Key Points
We examined 13 patients with KHE, a rare vascular tumor with thrombocytopenia and coagulation dysfunction.
Platelet CLEC-2 and GPVI-induced activation is disrupted in patients with KHE because of the tumor activity and can be restored upon treatment.
Abstract
Kaposiform hemangioendothelioma (KHE) is a rare vascular tumor of infancy that is commonly associated with a life-threatening thrombocytopenic condition, Kasabach-Merritt phenomenon (KMP). Platelet CLEC-2, tumor podoplanin interaction is considered the key mechanism of platelet clearance in these patients. Here, we aimed to assess platelet functionality in such patients. Three groups of 6 to 9 children were enrolled: group A with KHE/KMP without hematologic response (HR) to therapy; group B with KHE/KMP with HR; and group C with healthy children. Platelet functionality was assessed by continuous and end point flow cytometry, low-angle light scattering analysis (LaSca), fluorescent microscopy of blood smears, and ex vivo thrombi formation. Platelet integrin activation in response to a combination of CRP (GPVI agonist) and TRAP-6 (PAR1 agonist), as well as calcium mobilization and integrin activation in response to CRP or rhodocytin (CLEC-2 agonist) alone, were significantly diminished in groups A and B. At the same time, platelet responses to ADP with or without TRAP-6 were unaltered. Thrombi formation from collagen in parallel plate flow chambers was also noticeably decreased in groups A and B. In silico analysis of these results predicted diminished amounts of CLEC-2 on the platelet surface of patients, which was further confirmed by immunofluorescence microscopy and flow cytometry. In addition, we also noted a decrease in GPVI levels on platelets from group A. In KHE/KMP, platelet responses induced by CLEC-2 or GPVI activation are impaired because of the diminished number of receptors on the platelet surface. This impairment correlates with the severity of the disease and resolves as the patient recovers.
Introduction
Vascular neoplasms are tumors arising from blood vessel endothelial cells. Kaposiform hemangioendothelioma (KHE) is a rare (incidence 0.07: 100 000 per year1) vascular tumor of infancy with locally aggressive behavior.2 The most common KHE sites are the limbs and the trunk, followed by the head and neck.3 KHE is commonly (71% cases1) associated with Kasabach-Merritt phenomenon (KMP), a potentially life-threatening hematologic complication4 with reported mortality of up to 30%.1 KMP refers to severe thrombocytopenia, consumptive coagulation dysfunction, secondary fibrinogen reduction, and microangiopathic hemolytic anemia.5 Multiple studies of the KHE-associated hereditary factors did not reveal any common reason for anticipating the disease’s onset. There have been reports on mutations in multiple vascular neoplasms6,7 GNAQ genes and pathogenic variants in the PIK3CA gene.8 These mutations are supposedly associated with abnormal VEGF-R activation and downstream PI3K/Akt/mTOR signaling in lymphatic endothelial cells (LECs), which trigger KHE growth.6,8,9 However, the genetic factors are only partially responsible for KHE development.10
Histologically, KHE is composed of infiltrating nodules with slit-like or crescentic vessels lined by spindled endothelial cells.9 When tumor lymphatic vessels are dilated, this is called kaposiform lymphangiomatosis.11 KHE is primarily positive for endothelial markers CD31 and CD34 and lymphatic markers LYVE1, PROX1, and podoplanin.12 KHEs have also been shown to be positive for von Willebrand factor (VWF) in 80% of cases.13 KMP causes platelet trapping and platelet activation within the abnormal tumor vessels, which results in the formation of platelet thrombi and the initiation of plasma coagulation.14 The subsequent hyperactivation of the fibrinolytic system may cause intratumoral hemorrhage.9,15 Unlike disseminated intravascular coagulation, KMP causes localized coagulation disorder.15
KHE cells are known to express the platelet receptor CLEC-2 ligand podoplanin,16 which is needed to ensure the separation of blood and lymphatic circulations.16,17 CLEC-2 also contributes to vascular integrity,18,19 plays a role in thrombosis and wound healing,20,21 and megakaryocyte CLEC-2 is essential for normal platelet production.22 Thus, in patients with podoplanin-expressing tumors, long-term platelet exposure to podoplanin might potentially affect platelet functionality.
KHE/KMP treatment is often based on the introduction of vinca alkaloids, such as vincristine and vinblastine, as the first line of treatment, followed by mTOR inhibitors (eg, sirolimus) as the second line.9,10 Platelet transfusions in patients with KMP can lead not only to rapid tumor growth but also to severe deterioration of the coagulopathy.23,24 Upon activation, platelets secrete granule contents, which can lead to tumor growth and progression,25 that is why antiplatelet agents, such as aspirin or ticlopidine, were recommended as an adjuvant therapy,9 whereas others did not agree with this recommendation.26-28 Therefore, understanding platelet functioning in KHE/KMP is critically important for the therapeutic management of these pathologies.
Here, we characterized blood plasma coagulation parameters, platelet structure, and platelet functional responses in blood samples from 13 pediatric patients with KHE/KMP, including 3 patients assessed several times during therapy. We demonstrated that platelet responses to activation induced by tyrosine kinase–associated receptors (GPVI or CLEC-2) are significantly decreased in samples from patients with KMP. The copy numbers of CLEC-2 as well as GPVI receptors were reduced in these patients, but CLEC-2/GPVI responsiveness can be restored upon treatment.
Methods
Patients and blood collection
Pediatric patients who received treatment at the Dmitry Rogachev National Medical Research Center (Table 1) were enrolled in this study. The key inclusion criteria were the presence of a vascular tumor (KHE or congenital hemangioma) and associated coagulopathy and thrombocytopenia at diagnosis. Patients who received platelet transfusions within 3 weeks before enrollment were not included in the study. Age and treatment history were not exclusion criteria.
