Key Points
Compared with full-MTX, mini-MTX/MMF was associated with no significant differences in GVHD.
Mini-MTX was associated with faster engraftment, less mucositis, organ toxicities, and NRM.
Abstract
Tacrolimus (Tac)/methotrexate (MTX) is standard graft-versus-host disease (GVHD) prophylaxis; however, is associated with several toxicities. Tac, reduced-dose MTX (mini-MTX), and mycophenolate mofetil (MMF) have been used but never compared with standard MTX. We performed a randomized trial comparing Tac/MTX (full-MTX) with Tac/mini-MTX/MMF (mini-MTX/MMF) for GVHD prevention after allogeneic hematopoietic cell transplantation (HCT). Patients (pts) receiving first myeloablative HCT using an 8/8 HLA-matched donor were eligible. Primary end points were incidence of acute GVHD (aGVHD), mucositis, and engraftment. Secondary end points included chronic GVHD (cGVHD), organ toxicity, infection, relapse, nonrelapse mortality (NRM), and overall survival (OS). Ninety-six pts were randomly assigned to full-MTX (N = 49) or mini-MTX (N = 47). The majority (86%) used bone marrow grafts. There was no significant difference in grade 2-4 aGVHD (28% mini-MTX/MMF vs 27% full-MTX; P = .41); however higher incidence of grade 3-4 aGVHD (13% vs 4%; P = .07) with mini-MTX/MMF. Pts receiving mini-MTX/MMF had lower grade 3 or 4 mucositis and faster engraftment. There were no differences in moderate-to-severe cGVHD at 1 year or infections. Pts receiving mini-MTX/MMF experienced less nephrotoxicity and respiratory failure. There was no difference in the 1-year relapse (19% vs 21%; P = .89) and OS (72% vs 71%; P = .08), and mini-MTX/MMF was associated with lower but nonsignificant NRM (11% vs 22%; P = .06). Compared with full-MTX, mini-MTX/MMF was associated with no difference in grade 2-4 aGVHD and a more favorable toxicity profile. The higher severe aGVHD warrants further study to optimize this regimen. The trial was registered at www.clinicaltrials.gov as #NCT01951885.
Introduction
Allogeneic hematopoietic cell transplant (HCT) is an effective therapy for high-risk hematologic malignancies and bone marrow (BM) failure syndromes; however it is associated with a significant risk for transplant-related mortality, related largely to graft-versus-host disease (GVHD), infection, and end organ damage. The success of transplant is, thus, significantly influenced by the prevention of GVHD without relapse of underlying disease.
The use of a calcineurin inhibitor, most commonly tacrolimus (Tac), in combination with methotrexate (MTX), has been a standard practice over the past 3 decades for GVHD prevention. This approach, however, is associated with several toxicities; although several alternative approaches to GVHD prevention have been investigated, they have all failed to significantly improve transplant outcomes.1,2 Therefore, Tac/MTX has remained the most common standard of care for acute GVHD (aGVHD) prophylaxis.3 MTX has a long history of use as a component of GVHD prophylaxis agents in HCT. Initially used as a single agent, it was found to be complementary in combination with calcineurin inhibitors. As a result of severe mucositis and other toxicities, doses of MTX must often be held, which may further decrease the efficacy of GVHD prophylaxis.4 Although standard doses of MTX are typically 15 mg/m2 on day +1, followed by 10 mg/m2 on days +3, +6, and +11, reduced doses of MTX (5 mg/m2 on days +1, +3, and +6) have also been investigated in an effort to reduce the associated mucosal and hepatic complications.5,6 These studies demonstrated the safety and efficacy of the regimen in HLA-matched and 1 antigen–mismatched unrelated donors, with an incidence of grade 2 or 4 aGVHD from 33% to 59% and grade 3 or 4 acute GVHD of 17%. Tac and reduced-dose MTX (mini-MTX) have also been studied in combination with a third agent.7,8 Mizumoto et al used a combination of Tac, mini-MTX, and mycophenolate mofetil (MMF) as GVHD prophylaxis in reduced intensity transplants and demonstrated a safe and well tolerated profile with a low incidence of severe grade 3 or 4 aGVHD (5%). Although these studies have demonstrated the safety and efficacy of these regimens, there have been no studies directly comparing mini-MTX with standard dose MTX–based GVHD prophylactic regimens. We, therefore, undertook this randomized prospective trial comparing standard dose Tac/MTX with Tac, mini-MTX, and MMF.
