sanofi, IQVIA, and Verastem; and participation in Amgen and Servier data safety monitoring or advisory boards. A.J.M.F. reports speaker fees from Gilead and Roche; serves on advisory boards for Gilead, Juno, Novartis, PletixaPharm, and Roche; and receives research grants from ADC Therapeutics, Bayer HealthCare Pharmaceuticals, BeiGene, BMS, Genmab, Gilead, Hutchison Medipharma, Incyte, Janssen Research & Development, MEI Pharma, Novartis, PletixaPharm, Pharmacyclics, Protherics PLC, Roche, and Takeda. S.H., X.L., H.Y., J.P., W.N., W.Z., and H.Z. are employees of and own stock in BeiGene, Inc. P.L.Z. reports consultancy roles for MSD, Eusapharma, and Novartis; serves on speakers bureaus for Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte, and BeiGene; and has served on advisory boards for Secura Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte, and BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Pier Luigi Zinzani, Institute of Hematology “Seràgnoli,” University of Bologna, via Massarenti 9, 40138 Bologna, Italy; e-mail: pierluigi.zinzani@unibo.it.
References
Author notes
Individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated are available on request from BeiGene (datadisclosure@beigene.com).
Data availability will be subject to certain criteria, conditions, and exceptions.
The full-text version of this article contains a data supplement.