Patient characteristics
| Patient characteristics . | Disease characteristics . | Treatment (at enrollment) . | Hematologic parameters (at enrollment/at diagnosis) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age at enrollment (mo) . | Tumor type . | Tumor site . | Age at diagnosis (mo) . | Platelet (×109/L) . | Hb (g/L) . | Fibrinogen (g/L) . | D-dimer (ng/L) . | TB (μmol/L) . | DB (μmol/L) . | |
| 1 (D.)∗ | M | 3 | KHE | Soft tissue of the head and neck | 0 | LD (30 mg/m2), vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 54/5 | 88/75 | 2.6/1.3 | ND | 7.1/177.6 | 3.1/2.3 |
| 2 (K.) | F | 7 | KHE (CD34+, CD31+, PDPN+) | Soft tissue of the head | 0 | LD (30 mg/m2), vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 238/13 | 100/101 | 4.4/1.5 | 487/5325 | 3.3/7.1 | 1.4/2.2 |
| 3 (M.) | M | 17 | KHE | Soft tissue of the forearm | 0 | Sirolimus (1.6 mg/m2), prednisolone (5 mg/kg daily) | 294/36 | 111 /86 | 4.0/1.0 | 158/1619 | 5.7/4.9 | 2.6/1.8 |
| 4 (A.) | M | 11 | KHE (CD34+, CD31+, PDPN+) | Soft tissue of the upper back, thorax | 3 | vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 252/41 | 102/95 | 2.2/1.2 | 117/5423 | 8.2/8.8 | 3.8/3.1 |
| 5 (P.) | M | 7 | KHE (CD34+, CD31+, PDPN+, factor VIII+) | Soft tissue of the left axilla | 0 | LD (30 mg/m2), vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 380/78 | 111/74 | 2.1/1.0 | 138/6697 | 5.4/11.1 | 2.3/8.5 |
| 6 (F.) | M | 18 | Kaposiform lymphangiomatosis (CD34+, CD31+, PDPN+, CD61+) | Soft tissue of neck, upper chest; mediastinum | 13 | LD (30 mg//m2), sirolimus (0.5 mg x 2 daily) | 209/22 | 114/61 | 3.5/2.0 | 156/1069 | 2.1/6.2 | 1.3/0.7 |
| 7 (V.) | F | 36 | KHE (CD34+, CD31+, PDPN+, CD61+, factor VIII+) | Soft tissue of the neck, upper back and chest | 0 | LD (30 mg/m2), vincristine (1.5 mg/m2), atenolol (12.5 mg x 1 daily) | 293/110 | 98/80 | 3.2/0.3 | 718/7412 | 4.7/465.3 | 2.1/15.5 |
| 8 (B.) | F | 1 | KHE | Soft tissue of the right leg, perineum | 0 | prednisolone (2mg/kg daily), propranolol (2 mg/kg daily) | 6/4 | 77/72 | 0.9/0.3 | 5184/9412 | 5.1/7.3 | 4.1/5.5 |
| 9 (Pe.) | M | 5 | KHE | Soft tissue of the thorax | 3.5 | propranolol (0.6 mg/kg daily), LD (30 mg/m2), vinblastine (0.33 mg/m2), cyclophosphamide (50 mg/m2) | 10/3 | 107/75 | 1.0/0.3 | 23 042/52 722 | ND/ND | 1.8/4.1 |
| 10 (G.) | M | 2.5 | Rapidly involuting congenital hemangioma | Soft tissue of the forearm | 2.5 | propranolol (2.5 mg/kg daily) | 126/16 | 97/81 | 1.6/0.9 | 6223/ND | 17.2/328 | 6.2/15.5 |
| 11 (Ki.) | M | 3 | KHE | Soft tissue of the thorax | 3 | propranolol (3.5 mg/kg daily) | 6/6 | 108/88 | 0.8/0.8 | 16 542/16 542 | 8.7/8.7 | ND/ND |
| 12 (Ch.) | F | 3 | KHE | Soft tissue of the thorax | 2.5 | prednisolone (5 mg/kg daily), propranolol (2 mg/kg daily) | 49/10 | 92/75 | 0.2/0.2 | 9835/9835 | ND/ND | 2.8/ND |
| 13 (Ty.) | F | 12 | KHE | Soft tissue of the forearm | 6 | LD (5 mg/m2), vinblastine (1 mg), cyclophosphamide (50 mg/m2) | 295/71 | 107/95 | 2.03/0.2 | 239/8503 | 7.9/8.1 | 2.9/3 |
| Patient characteristics . | Disease characteristics . | Treatment (at enrollment) . | Hematologic parameters (at enrollment/at diagnosis) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age at enrollment (mo) . | Tumor type . | Tumor site . | Age at diagnosis (mo) . | Platelet (×109/L) . | Hb (g/L) . | Fibrinogen (g/L) . | D-dimer (ng/L) . | TB (μmol/L) . | DB (μmol/L) . | |
| 1 (D.)∗ | M | 3 | KHE | Soft tissue of the head and neck | 0 | LD (30 mg/m2), vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 54/5 | 88/75 | 2.6/1.3 | ND | 7.1/177.6 | 3.1/2.3 |
| 2 (K.) | F | 7 | KHE (CD34+, CD31+, PDPN+) | Soft tissue of the head | 0 | LD (30 mg/m2), vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 238/13 | 100/101 | 4.4/1.5 | 487/5325 | 3.3/7.1 | 1.4/2.2 |
| 3 (M.) | M | 17 | KHE | Soft tissue of the forearm | 0 | Sirolimus (1.6 mg/m2), prednisolone (5 mg/kg daily) | 294/36 | 111 /86 | 4.0/1.0 | 158/1619 | 5.7/4.9 | 2.6/1.8 |
| 4 (A.) | M | 11 | KHE (CD34+, CD31+, PDPN+) | Soft tissue of the upper back, thorax | 3 | vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 252/41 | 102/95 | 2.2/1.2 | 117/5423 | 8.2/8.8 | 3.8/3.1 |
| 5 (P.) | M | 7 | KHE (CD34+, CD31+, PDPN+, factor VIII+) | Soft tissue of the left axilla | 0 | LD (30 mg/m2), vinblastine (1 mg/m2), cyclophosphamide (50 mg/m2) | 380/78 | 111/74 | 2.1/1.0 | 138/6697 | 5.4/11.1 | 2.3/8.5 |
| 6 (F.) | M | 18 | Kaposiform lymphangiomatosis (CD34+, CD31+, PDPN+, CD61+) | Soft tissue of neck, upper chest; mediastinum | 13 | LD (30 mg//m2), sirolimus (0.5 mg x 2 daily) | 209/22 | 114/61 | 3.5/2.0 | 156/1069 | 2.1/6.2 | 1.3/0.7 |
| 7 (V.) | F | 36 | KHE (CD34+, CD31+, PDPN+, CD61+, factor VIII+) | Soft tissue of the neck, upper back and chest | 0 | LD (30 mg/m2), vincristine (1.5 mg/m2), atenolol (12.5 mg x 1 daily) | 293/110 | 98/80 | 3.2/0.3 | 718/7412 | 4.7/465.3 | 2.1/15.5 |
| 8 (B.) | F | 1 | KHE | Soft tissue of the right leg, perineum | 0 | prednisolone (2mg/kg daily), propranolol (2 mg/kg daily) | 6/4 | 77/72 | 0.9/0.3 | 5184/9412 | 5.1/7.3 | 4.1/5.5 |
| 9 (Pe.) | M | 5 | KHE | Soft tissue of the thorax | 3.5 | propranolol (0.6 mg/kg daily), LD (30 mg/m2), vinblastine (0.33 mg/m2), cyclophosphamide (50 mg/m2) | 10/3 | 107/75 | 1.0/0.3 | 23 042/52 722 | ND/ND | 1.8/4.1 |
| 10 (G.) | M | 2.5 | Rapidly involuting congenital hemangioma | Soft tissue of the forearm | 2.5 | propranolol (2.5 mg/kg daily) | 126/16 | 97/81 | 1.6/0.9 | 6223/ND | 17.2/328 | 6.2/15.5 |
| 11 (Ki.) | M | 3 | KHE | Soft tissue of the thorax | 3 | propranolol (3.5 mg/kg daily) | 6/6 | 108/88 | 0.8/0.8 | 16 542/16 542 | 8.7/8.7 | ND/ND |
| 12 (Ch.) | F | 3 | KHE | Soft tissue of the thorax | 2.5 | prednisolone (5 mg/kg daily), propranolol (2 mg/kg daily) | 49/10 | 92/75 | 0.2/0.2 | 9835/9835 | ND/ND | 2.8/ND |
| 13 (Ty.) | F | 12 | KHE | Soft tissue of the forearm | 6 | LD (5 mg/m2), vinblastine (1 mg), cyclophosphamide (50 mg/m2) | 295/71 | 107/95 | 2.03/0.2 | 239/8503 | 7.9/8.1 | 2.9/3 |
ALT, alanine aminotransferase; DB, direct bilirubin; Hb, hemoglobin, ND, no data; PDPN, podoplanin; PLT, platelet count.