Methods
Study design
This was an open-label, phase 3, prospective randomized trial conducted at a single center designed to test 2 GVHD prevention strategies: Tac/MTX (full-MTX) vs Tac, mini-MTX and MMF (mini-MTX/MMF) after myeloablative HLA-matched allogeneic transplants. The target enrollment was 100 participants. The primary end points were the incidence of aGVHD by day 100, including incidence and severity of grade 2 or 4 and grade 3 or 4 aGVHD, incidence and severity of mucositis during transplant hospitalization on day 28, and incidence and timing of neutrophil and platelet engraftment by day 28. Prespecified secondary end points included incidence and severity of chronic GVHD (cGVHD) at 6 and 12 months, incidence of infection, hepatotoxicity, nephrotoxicity during the first 100 days and pulmonary toxicity during the first 180 days, length of hospitalization, use of total parenteral nutrition within 100 days, length of time on and cumulative doses of continuous infusion narcotics by day 28, 1-year incidence of relapse, nonrelapse mortality (NRM), and overall survival (OS). Given the adequate follow-up time, we also report 2-year incidence of relapse, NRM, and OS in post hoc analyses. Post hoc analyses also included 1-year GVHD relapse–free survival (GRFS), defined as survival without grade 3 or 4 aGVHD, cGVHD requiring immunosuppression, or disease relapse.9
Enrollment began on 21 May 2014 and ended on 7 July 2020, and all participants were followed up with for at least 1 year after HCT. The trial was clinically registered as NCT01951885. It was approved by the Case Comprehensive Cancer Center Protocol Review and Monitoring Committee and the Cleveland Clinic Institutional Review Board. All participants gave written informed consent before enrollment. The study was conducted in accordance with the Declaration of Helsinki. The authors had access to and reviewed clinical trial results.
Participants
Eligible participants were aged <70 years undergoing first myeloablative allogeneic transplant for acute leukemia in remission, myelodysplastic syndromes, chronic myeloid leukemia in chronic phase, myeloproliferative neoplasm, non-Hodgkins lymphoma, Hodgkins lymphoma, or multiple myeloma. All participants had an HLA-matched sibling or unrelated donor, defined as HLA-A, -B, -C, and -DRB1 high resolution molecular typing, meeting institutional guidelines for donation. Patients should have had an adequate performance status and met institutional criteria for the transplant. Exclusion criteria included prior allogeneic or autologous transplant, HIV or other uncontrolled active infection, and pregnancy or lactation in females.
Treatment
Participants received pretransplant myeloablative conditioning with busulfan (12.8 mg/kg, IV) and cyclophosphamide (Cy; 120 mg/kg); total body irradiation (TBI) (1320 cGy) and etoposide (60 mg/kg); or TBI (1200 cGy) and Cy (120 mg/kg). Busulfan pharmacokinetics were performed targeting a daily area under the curve of 5000 μmol-min/L, per institutional standard of practice, which began in April 2018. GVHD prophylaxis consisted of full-MTX or mini-MTX. In both groups, Tac was given at 0.03 mg/kg per day IV beginning on day −1. Tac levels were obtained to maintain a recommended target serum level between 5 and 15 mg/mL, per institutional guidelines. Dose adjustments of Tac were based on clinical judgment of the treating physician after considering clinical toxicity, serum levels, GVHD, concomitant drug use, and rate of rise or decline of serum level. In the absence of GVHD, Tac was tapered at approximately day +100 per the investigator’s discretion. In the full-MTX group, MTX was given at 15 mg/m2 IV on day +1, followed by 10 mg/m2 on days +3, +6, and +11. In the mini-MTX/MMF arm, MTX was given at 5 mg/m2 IV on day +1, +3, and +6. Dose reductions or held doses of MTX were per institutional guidelines for renal and liver toxicities and per the treating physician’s discretion for severe mucositis. MMF was administered orally on day +1 at 1000 mg, twice daily. For patients who weighed <40 kg, MMF was administered at 15 mg/kg, 3 times a day. MMF was discontinued approximately between day +36 and +45 in the absence of GVHD, per institutional standard. Posttransplant supportive care was provided per institutional standards, including routine use of granulocyte colony–stimulating factor.
Outcome assessments and definitions
aGVHD and cGVHD was graded prospectively, but for analysis, all GVHD events were reviewed retrospectively and graded as per MAGIC10 and National Institute of Health consensus criteria,11 respectively. Oropharyngeal mucositis was scored 3 times a week by the transplant provider from day 0 to day +28 or to discharge from transplant hospitalization (whichever occurred first), according to the World Health Organization Mucositis grading scale: grade 0, no mucositis; grade 1, soreness ± erythema and ability to eat and swallow solids; grade 2, erythema, ulcers, and ability to eat solids; grade 3, ulcers and inability to eat solids (required liquid diet); grade 4, alimentation not possible. Neutrophil engraftment was defined as the first of 3 consecutive measurements, with an absolute neutrophil count of ≥500 cells/μL. Platelet engraftment was defined as the first of 3 consecutive measurements, with a platelet count of ≥20 000/μL without transfusion of platelets in the preceding 72 hours. Disease risk was defined by the disease risk index developed by Armand et al.12 Hepatotoxicity was measured as incidence of veno-occlusive disease and maximum values of bilirubin, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase. Nephrotoxicity was measured based on the need for renal replacement therapy and maximum values of creatinine. Pulmonary toxicity was measured as the change in FEV1 from pretransplant period to day 100, any Common Terminology Criteria for Adverse Events grade ≥3 pulmonary edema, bronchopulmonary hemorrhage, and grade ≥4 respiratory failure.
Statistical analysis
The primary analysis aimed to demonstrate a reduction in incidence of severe mucositis and time to hematopoietic engraftment while not increasing the incidence or severity of aGVHD. The study was thus powered on these primary end points of GVHD, mucositis, and engraftment. Sample size calculations were based on 80% power and P = .05 significance level using a 1-sided test. Based on institutional data, the rate of severe mucositis with standard full-MTX was estimated to be 41%. A sample size of 94 (47 in each arm) was estimated to demonstrate a 25% improvement in mucositis to 16%. Using the same methods, a sample size of 100 patients (50 in each group) was calculated to demonstrate a 4-day improvement in time to neutrophil engraftment (based on the current estimate of 19 days), and a sample size of 98 (49 in each group) was needed to demonstrate a 25% improvement in platelet engraftment (current estimate, 26%). Based on our institutional incidence rates of aGVHD of 62%, 45 patients per arm were needed for the detection of a hazard ratio (HR) of ≤1.7% or ≤25% bound, using a noninferiority log-rank test with 5% significance and 80% power. Therefore, a sample size of 100 patients was proposed to have adequate power for all primary analyses. Patients were randomly assigned to 2 treatment groups using block-randomization method with 3 stratification factors, including TBI in the preparative regimen (yes or no), donor relationship (related or unrelated), and source of hematopoietic cells (peripheral blood stem cells [PBSCs] or marrow). The randomization was examined using 2-sample Wilcoxon test and χ2 test. Mucositis and engraftment were compared between the 2 arms using χ2 test and 2-sample Wilcoxon rank test. The median time to neutrophil and platelet engraftment were also analyzed using the Kaplan-Meier method and compared using the long-rank test. aGVHD and cGVHD, relapse, and NRM were estimated using cumulative incidence with competing risk and compared using the Gray test. OS, progression-free survival, and GRFS were estimated using the Kaplan-Meier method and compared between patients receiving full-MTX and those receiving mini-MTX/MMF using the log-rank test. Hospital stay was compared among groups using the Wilcoxon rank sum test. Total parenteral nutrition use and 100-day incidence of complications were compared using the χ2 test and Wilcoxon rank test. Two-sided P-values are presented, and P < .05 is considered as statistically significant. All statistical analyses were performed using Statistical Analysis System software (version 9.4).
Results
Patient characteristics
A total of 101 patients were enrolled and randomly assigned; 5 patients were excluded from analysis because of change in eligibility or withdrawal of consent before HCT procedure (supplemental Figure 1). The final analysis was based on data from 96 patients who were randomly assigned to receive mini-MTX/MMF (N = 47) or full-MTX (N = 49). The median follow-up period after transplant was 19.9 months (range, 0.5-48 months). Patient, disease, and transplant characteristics are shown in Table 1. The median age at the time of transplantation was similar between the 2 groups, 45 years (range, 2-62) and 47 years (range, 5-59) in mini-MTX/MMF and full-MTX groups, respectively (P = .47). There was a greater proportion of males (64%) than females (36%) in the mini-MTX/MMF cohort, compared with that of 43% males and 57% females in the full-MTX group (P = .04). The majority of patients had an intermediate (1-2) or high (≥3) HCT comorbidity index and had undergone transplantation for acute myeloid leukemia. Although there were slight differences in distribution of HCT comorbidity index and primary malignancy, these were not statistically significant. The cohorts were otherwise generally well-balanced with regards to disease risk, donor type, conditioning, and graft source. All patients in the mini-MTX/MMF arm received their 3 planned doses of MTX. The median number of days for receiving MMF was 57 days (range, 30-727). In the full-MTX arm, 71% received all 4 doses, 26% received 3 doses, and 1 patient received 2 doses of MTX. No additional immunosuppression was administered for omitted doses of MTX.