The capital letters have been introduced to distinguish patients and do not reflect their personal information.
Nine pediatric healthy donors were recruited as controls for this study. The median age of healthy donors was 7 months (range, 0.25-33) overall, with female donors predominating (55%). Neither of the donors nor their parents had any kind of platelet dysfunction or other diseases of a hematologic nature in their history.
At enrollment, 1.6 mL of blood were collected into hirudin-containing S-Monovette tubes, and 4.3 mL of blood were collected into sodium citrate–containing S-Monovette tubes (Monovette, Sarstedt, Newton, NC). Blood samples were processed within 1.5 hours after collection.
The study protocol was approved by the independent ethics committee of the CTP PCP RAS (approval number 3/1-21; date: 5 October 2021). Legal representatives of all participants provided written informed consent before enrollment. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines.
Materials
Flow cytometry
Functional testing of platelets was performed as described earlier.31 Briefly, blood was collected into sodium citrate (3.8% v/v) vacuum tubes and then diluted 20 times by Tyrode’s buffer. Platelets were activated with either 12.5 μM TRAP-6 and 10 μg/mL collagen-related peptide (CRP) or 100 μM TRAP-6, 100 μM AYGPKF, and 5 μM ADP for 10 minutes, then stained with anti-CD42b, anti-CD61, PAC1, anti-CD62p, and Annexin-V for 10 minutes. Alternatively, platelets were incubated with 10 μM mepacrine for 30 minutes. Samples were studied using the NovoCyte Acea flow cytometer (ACEA Biosciences, San Diego, CA). Assays of platelet signaling were performed as described earlier.32 Briefly, platelets were loaded with Fura-Red, mixed with Alexa-488–labeled human fibrinogen, and studied by the BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) in a continuous mode. The Fura-Red MFI Ratio was recalculated into platelet cytosolic calcium concentration using the Grynkiewicz formula.33
Flow cytometry assessment of platelet CLEC-2 and GPVI expression for patients 9, 11, and 13 was performed as described previously.34,35 Briefly, diluted to 150 × 106 platelet (plt)/mL platelet-rich plasma was incubated with anti–CLEC-2 or anti-GPVI antibodies for 10 minutes. After incubation with primary antibodies, Alexa-488–labeled secondary antibodies were added to the suspension and incubated for 10 minutes. After the second incubation, platelets were analyzed by a BD FACS Canto II flow cytometer.
Immunofluorescence microscopy of blood smears
Immunofluorescence labeling of standard air-dried peripheral blood smears with antibodies (Sigma-Aldrich, St Louis, MO) against glycoprotein (GP) Ib, GPIIb, VWF, P-selectin, lysosomal-associated membrane protein (LAMP)-1, LAMP-2, LAMP-3, CLEC-2, GP-VI, nonmuscle myosin IIA (NMMIIa), and β1-tubulin, was performed as described by A. Greinacher and others.36 The relative amounts of CLEC-2 and GPVI receptors were assessed in the following manner: platelets were identified using ilastic software (https://www.ilastik.org/) and protein expression was assessed as the antibody-bound dye intensity over a circle with a radius of 5 pixels centered in the recognized platelet center.
Ex vivo thrombus growth analysis, thrombodynamics, and LaSca assays
Ex vivo thrombus growth in parallel plate flow chambers with fibrillary collagen at a 100 s-1 shear rate. The analysis was conducted as described previously.32,37 Thrombus growth was observed using a Nikon Eclipse Ti-E inverted microscope (Nikon, Tokyo, Japan). Thrombodynamics assay for registering spatial fibrin clot formation from a tissue factor–covered surface was performed as previously described.38 Low-angle scattering analysis (LaSca) of platelet di-aggregate formation in diluted to 107 plt/mL platelet-rich plasma was performed as described elsewhere.39 The parameters of the tests are given in the supplemental Information.
Computational model construction and integration
Statistical analysis
Flow cytometry data were analyzed by means of FlowJo (https://www.flowjo.com) and Python 3.8. Aggregation data were analyzed using Python 3.8. Statistical analysis of the data was performed by means of Python 3.8. The data were analyzed using the nonparametric Mann-Whitney U test. The significance level was set as 95%.
Results
Patient characteristics, coagulation testing, and platelet morphology
Thirteen pediatric patients (Table 1, 61.5% male, median age 6 months, tumor site was soft tissues of the upper body) were enrolled in the study. The disease has predominantly manifested since birth with a deep thrombocytopenia, and in all cases, with an associated coagulopathy (KMP). Eleven out of 13 patients had KHE; 1 patient (patient 6) had kaposiform lymphangiomatosis; and 1 patient (patient 10) had rapidly involuting congenital hemangioma. In 6 of 13 cases, immunohistochemistry was performed and revealed positive staining for podoplanin, CD31, and CD34. At the time of enrollment, 6 patients were presented with KMP, and others had achieved hematologic response (HR) before enrollment. HR was defined as an adequate platelet count (>100 × 109 plt/L), hemoglobin (>10.0 gm/dL), and fibrinogen (>200 mg/dL) levels. The treatment response was defined as follows: (1) hematologic complete response, platelet count >130 × 109 plt/L without transfusion; and (2) clinical complete response, complete tumor disappearance or small residual vascular tumor displaying lack of proliferation for at least 6 months after treatment discontinuation.42
Patients 1, 2, 4, 5, 9, and 13 received cyclophosphamide and vinblastine with/without liposomal doxorubicin (LD), patient 3 received sirolimus and prednisolone, patient 6 received LD and sirolimus, patient 7 received LD and vincristine, and patients 8 and 12 received prednisolone at enrollment. Six patients also received β-blockers: atenolol (patient 7) or propranolol (patients 8, 9-12).
In thrombodynamic test of blood plasma coagulation, 4 out of 8 patients (patients 2, 3, 6, and 7) demonstrated modest hypercoagulation, patient 1 demonstrated significant hypercoagulation, and patients 4, 5, and 6 demonstrated mild hypocoagulation (supplemental Figure 1). In patient 8, we observed a significant decrease in clot density (supplemental Figure 1E). These data are consistent with the presence of a DIC disseminated intravascular coagulation-like phenotype in patients with KMP (patients 1 and 8) and the previously reported reduction of D-dimer levels upon sirolimus administration.43
Immunofluorescence microscopy of peripheral blood smears was performed for all patients and did not reveal abnormalities in platelet staining for GPIb, GPIIb, vWF, P-selectin, LAMP-1, -2, -3, and NMMIIa in all the patients (data not shown). In patients 4, 5, and 7, abnormal diffuse distribution of platelet β1-tubulin was observed (supplemental Figure 2). This phenomenon could be explained by treatment with tubulin-targeted agents, vincristine, or vinblastine.44
Platelet aggregation is impaired in patients with KHE/KMP
Patients and healthy donors enrolled in our study were mostly <15 months old. This has significantly limited amounts of whole blood that could be safely collected.45,46 Thus, we were unable to use conventional platelet aggregation assays and had to switch to methods that require less sample volume: platelet thrombus growth assays37,47 and platelet low-angle light scattering analysis (LaSca39).
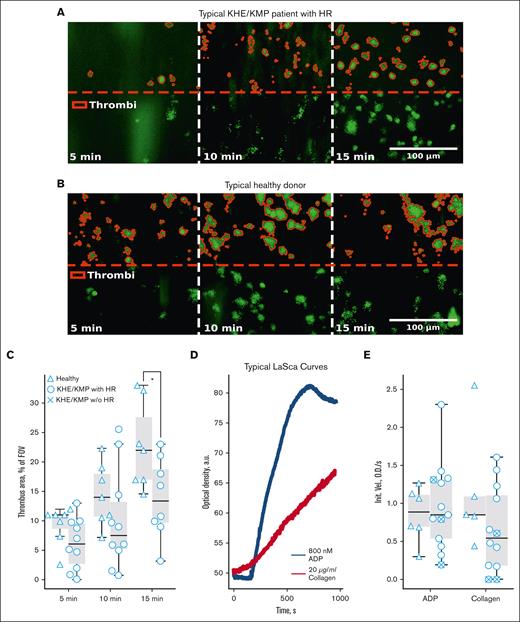
Platelet thrombus growth under flow condition assays is an emerging and attractive option for the characterization of platelet capability to form aggregates.47 Here, we performed ex vivo thrombus growth in collagen-covered parallel-plate flow chambers, as described previously.32,37 Thrombus growth assays were performed only for patients with platelet counts >150 × 106 plt/mL. Typical platelet thrombi images at the fifth, 10th, and 15th minutes for a nonthrombocytopenic patient with KHE/KMP with HR and healthy donor are shown in Figure 1A-B, correspondingly. The percentage of the area covered by platelet thrombi was used as a measure for the quantification of the experimental data. No significant difference between patients with KHE/KMP with HR and healthy controls at the initial stages of thrombi formation (Figure 1C) was present. However, after 15 minutes of blood perfusion, thrombi areas in patients with KHE/KMP with HR were significantly decreased compared with those in healthy controls (Figure 2C).
Platelet thrombus formation and aggregation in KHE/KMP. Analysis of platelets from blood samples from healthy children (triangles) and patients with KHE/KMP with (circles) or without (crosses) HR. (A-B) Typical microscopy images of the growing thrombi of patients with KHE/KMP with HR (A) and healthy donors (B) after 5, 10, and 15 minutes of the whole hirudin-anticoagulated blood perfusion through fibrillar collagen-coated flow chamber. Thrombi (highlighted in red) were identified by DiOC-6 fluorescence (green). (C) Scaled thrombi areas at different time points. (D) Typical aggregation curves for the LaSca assays after activation with 800 nM ADP (black curves) or 20 μg/mL collagen (red curve). (E) Initial velocities of platelet aggregation induced by 800 nM ADP or 20 μg/mL collagen. Statistical significance was calculated using Mann-Whitney U test; ∗ P < .05.
Platelet thrombus formation and aggregation in KHE/KMP. Analysis of platelets from blood samples from healthy children (triangles) and patients with KHE/KMP with (circles) or without (crosses) HR. (A-B) Typical microscopy images of the growing thrombi of patients with KHE/KMP with HR (A) and healthy donors (B) after 5, 10, and 15 minutes of the whole hirudin-anticoagulated blood perfusion through fibrillar collagen-coated flow chamber. Thrombi (highlighted in red) were identified by DiOC-6 fluorescence (green). (C) Scaled thrombi areas at different time points. (D) Typical aggregation curves for the LaSca assays after activation with 800 nM ADP (black curves) or 20 μg/mL collagen (red curve). (E) Initial velocities of platelet aggregation induced by 800 nM ADP or 20 μg/mL collagen. Statistical significance was calculated using Mann-Whitney U test; ∗ P < .05.
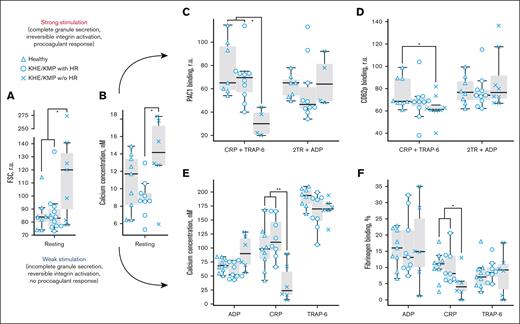
KHE/KMP affects platelet responsiveness to GPVI-mediated activation. Flow cytometry analysis of platelets and designations of groups of patients are the same as in Figure 1. (A-B) characteristics of platelets in resting state: forward light scattering (FSC) indicating platelet size (A) and cytosolic calcium concentration (B). (C-D) platelet responses to strong activation with a combination of 10 μg/mL CRP and 12.5 μM TRAP-6 (CRP + TRAP-6); or 100 μM of TRAP-6, 100 μM of AYGPKF, and 5 μM of ADP (2TR+ADP): platelet PAC-1 binding (C) and CD62p expression (D). (E-F) platelet responses to mild activation with either 2 μM of ADP (ADP), 2 μg/mL CRP (CRP) or 5 μM of TRAP-6 (TRAP-6): cytosolic calcium concentration (H) and fibrinogen binding (I). Statistical significance was calculated by means of Mann-Whitney U test. ∗P < .05; ∗∗P < .01.
KHE/KMP affects platelet responsiveness to GPVI-mediated activation. Flow cytometry analysis of platelets and designations of groups of patients are the same as in Figure 1. (A-B) characteristics of platelets in resting state: forward light scattering (FSC) indicating platelet size (A) and cytosolic calcium concentration (B). (C-D) platelet responses to strong activation with a combination of 10 μg/mL CRP and 12.5 μM TRAP-6 (CRP + TRAP-6); or 100 μM of TRAP-6, 100 μM of AYGPKF, and 5 μM of ADP (2TR+ADP): platelet PAC-1 binding (C) and CD62p expression (D). (E-F) platelet responses to mild activation with either 2 μM of ADP (ADP), 2 μg/mL CRP (CRP) or 5 μM of TRAP-6 (TRAP-6): cytosolic calcium concentration (H) and fibrinogen binding (I). Statistical significance was calculated by means of Mann-Whitney U test. ∗P < .05; ∗∗P < .01.
LaSca allows analyzing platelet aggregate formation by measuring laser beam scattering at different angles, which correspond to platelet aggregates of different sizes. This allows to analyze platelet aggregation at concentrations <104 plt/μL.39 Typical plots of overall platelet aggregation upon stimulation with 800 nM ADP (black curves) and 20 μg/mL collagen (red curves) in KHE/KMP are shown in Figure 1D. We found no significant differences in the initial velocities of platelet aggregation upon stimulation with ADP or collagen between healthy donors and patients with KHE/KMP (Figure 1E). However, several patients with KHE/KMP had severely impaired collagen-induced platelet aggregation (Figure 1E).