Patient, disease, and transplant characteristics
| Variable . | Tac/MTX (N = 49) N (%) . | Tac/mini-MTX/MMF (N = 47) N (%) . | P value . |
|---|---|---|---|
| Age at transplant, y | |||
| Median (range) | 47 (5-59) | 45 (2-62) | .47 |
| Sex | |||
| Male | 21 (42.9) | 30 (63.8) | .04 |
| Female | 28 (57.1) | 17 (36.2) | |
| Race | |||
| White | 48 (98.0) | 47 (100.0) | .32 |
| Black | 1 (2.0) | 0 (0.0) | |
| HCT-CI | |||
| Low | 4 (8.2) | 8 (17) | .30 |
| Intermediate | 19 (38.8) | 20 (42.6) | |
| High | 26 (53.1) | 19 (40.4) | |
| Diagnosis | |||
| AML | 30 (61.2) | 19 (40.4) | .14 |
| ALL | 6 (12.2) | 8 (17.0) | |
| MDS | 3 (6.1) | 8 (17.0) | |
| CML | 3 (6.1) | 6 (12.8) | |
| MPN | 2 (4.1) | 4 (8.5) | |
| NHL | 2 (4.1) | 2 (4.3) | |
| Other (acute leukemia) | 3 (6.1) | 0 (0.0) | |
| Disease risk index | |||
| Low | 9 (18.4) | 14 (29.8) | .46 |
| Intermediate | 27 (55.1) | 22 (46.8) | |
| High | 13 (26.5) | 11 (23.4) | |
| Donor | |||
| Matched unrelated | 38 (77.6) | 34 (72.3) | .56 |
| Matched sibling | 11 (22.4) | 13 (27.7) | |
| Conditioning | |||
| Bu/Cy | 40 (81.6) | 39 (83.0) | .54 |
| TBI/VP | 9 (18.4) | 7 (14.9) | |
| Cy/TBI | 0 (0.0) | 1 (2.1) | |
| Graft source | |||
| BM | 44 (89.8) | 39 (83.0) | .33 |
| Peripheral blood | 5 (10.2) | 8 (17.0) | |
| Donor-recipient sex | |||
| From F to M | 5 (10.4) | 8 (17.8) | .10 |
| Baseline CMV status | |||
| D+/R+ | 13 (26.5) | 6 (12.8) | .17 |
| D+/R– | 4 (8.2) | 5 910.6) | |
| D–/R+ | 24 (49.0) | 21 (44.7) | |
| D–/R– | 8(16.3) | 15 (31.9) | |
| MTX doses | |||
| 2 | 1 (2.0) | 0 (0.0) | < .001 |
| 3 | 13 (26.5) | 47 (100.0) | |
| 4 | 35 (71.4) | -- |
| Variable . | Tac/MTX (N = 49) N (%) . | Tac/mini-MTX/MMF (N = 47) N (%) . | P value . |
|---|---|---|---|
| Age at transplant, y | |||
| Median (range) | 47 (5-59) | 45 (2-62) | .47 |
| Sex | |||
| Male | 21 (42.9) | 30 (63.8) | .04 |
| Female | 28 (57.1) | 17 (36.2) | |
| Race | |||
| White | 48 (98.0) | 47 (100.0) | .32 |
| Black | 1 (2.0) | 0 (0.0) | |
| HCT-CI | |||
| Low | 4 (8.2) | 8 (17) | .30 |
| Intermediate | 19 (38.8) | 20 (42.6) | |
| High | 26 (53.1) | 19 (40.4) | |
| Diagnosis | |||
| AML | 30 (61.2) | 19 (40.4) | .14 |
| ALL | 6 (12.2) | 8 (17.0) | |
| MDS | 3 (6.1) | 8 (17.0) | |
| CML | 3 (6.1) | 6 (12.8) | |
| MPN | 2 (4.1) | 4 (8.5) | |
| NHL | 2 (4.1) | 2 (4.3) | |
| Other (acute leukemia) | 3 (6.1) | 0 (0.0) | |
| Disease risk index | |||