Platelets of the patients with KHE/KMP retain normal response to G-protein coupled receptor–mediated, but impaired GPVI-induced activation
Because the pathogenesis of KHE/KMP is driven by platelet-tumor interactions, we expected altered platelet functionality in KHE. This was supported by the impaired platelet thrombus formation in patients (Figure 1C). Further analysis was performed by means of live-cell flow cytometry. Resting platelets of the patients with KHE/KMP without HR increased in size (Figure 2A) and had a higher cytosolic calcium concentration (Figure 2B). CD61 expression in the quiescent state (supplemental Figure 3C) was normal for all patients. Patients with KHE/KMP without HR had increased CD62p binding in the resting state, but these differences were not statistically significant (supplemental Figure 3D).In combination with the increased amounts of PS-positive platelets (supplemental Figure 3E), these results could be interpreted so that platelets are preactivated,48 probably because of their contact with the tumor and consumption by it, and this resulted in decreased platelet counts and platelet age.49
We analyzed platelet responses to 2 types of stimulation: a strong one, that platelets are likely to encounter in thrombus “core,” and a mild one, characteristic for thrombus “shell.”50 Platelets in the thrombus core are simultaneously activated by combinations of ADP (from platelet granules and damaged endothelium), collagen (from extracellular matrix), and thrombin, generated because of coagulation cascade activity. Therefore, we studied platelet functional responses (GPIb “shedding,” granule release, integrin activation, and phosphatidylserine exposure) in response to stimulation with either a combination of CRP (GPVI agonist) and TRAP-6 (PAR1 agonist) or a combination of AYGPKF (PAR4 agonist), TRAP-6, and ADP by end point flow cytometry (Figures 2C-D; supplemental Figure 3). In response to strong stimulation with CRP and TRAP-6, platelets from patients without HR demonstrated normal GPIb expression (supplemental Figure 3A), but diminished GPIb “shedding” (supplemental Figure 3B), diminished integrin activation (Figure 2C), decreased α- (Figure 2D) and dense (supplemental Figure 3F) granule release, and less procoagulant platelets (S3G) than platelets from healthy children or patients with HR. It is noteworthy that platelet responses to other types of strong stimulation (thrombin receptor agonists and ADP) were within normal ranges (Figures 2C-D; supplemental Figures 3B,F,G).
Platelet responses to weak activation encountered in the thrombus “shell” generally do not include a profound granule release,51 therefore, to assess such activation, we used cytosolic calcium (Figure 2E) and fibrinogen binding (Figure 2F) as the observed responses. For both markers, no significant differences were observed between the platelets of healthy pediatric donors and those of both groups of patients with KHE/KMP upon stimulation with 2 μM of ADP or 5 μM of TRAP-6 (Figure 2E-F). However, platelets of the patients with KHE/KMP without HR had significantly impaired responses to activation with 2 μg/mL of CRP (Figure 2E-F).
CLEC-2 signaling is impaired in all groups of patients with KHE/KMP, but improves upon HR
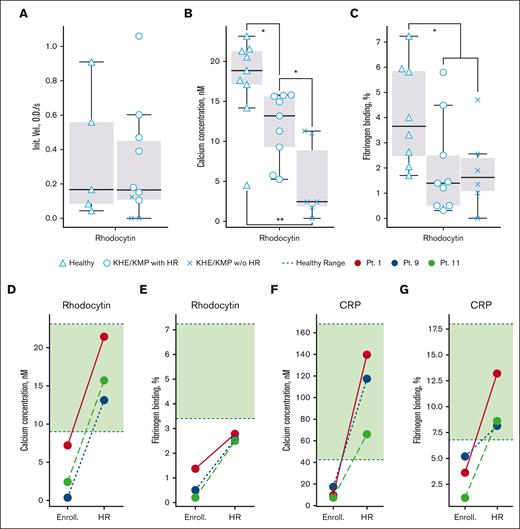
Because the CLEC-2-podoplanin axis is among the key drivers of the KHE/KMP pathogenesis, aberrant CLEC-2 signaling in patients with KHE/KMP could have been expected. We analyzed platelet di-aggregate formation upon weak stimulation by 10 nM of rhodocytin by means of LaSca. No significant differences were observed between patients with KHE/KMP and healthy donors in this assay (Figure 3A), although patients without HR appeared to be less responsive. Here, it should be noted that although platelet concentration is normalized in the LaSca assay, the ability of platelets to form aggregates in response to weak activation is highly variable, probably reflecting the necessity for secondary activation for CLEC-2-induced platelet aggregation.16 Thus, to avoid method-associated uncertainties, we analyzed rhodocytin-induced platelet activation by flow cytometry (Figure 3). The calcium mobilization upon CLEC-2 stimulation was significantly decreased in samples from patients with KHE/KMP with HR and was almost absent in patients with KHE/KMP without HR (Figure 3B). Fibrinogen binding was significantly decreased in all the studied patients (Figure 3C).
Platelet activation via CLEC-2 and GPVI receptors. Flow cytometry and low-angle light scattering aggregometry analysis and designations of groups of patients are the same as in Figure 1. (A) Initial velocity of platelet aggregation upon activation with 10 nM of rhodocytin. (B-C) Calcium mobilization (B) and fibrinogen binding (C) upon activation with 200 nM of rhodocytin. (D-G) Calcium mobilization (D,F) and fibrinogen binding (E,G) upon platelet activation with 200 nM of rhodocytin (D-E) or 2 μg/mL of CRP (F-G) for patients at the time point of enrollment (without HR, “Enroll.”) and upon HR (“HR”). Red lines correspond to patient 1, blue lines correspond to patient 9, and green lines correspond to patient 11. Green regions correspond to healthy-donor ranges. Statistical significance was calculated using Mann-Whitney U test. ∗P < .05; ∗∗P < .01.
Platelet activation via CLEC-2 and GPVI receptors. Flow cytometry and low-angle light scattering aggregometry analysis and designations of groups of patients are the same as in Figure 1. (A) Initial velocity of platelet aggregation upon activation with 10 nM of rhodocytin. (B-C) Calcium mobilization (B) and fibrinogen binding (C) upon activation with 200 nM of rhodocytin. (D-G) Calcium mobilization (D,F) and fibrinogen binding (E,G) upon platelet activation with 200 nM of rhodocytin (D-E) or 2 μg/mL of CRP (F-G) for patients at the time point of enrollment (without HR, “Enroll.”) and upon HR (“HR”). Red lines correspond to patient 1, blue lines correspond to patient 9, and green lines correspond to patient 11. Green regions correspond to healthy-donor ranges. Statistical significance was calculated using Mann-Whitney U test. ∗P < .05; ∗∗P < .01.
To study the impact of the HR on platelet functioning, we performed the analysis for the same patients (patients 1, 9, and 11) at enrollment and at the point of HR (Figure 3D-F). At the point of HR, both CLEC-2 (Figure 3D) and GPVI-induced (Figure 3F) calcium mobilization were restored to normal values. Rhodocytin-induced fibrinogen binding did not restore to normal values (Figure 3E), whereas CRP-induced did (Figure 3G). These findings prove that platelet signaling in these patients was altered because of tumor activity instead of hereditary factors. Platelet size, cytosolic calcium concentration, and amounts of procoagulant platelets, as well as platelet responsiveness to CRP and TRAP-6 stimulation, also restored to normal values upon HR (supplemental Figure 4).