| Low | 9 (18.4) | 14 (29.8) | .46 |
| Intermediate | 27 (55.1) | 22 (46.8) | |
| High | 13 (26.5) | 11 (23.4) | |
| Donor | |||
| Matched unrelated | 38 (77.6) | 34 (72.3) | .56 |
| Matched sibling | 11 (22.4) | 13 (27.7) | |
| Conditioning | |||
| Bu/Cy | 40 (81.6) | 39 (83.0) | .54 |
| TBI/VP | 9 (18.4) | 7 (14.9) | |
| Cy/TBI | 0 (0.0) | 1 (2.1) | |
| Graft source | |||
| BM | 44 (89.8) | 39 (83.0) | .33 |
| Peripheral blood | 5 (10.2) | 8 (17.0) | |
| Donor-recipient sex | |||
| From F to M | 5 (10.4) | 8 (17.8) | .10 |
| Baseline CMV status | |||
| D+/R+ | 13 (26.5) | 6 (12.8) | .17 |
| D+/R– | 4 (8.2) | 5 910.6) | |
| D–/R+ | 24 (49.0) | 21 (44.7) | |
| D–/R– | 8(16.3) | 15 (31.9) | |
| MTX doses | |||
| 2 | 1 (2.0) | 0 (0.0) | < .001 |
| 3 | 13 (26.5) | 47 (100.0) | |
| 4 | 35 (71.4) | -- |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; Bu/Cy, busulfan and Cy; CML, chronic myeloid leukemia; CMV, cytomegalovirus; D, donor; F, female; HCT-CI, HCT comorbidity index; M, male; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; R, recipient; TBI/VP, TBI with etoposide.
GVHD outcomes
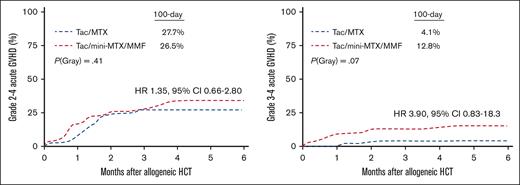
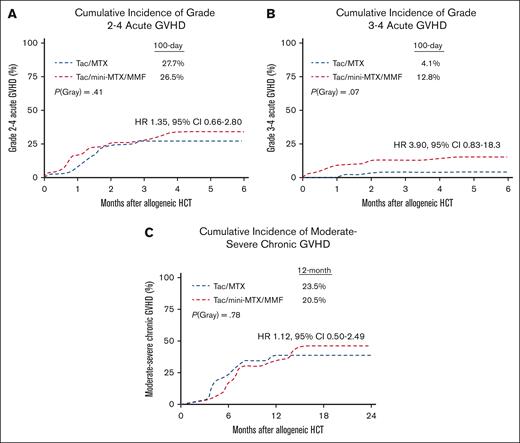
The cumulative incidence of grade 2 or 4 aGVHD by day 100 was 28% in the mini-MTX/MMF vs 27% in the full-MTX arm, (HR 1.35; 95% confidence interval [CI] 0.66-2.80; P = .41; Figure 1A). There was higher incidence of grade 3 or 4 aGVHD with mini-MTX/MMF, that is, 13% compared with 4% in the full-MTX group; however, this did not reach statistical significance (HR 3.90; 95% CI, 0.83-18.3; P = .07; Figure 1B). In the mini-MTX arm, 5 patients developed grade 4, and 2 patients developed grade 3 aGVHD primarily involving the gastrointestinal tract and/or liver. There were no significant differences between the 2 arms in the 1-year incidence of any cGVHD (36% in mini-MTX/MMF vs 25% in full-MTX; P = .09). There was also no difference in moderate-to-severe cGVHD at 1 year (24% in mini-MTX/MMF vs 21% in full-MTX), with an HR of 1.80, 95% CI from 0.82 to 3.94, and P = .08. There was an increasing incidence of moderate-to-severe cGVHD in the mini-MTX/MMF cohort at 32% vs 21% in the full-MTX arm at 2 years (Figure 1C).
GVHD outcomes. (A) Cumulative incidence of grade 2 or 4 aGVHD. (B) Cumulative incidence of grade 3 or 4 aGVHD. (C) Cumulative incidence of moderate-to-severe cGVHD.
GVHD outcomes. (A) Cumulative incidence of grade 2 or 4 aGVHD. (B) Cumulative incidence of grade 3 or 4 aGVHD. (C) Cumulative incidence of moderate-to-severe cGVHD.
Engraftment and mucositis
One patient failed to achieve engraftment in the full-MTX group. Among patients who achieved engraftment, mini-MTX/MMF recipients had faster engraftment of both neutrophils (median, 15 days; 95% CI, 14-16 vs median, 17 days; 95% CI 16-19; P < .001) and platelets (median, 23 days; 95% CI, 20-26 vs median, 28 days; 95% CI, 26-33; P = .01). In all patients, the cumulative incidence of neutrophil and platelet engraftment by day 28 was 100% vs 96% (P < .001) and 72% vs 53% (P = .002), respectively. This translated into significantly shorter length of transplant-related hospital stay with mini-MTX/MMF than that with full-MTX (median, 27 days; inter quartile range [IQR], 23-30 vs median, 31 days; IQR 28-37; P < .001).
For mucositis, patients receiving mini-MTX/MMF had significantly lower grade 3 or 4 mucositis (N = 27, 57%) than those receiving full-MTX (N = 40, 82%; P = .01) per World Health Organization criteria. The duration of mucositis was also significantly less with mini-MTX/MMF than that with full-MTX (median, 11 days vs 18 days; P < .001). Despite this, the use of patient-controlled analgesia was not statistically different (N = 18, 38%) for mini-MTX/MMF group and for full-MTX (N = 20, 41%). However, the total narcotic usage based on morphine equivalents was lower in the mini-MTX/MMF group (893.2 mg; IQR, 210.8-14525.5) than in full-MTX group (3230.4 mg; IQR, 191.0-14173.2), although it did not reach statistical significance (P = .80).
Infection and organ toxicities
For secondary end points, there was no difference in the incidence of any infections between the 2 groups (HR, 0.90; 95% CI, 0.57-1.41; P = .63). Specifically, the incidence of bacterial infections was lower with mini-MTX/MMF, although not statistically significant (34% vs 53%; P = .18), whereas fungal infections (2% vs 4%), viral infections (28% vs 20%), and cytomegaloviral infection requiring treatment (13% vs 18%) were similar with mini-MTX/MMF vs full-MTX, respectively.
With regard to hepatotoxicity within the first 100 days, there was no difference in the incidence of veno-occlusive disease (VOD; n = 5 [11%]) in the mini-MTX/MMF arm compared with that in the full-MTX arm (n = 4, 8%) (P = .18). There were also no differences in maximum median level of bilirubin (1.1; range, 0.7-1.8 vs 1.5; range, 0.8-3.2), alanine aminotransferase (median, 82; range, 54-179 vs median, 91; range, 54-170), and alkaline phosphatase (median, 131; range, 92-209 vs median, 135; range, 101-207) between the groups. There was a lower median maximum level of aspartate aminotransferase in the mini-MTX/MMF arm than that in the full-MTX (median, 82; range, 54-179 vs median, 91; range, 54-170; P = .023). With regard to nephrotoxicity, there was no difference in renal failure requiring dialysis between the groups, with n = 2 (4%) in mini-MTX/MMF arm compared with n = 6 (12%) in the full-MTX arm (P = .16). However, there was significantly lower maximum creatinine (median, 1.26; IQR, 1.00-1.63) in the mini-MTX/MMF arm than in the full-MTX arm (median, 1.50; IQR, 1.11-2.39; P = .040). There was also a significantly smaller number of patients in the mini-MTX/MMF arm (n = 1 [2%]) experiencing a maximum creatinine level ≥3 times the upper limit range of normal than in the full-MTX arm (n = 13 [22%]; P < .001). Lastly, we evaluated the pulmonary toxicity based on the change in the median FEV1 from pretransplant to day 100 and found no significant differences between groups (median, 1.1 [IQR 0.64-1.13] vs 1.02 [IQR, 0.66-1.14]; P = .74), in mini-MTX/MMF and full-MTX, respectively. However, there was a nonsignificant but lower incidence of respiratory failure requiring intubation in the mini-MTX/MMF group (n = 3 [6%]) than in full-MTX group (n = 9 [18%]; P = .076).