CLEC-2 and GPVI numbers are decreased on the platelet surface of patients with KHE/KMP
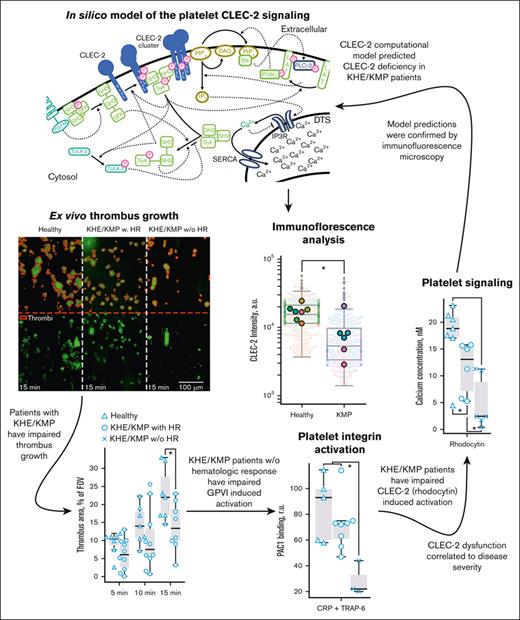
To study the causes of the observed diminished platelet responsiveness to CLEC-2 stimulation, we simulated platelet activation by means of our previously developed computational model41 (Figure 4A). The model predicted that even a modest decrease in the number of CLEC-2 copies per platelet could result in more than a double decrease in calcium mobilization (Figure 4B); therefore, platelets of patients with KHE/KMP could have fewer CLEC-2 molecules.
Patients with KHE/KMP have decreased levels of CLEC-2 and GPVI on the platelet surface. (A) Scheme of the computational model of CLEC-2 induced signaling in platelets: CLEC-2 activation results in the receptor clustering and phosphorylation by Syk kinases. In resting platelets, small amount of active Syk is maintained by active SFK kinases, which, in turn, are maintained active by CD148 phosphatases. Nonactive Syk bind to phosphorylated and clustered CLEC-2 receptors and also become active. Active Syk phosphorylate LAT and TULA-2. TULA-2 is the negative regulator of platelet Syk activation.40 PLCγ2 and PI3K bind to phosphorylated LAT, and PI3K becomes active. PI3K produces PIP3 from PIP2, which is bound by PH-domain of Btk. Hereby, Btk becomes active and activates PLCγ2, which hydrolyzes PIP2 and produces IP3, which initiates cytosolic calcium signaling. (B) Dependance of the maximally achievable cytosolic calcium concentration from CLEC-2 number on the resting platelets, predicted by the computational model. Physiological number of CLEC-2 per platelet35 is highlighted in red. (C) Typical results of the microscopic immunofluorescence analysis of the CLEC-2 expression on the platelet surface of the patients with KHE/KMP and healthy donors. (D-E) Fluorescence intensities of the anti–CLEC-2 antibodies (D) and anti-NMII antibodies (E) of the patients with KHE/KMP and healthy donors. Blurred corresponds to unique platelet measurements, and bright dots correspond to patients. Each color corresponds to unique patients. Seven healthy donors and 6 patients with KHE/KMP were analyzed. (F) Typical results of the microscopic immunofluorescence analysis of the GPVI expression on the platelet surface (GPVI; NMII) of the patients with KHE/KMP and healthy donors. (G-H) Fluorescence intensities of the anti-GPVI antibodies (G) and anti-NMII antibodies (H) of the KHE/KMP patients and healthy donors. Blurred corresponds to unique platelet measurements, and bright dots correspond to patients. Each color corresponds to unique patients. Seven healthy donors and 6 patients with KHE/KMP were analyzed. Statistical significance was calculated using Mann-Whitney U test. ∗P < .05; ∗∗P < .01. CLEC-2, anti–CLEC-2 antibodies; GPVI, anti-GPVI antibodies; NMII, nonmuscular myozin II (used for platelet identification).
Patients with KHE/KMP have decreased levels of CLEC-2 and GPVI on the platelet surface. (A) Scheme of the computational model of CLEC-2 induced signaling in platelets: CLEC-2 activation results in the receptor clustering and phosphorylation by Syk kinases. In resting platelets, small amount of active Syk is maintained by active SFK kinases, which, in turn, are maintained active by CD148 phosphatases. Nonactive Syk bind to phosphorylated and clustered CLEC-2 receptors and also become active. Active Syk phosphorylate LAT and TULA-2. TULA-2 is the negative regulator of platelet Syk activation.40 PLCγ2 and PI3K bind to phosphorylated LAT, and PI3K becomes active. PI3K produces PIP3 from PIP2, which is bound by PH-domain of Btk. Hereby, Btk becomes active and activates PLCγ2, which hydrolyzes PIP2 and produces IP3, which initiates cytosolic calcium signaling. (B) Dependance of the maximally achievable cytosolic calcium concentration from CLEC-2 number on the resting platelets, predicted by the computational model. Physiological number of CLEC-2 per platelet35 is highlighted in red. (C) Typical results of the microscopic immunofluorescence analysis of the CLEC-2 expression on the platelet surface of the patients with KHE/KMP and healthy donors. (D-E) Fluorescence intensities of the anti–CLEC-2 antibodies (D) and anti-NMII antibodies (E) of the patients with KHE/KMP and healthy donors. Blurred corresponds to unique platelet measurements, and bright dots correspond to patients. Each color corresponds to unique patients. Seven healthy donors and 6 patients with KHE/KMP were analyzed. (F) Typical results of the microscopic immunofluorescence analysis of the GPVI expression on the platelet surface (GPVI; NMII) of the patients with KHE/KMP and healthy donors. (G-H) Fluorescence intensities of the anti-GPVI antibodies (G) and anti-NMII antibodies (H) of the KHE/KMP patients and healthy donors. Blurred corresponds to unique platelet measurements, and bright dots correspond to patients. Each color corresponds to unique patients. Seven healthy donors and 6 patients with KHE/KMP were analyzed. Statistical significance was calculated using Mann-Whitney U test. ∗P < .05; ∗∗P < .01. CLEC-2, anti–CLEC-2 antibodies; GPVI, anti-GPVI antibodies; NMII, nonmuscular myozin II (used for platelet identification).
To test this hypothesis, we performed an analysis of platelet CLEC-2 expression using immunofluorescent microscopy of the blood smears.36 Typical platelets of the patients with KHE/KMP and healthy donors are given in Figure 4C. It appeared that the number of CLEC-2 receptors on platelet surface was significantly decreased in patients with KHE/KMP as compared with healthy donors (Figure 4D), whereas NMII levels were normal (Figure 4E). We have additionally tested the GPVI expression on the platelet surface and observed that GPVI levels were also decreased in patients (Figure 4F-H), but not to the same extent as the CLEC-2 levels. Additional validation of the used method by flow cytometry confirmed that patients with KHE/KMP had diminished CLEC-2 (supplemental Figure 5A-B) and GPVI (supplemental Figure 5C-D) levels on the platelet surface.
Discussion
Here, we performed a comprehensive evaluation of platelet functionality for patients with podoplanin-expressing KHE. Platelet aggregation ability evaluated both by low-angle light scattering analysis (LaSca) and thrombus formation in flow chambers revealed that KHE indeed affects platelet functionality (Figure 1). Although thrombin- or ADP-induced platelet responses were within normal ranges (Figure 2), GPVI- and, especially, CLEC-2–mediated platelet activation were impaired (Figure 3). In silico analysis predicted that a decreased platelet CLEC-2 expression could cause the observed alterations (Figure 4A,B), which was confirmed by immunofluorescence and flow cytometry analysis (Figure 4C-E). Similar results were obtained for the GPVI receptors on the patient platelets (Figure 4F-H).