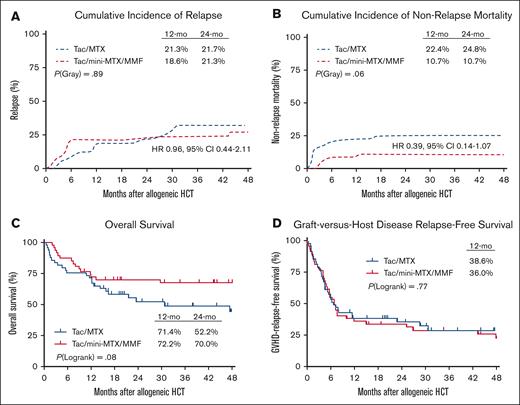
Relapse and survival outcomes
Primary analysis of 1-year outcomes are shown in Figure 2. The duration of follow-up allowed for 2-year post hoc analysis, as described in this study. There was no difference in the cumulative incidence of relapse at 2 years between mini-MTX/MMF (21%) and full-MTX groups (22%) (HR, 1.04; 95% CI, 0.47-2.28; P = .89; Figure 2A). The cumulative incidence of NRM was lower with mini-MTX/MMF (11%) than with full-MTX (25%) (HR, 0.39; 95% CI, 0.14-1.08), although this did not reach statistical significance (P = .06; Figure 2B). Similarly, OS at 2 years was higher with mini-MTX/MMF (70%; 95% CI, 57-83) than with full-MTX (52%; 95% CI, 37%-67%; HR 1.78; 95% CI, 0.93-3.39); however, this also did not reach statistical significance (P = .08; Figure 2C). There was no difference in 1-year GRFS between mini-MTX/MMF (36%; 95% CI, 22%-50%) and full-MTX (39%; 95% CI, 25%-52%; P = .77; Figure 2D). The most common cause of death in both arms was relapse (n = 10 [67% of deaths with mini-MTX/MMF] and n = 12 [50% of deaths with full-MTX]); followed by GVHD (n = 4 with mini-MTX/MMF) and infection (n = 4 with full-MTX); VOD (n = 4 with full-MTX); pulmonary toxicity (n = 2 full-MTX); other organ failure (n = 1 for both mini-MTX/MMF and full-MTX); and graft failure (n = 1 in full-MTX).
Relapse and survival outcomes. (A) Cumulative incidence of relapse. (B) Cumulative incidence of NRM. (C) OS. (D) GRFS.
Relapse and survival outcomes. (A) Cumulative incidence of relapse. (B) Cumulative incidence of NRM. (C) OS. (D) GRFS.
Discussion
In this randomized trial comparing standard full-MTX with mini-MTX/MMF for the prevention of GVHD after matched related and unrelated donor transplant, we demonstrate significant improvement in severe mucositis and end organ toxicities with Tac/mini-MTX/MMF without a significant difference in GVHD outcomes compared to that with full-MTX. In addition, patients who received mini-MTX/MMF achieved engraftment significantly earlier and experienced subsequent decrease in length of hospital stay. Although not reaching statistical significance, this translated into lower NRM and improved the OS in patients receiving mini-MTX/MMF. One-year GRFS was not different between the 2 cohorts, although our study was not powered to detect differences in survival outcomes.
Our 1- and 2-year survival with full-MTX was lower than that reported in the recent BMT CTN 1301 clinical trial but is in line with previous benchmarks,9 which may be related to the use of peripheral blood as a graft source in some patients and a more heterogeneous cohort of patients in regard to disease and comorbidity. We acknowledge a relatively high incidence of VOD and respiratory failure as causes of death in this study. Although reasons for this are not completely clear, ∼65% of the cohort did not have busulfan pharmacokinetic monitoring because this did not become an institutional standard until April 2018, which might have impacted these observed toxicities and outcomes.
Although most patients in our study received all 4 doses of MTX in the full-MTX arm, ∼29% of the patients had at least 1 dose omitted because of toxicities determined by the physician. Although previous studies have demonstrated worse outcomes with omission of MTX doses,4,13,14 we did not find any significant differences in GVHD or survival outcomes in patients who received all 4 doses of MTX compared to those who missed a dose in this study (data not shown). Many centers do administer additional immunosuppression in cases of MTX dose omission; however, we did not add or substitute alternative immunosuppression because this had not been a standard practice at our institution. Given the known toxicities and challenges of MTX administration, many centers have thus empirically reduced doses of MTX, typically between 5 and 10 mg/m2, for 1 or all doses of MTX; however, it is important to note that these mini-MTX regimens have never been directly compared with full-MTX regimens.
We demonstrate a potentially higher risk of severe grade 3 or 4 aGVHD and chronic GVHD beyond 1 year in the mini-MTX/MMF arm compared with that in the full-MTX arm. Despite this, the post hoc analysis demonstrated similar 1-year GRFS in both arms, which was similar to prior studies and benchmarks.9,15 Upon further review of the severe aGVHD cases, the majority of cases were driven by severe lower gastrointestinal GVHD. Of the 7 patients with grade 3 or 4 aGVHD within the mini-MTX group, 5 received some combination of high dose TBI, PBSC grafts and/or transplant from a matched unrelated donor (MUD), all factors previously shown to be associated with both acute and chronic GVHD, particularly with combined effect (2 patients with TBI + PBSC + MUD; 2 with non-TBI + PBSC + MUD; and 1 with TBI + BM + MUD).16,17 Both cases of grade 3 or 4 aGVHD in the full-MTX arm were non-TBI + BM + matched sibling donor. Although the use of TBI and PBSC represented a minority of patients in this study, their inclusion likely had some impact on GVHD outcomes. As several studies have consistently demonstrated favorable outcomes with BM grafts,18,19 this was a preferred graft source for this study and our institution. It is important to acknowledge, however, that PBSCs has increasingly been favored as a graft source within the transplant community.3
The trend of higher grade 3 or 4 aGVHD may also be due to the decreased MTX dose as well as lower MMF dosing. A recent study by Lin et al reported on missed doses of mini-MTX, demonstrating that missed doses were associated with increased cGVHD, although not necessarily acute. Notably, MMF at 3 g per day was typically substituted when MTX doses were omitted.20 Previous studies have evaluated the impact of MMF dosing on GVHD outcomes,21,22 supporting higher weight-based dosing to mitigate aGVHD risk. This study included MMF at a dose of just 2 g per day, which may be insufficient to achieve an adequate mycophenolic acid concentration for optimal GVHD prevention. Because we adopted the mini-MTX/MMF strategy at our institution, we have also increased MMF dosing to a total of 3 g per day.
Despite the increasing use of several novel approaches to GVHD prophylaxis, Tac/MTX remains the most common approach to GVHD prevention in both matched related and unrelated donor transplant. Data from the Center for International Blood and Marrow Transplant Research demonstrated that even in the current era (2018-2020), Tac/MTX is being used in the majority of HLA-matched related (63%) and unrelated (64%) donor transplants.3 To date, there have been no GVHD prevention strategies found to be clearly superior to Tac/MTX in the myeloablative setting. A recent randomized phase 3 study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 1301) comparing calcineurin-free approaches with Tac/MTX treatment, again, did not demonstrate any advantage to CD34+ selection or posttransplant Cy alone compared with standard Tac/MTX.23 This trial reported favorable outcomes with Tac/MTX using the BM, with an OS of 76% at 2 years; confirming the continued relevance of Tac/MTX as the backbone of GVHD prophylaxis.
Whether platforms using posttransplant Cy or novel graft manipulation are superior to MTX-based approaches in the myeloablative and PBSC graft setting remain to be seen and still need investigation in large randomized prospective clinical trials.
This study focused on investigating a GVHD prophylaxis approach to minimize the toxicities of standard MTX dosing in conjunction with calcineurin inhibitor. Although there remains a continued need to optimize GVHD prevention to mitigate severe GVHD and disease relapse, this study demonstrates that a Tac/mini-MTX/MMF regimen is a safe and effective alternative to standard Tac/MTX in myeloablative related and unrelated donor transplant.
Acknowledgments
The authors thank the patients and their families for their participation in this study.
Authorship
Contribution: B.K.H. designed and performed the research, enrolled patients, analyzed data, and wrote the manuscript; L.A.R. and H.L. performed statistical analysis; T.L. and D.C. contributed to data collection and performed research; M.K., R.S., R.H., S.J.R., R.M.D., A.T.G., D.J., and N.S.M. enrolled patients, performed research, contributed to analysis, and reviewed the manuscript; C.B. and C.S.S. contributed to analysis and critically reviewed the manuscript; and E.A.C. designed research, contributed to analysis, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Betty Ky Hamilton, Cleveland Clinic, Taussig Cancer Institute, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: hamiltb2@ccf.org.
References
Author notes
This study was presented at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2021.
Protocol and de-identified participant data that underlie reported results are available, for a period of 5 years after publication date, on request from the corresponding author, Betty K. Hamilton (hamiltb2@ccf.org).
The full-text version of this article contains a data supplement.