Previously,9 it was shown that platelets are involved in the pathogenesis of KHE/KMP, and it is assumed that platelets are activated because of direct interaction with tumor cells.9,10 Here, we demonstrated that the ability of platelets to form aggregates is severely diminished for patients with KHE (Figure 1), whereas for patients without HR, thrombi did not form (Figure 1C), in contrast to artificial thrombocytopenia, when platelet thrombus growth was impaired but the initial layer of platelets still formed.32,37 Thus, in patients with KHE/KMP, the defect in the platelets themselves must be present. Platelet exhaustion due to a previous activation, as in patients with COVID-19,48 could be the cause. Although GPIb shedding was reduced (Figure 2E), their CD62p expression (supplemental Figure 3D) and the fraction of PS+ platelets (Figure 2C) were mostly normal. In addition, unlike patients with COVID-19,48 none of the patients had severely enhanced plasma coagulation (supplemental Figure 1). The platelet responses to GPCR stimulation were unaltered (Figure 2). Therefore, altogether platelets from patients with KHE/KMP do not demonstrate the exhausted phenotype.
The most significant differences were observed for platelet activation via GPVI and CLEC-2 (Figures 2-3). The first thing that comes to mind is that the SFK-Syk-PLC2 signaling axis is aberrant.20,21 However, the demonstrated decreased levels of the corresponding receptors (Figure 4) and unaltered ADP-induced aggregation (Figure 1), together with the restoration of GPVI signaling and the HR, suggest that these kinases function normally in patients.
Theoretical and experimental approaches revealed that GPVI and CLEC-2 expression on the surface of the patients’ platelets decreased (Figure 4). We have experimentally demonstrated it using the immunofluorescence analysis of blood smears method,36,52 where signals from antibodies to CLEC-2 and GPVI were significantly different between patients and healthy donors, whereas the signal from antibody to NMII was preserved (Figure 4E,H). The findings of the immunofluorescent microscopy were additionally confirmed by means of flow cytometry on a selected group of patients (supplemental Figure 5). It should be noted that even though the platelets of the patients with KHE/KMP had restored their responsiveness to CRP (Figure 3), their GPVI levels were still decreased (supplemental Figure 5), indicating that platelets require fewer numbers of GPVI than CLEC-2 to maintain normal responsiveness, possibly because of their clustering.41
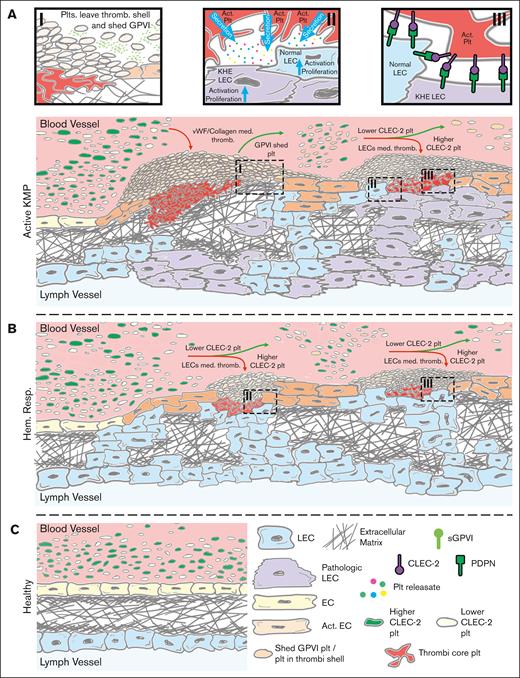
Based on the obtained results and the known KHE composition,9,13 we propose the following mechanisms for the cause of platelet dysfunction in KHE/KMP. Tonic signal from VEGFR36,7 on the LECs causes their pathological proliferation and exposure to the blood flow, where they interact with platelets through the podoplanin-CLEC-2 axis.9,10,14,53 Activated platelets secrete significant amounts of VEGF-A and PDGF9,53 leading to tumor growth (Figure 5A). Because of the LECs’ activity and subsequent aberrant neoangiogenesis, collagen is also exposed to the blood flow, leading to more platelet recruitment9,10 and a positive feedback loop.9 Platelet population is nonuniform because of the heterogeneity of the megakaryocyte population and the stochastic nature of platelet production,54 which leads to variation in receptor expression. We propose that the tumor accumulates platelets with normal CLEC-2 expression, whereas the ones with decreased CLEC-2 numbers remain in circulation (Figure 5A). This leads to the observed decrease in platelet CLEC-2 expression (Figure 4) among the patients with KHE/KMP without HR. Furthermore, KHE tumors contain regions with impaired endothelial integrity where tissue factor, VWF, and collagen are exposed to blood flow.9 This also leads to platelet activation via GPVI and subsequent GPVI shedding,55 which is in line with the observed increased CD62p (supplemental Figure 3) levels on the quiescent platelets of the patients with KHE/KMP without HR.55
Proposed scheme of the KHE/KMP impact on patient platelets. (A) KHE pathogenesis is mostly based on aberrant VEGFR signaling in LECs.9 These LECs are constitutively proliferating and subsequently intervene in the blood vessel endothelium, thus causing blood endothelium activation, disruption, and extracellular matrix exposure to the blood flow (Ai).9,10 Exposed collagen causes the initiation of platelet thrombus formation and subsequent thrombocytopenia due to platelet consumption. Thrombi are heterogenous in nature; although platelets in the thrombus core are highly activated by a combination of collagen, thrombin, and platelet granule contents, platelets in the thrombus shell are stimulated mostly by ADP and thromboxane.50 Here, platelets in the outer layers of the thrombus can leave the thrombus shell and return to the circulation.50,56 Meanwhile, even moderate platelet activation can cause platelet GPVI receptor shedding.57 Thus, these preactivated platelets (orange platelets on the scheme) become less responsive to further GPVI-mediated activation (Ai). In the meantime, pathologically proliferating LECs also intrude the blood flow and, via the podoplanin-CLEC-2 axis, initiate platelet adhesion and activation (Aii-iii).9,10 Active platelets secrete growth factors stored in their granules, which causes additional LEC and EC activation and proliferation, thus acting as a positive feedback loop (Aii).9,58 Finally, platelets are heterogenous because of the mechanisms of their production: platelets are “ripped” from the megakaryocytes, and thus, platelet membrane receptor expression is not uniform.54 Hereby, it can be expected that some of the platelets in the blood flow have higher CLEC-2 levels (green platelets on the scheme) and some have lesser CLEC-2 levels (white platelets on the scheme). LECs, by exposing podoplanin to the blood flow, capture platelets with higher CLEC-2 levels, acting like filters. Hence, only platelets with lesser CLEC-2 on the surface, those cannot be effectively captured by the LECs, remain in circulation (Aiii). (B) Upon HR, proliferation of the LECs is abrogated because chemotherapy inhibits their VEGF signaling. This allows the blood vessel endothelial cells to cover the exposed extracellular matrix and thus significantly reduce pathological thrombus formation. However, despite being inhibited, LECs still remain in the blood vasculature and still filter platelets with higher CLEC-2 levels (Biii). These platelets secrete growth factors from their granules and thus activate remaining LECs, which maintain LECs’ presence in the blood flow (Bii). (C) Finally, upon recovery, no LECs are exposed to blood flow.
Proposed scheme of the KHE/KMP impact on patient platelets. (A) KHE pathogenesis is mostly based on aberrant VEGFR signaling in LECs.9 These LECs are constitutively proliferating and subsequently intervene in the blood vessel endothelium, thus causing blood endothelium activation, disruption, and extracellular matrix exposure to the blood flow (Ai).9,10 Exposed collagen causes the initiation of platelet thrombus formation and subsequent thrombocytopenia due to platelet consumption. Thrombi are heterogenous in nature; although platelets in the thrombus core are highly activated by a combination of collagen, thrombin, and platelet granule contents, platelets in the thrombus shell are stimulated mostly by ADP and thromboxane.50 Here, platelets in the outer layers of the thrombus can leave the thrombus shell and return to the circulation.50,56 Meanwhile, even moderate platelet activation can cause platelet GPVI receptor shedding.57 Thus, these preactivated platelets (orange platelets on the scheme) become less responsive to further GPVI-mediated activation (Ai). In the meantime, pathologically proliferating LECs also intrude the blood flow and, via the podoplanin-CLEC-2 axis, initiate platelet adhesion and activation (Aii-iii).9,10 Active platelets secrete growth factors stored in their granules, which causes additional LEC and EC activation and proliferation, thus acting as a positive feedback loop (Aii).9,58 Finally, platelets are heterogenous because of the mechanisms of their production: platelets are “ripped” from the megakaryocytes, and thus, platelet membrane receptor expression is not uniform.54 Hereby, it can be expected that some of the platelets in the blood flow have higher CLEC-2 levels (green platelets on the scheme) and some have lesser CLEC-2 levels (white platelets on the scheme). LECs, by exposing podoplanin to the blood flow, capture platelets with higher CLEC-2 levels, acting like filters. Hence, only platelets with lesser CLEC-2 on the surface, those cannot be effectively captured by the LECs, remain in circulation (Aiii). (B) Upon HR, proliferation of the LECs is abrogated because chemotherapy inhibits their VEGF signaling. This allows the blood vessel endothelial cells to cover the exposed extracellular matrix and thus significantly reduce pathological thrombus formation. However, despite being inhibited, LECs still remain in the blood vasculature and still filter platelets with higher CLEC-2 levels (Biii). These platelets secrete growth factors from their granules and thus activate remaining LECs, which maintain LECs’ presence in the blood flow (Bii). (C) Finally, upon recovery, no LECs are exposed to blood flow.
The observed dysfunction of platelet GPVI and, to a lesser extent, CLEC-2 was resolved at the point of HR42 in most of the studied patients with KHE/KMP (Figure 3). Most of the patients were treated with LD, accompanied by vinca alkaloids, which inhibit the proliferation of the actively growing tumor cells.44,59 Some of the treatment strategies also included mTORc inhibitors, such as sirolimus (Table 1). Upon abrogation of the tumor growth, normalization of the blood vascular endothelium occurs, reducing the exposure of the collagen in the extracellular matrix to the blood flow and subsequent platelet consumption to actively growing thrombi9,10 (Figure 5B). In contrast, Tamura et al22 have demonstrated that CLEC-2 deletion from the platelets/megakaryocytes in mice also resulted in thrombocytopenia because of the abnormal megakaryopoiesis. However, the observed restoration of the platelet count and function with an incomplete restoration of the platelet responsiveness to rhodocytin upon HR allows us to claim that this mechanism is not the key one in the development of profound thrombocytopenia in KHE/KMP.
This study has several limitations. Above all, not all the patients included in the study were treatment-naïve. Limited impact on platelets were earlier demonstrated for most of the drugs used.60,61 Doxorubicin is known to be cytotoxic,59 but in liposomal form it minimally affects platelets.62 In 5% of cases, cyclophosphamide could cause thrombocytopenia.63 Vinca alkaloids inhibit platelet activation in vitro,64 however, we have not seen this in our study (Figure 3C-D). Finally, sirolimus could cause platelet preactivation,65,66 but for 2 patients receiving it, increased CD62p levels were not observed (supplemental Figure 3D). Another limitation is associated with the overall age of the studied patients and donors: because of their young age, we were significantly limited in the available blood samples.45,46 Moreover, all of the patients with KHE/KMP without HR had profound thrombocytopenia (Table 1). Thus, we were not able to apply conventional methods, for example, platelet aggregometry, to characterize these patients’ platelets.
Altogether, investigating the role of platelets in solid podoplanin-expressing tumors can help reveal novel mechanisms of tumor progression and improve therapeutic strategies.
Acknowledgments
The authors are grateful to Antonina Boldyreva (CTP PCP RAS) and Evgenia Ponomarenko (NMRC PHOI) for the technical assistance during experiments. The experiments on platelet functional responses (Figures 2 and 3) and computational modeling (Figure 4A,B) were supported by the Russian Science Foundation Grant 21-74-20087; patient recruitment, platelet aggregation (Figure 1), and platelet immunostaining (Figure 4C,D) were supported by the endowment foundation “Science for Children”; J.A.E. is financially supported by the Interdisciplinary Center for Clinical Research (IZKF) Münster (grant: IZKF: Ebl-A/009/21). We acknowledge support from the open access publication fund of the University of Münster.
Authorship
Contribution: A.A.M. designed the experiments, performed experiments on continuous flow cytometry, constructed and analyzed the computational model, analyzed the data, and wrote the manuscript; I.P.T. and L.A.K. proposed the study, recruited the patients, analyzed the data, and wrote the paper; O.I.A. performed continuous flow cytometry experiments and edited the paper; A.A.I. performed end point flow cytometry experiments and edited the manuscript. E.M.K. designed the thrombodynamics experiments, performed the experiments, and analyzed the data; J.-J.D.K. performed experiments on thrombi growth in flow chambers and analyzed the data; A.E.B. performed and analyzed experiments on platelet observation in blood smears; N.A.P. designed experiments on platelet observation in blood smears and analyzed the data; G.S.S. performed experiments on low-angle light scattering aggregometry; E.Y. performed experiments on platelet observation in blood smears; G.A.N., M.A.P., J.A.E., D.V.K., and A.N.S. designed the study, analyzed the data, and edited the manuscript; J.A.E., D.V.K., and A.N.S. recruited the resources for the study; and A.N.S. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anastasia N. Sveshnikova, Dmitry Rogachev National Medical Research Centеr of Pediatric Hematology, Oncology and Immunology, 1 Samory Mashela St, 117997, Moscow, Russia; e-mail: a.sveshnikova@physics.msu.ru; and Dmitrii V. Kalinin, University of Münster Institut für Pharmazeutische und Medizinische, Chemie Corrensstraße, 48 48149, Münster, Germany; e-mail: dmitrii.kalinin@uni-muenster.de.
References
Author notes
Data will be available on request from the corresponding authors, Ekaterina M. Koltsova (ekaterina_koltsova@bk.ru) for Thrombodynamics, Lili A. Khachatryan (lili.2510@yandex.ru) for information about the patients, Mikhail A. Panteleev (mapanteleev@yandex.ru) for LaSca and end point flow cytometry, and Anastasia N. Sveshnikova (a.sveshnikova@physics.msu.ru) for other data. LaSca - low-angle light scattering analysis.
The full-text version of this article contains a data supplement.