Abstract
Follicular lymphoma (FL) is a neoplasm derived from germinal center B cells, composed of centrocytes and centroblasts, with at least a focal follicular growth pattern. The t(14;18) translocation together with epigenetic deregulation through recurrent genetic alterations are now recognized as the hallmark of FL. Nevertheless, FL is a heterogeneous disease, clinically, morphologically, and biologically. The existence of FL lacking the t(14;18) chromosomal alteration highlights the complex pathogenesis of FL, and indicates that there are alternative pathogenetic mechanisms that can induce a neoplasm with follicular center B-cell phenotype. Based on their clinical presentation, t(14;18)-negative FLs can be divided into 3 broad groups: nodal presentation, extranodal presentation, and those affecting predominantly children and young adults. Recent studies have shed some light into the genetic alterations of t(14;18)-negative FL. Within the group of t(14;18)-negative FL with nodal presentation, cases with STAT6 mutations are increasingly recognized as a distinctive molecular subgroup, often cooccurring with CREBBP and/or TNFRSF14 mutations. FL with BCL6 rearrangement shows clinicopathological similarities to its t(14;18)-positive counterpart. In contrast, t(14;18)-negative FL in extranodal sites is characterized mainly by TNFRSF14 mutations in the absence of chromatin modifying gene mutations. FL in children have a unique molecular landscape when compared with those in adults. Pediatric-type FL (PTFL) is characterized by MAP2K1, TNFRSF14, and/or IRF8 mutations, whereas large B-cell lymphoma with IRF4 rearrangement is now recognized as a distinct entity, different from PTFL. Ultimately, a better understanding of FL biology and heterogeneity should help to understand the clinical differences and help guide patient management and treatment decisions.
Introduction
Follicular lymphoma (FL) is by definition a neoplasm composed of follicle center B cells, which usually has at least a partially follicular pattern (Figure 1A-B).1 In recent years, the molecular alterations of several disease entities fulfilling this definition but lacking the t(14;18) chromosomal alteration, the genetic hallmark of conventional FL, have been described. The existence of t(14;18)-negative FL indicates that alternative neoplastic programs can induce a follicular center B-cell phenotype (Figure 1C-I). These alternative forms of t(14;18)-negative FL include primary nodal and extranodal diseases that affect either predominantly children or adults (Figure 2). New studies have also unraveled the heterogeneity of FL, which is greater than originally appreciated, with newly recognized t(14;18)-negative FL variants.
Morphological and immunophenotypic features of nodal t(14;18)-positive and t(14;18)-negative FLs. (A) t(14;18)-positive FL showing a nodular growth pattern, with follicles growing back to back and the lack of a defined mantle zone, (original magnification ×50; hematoxylin and eosin [H&E] stain). (B) Higher magnification demonstrates the presence of predominantly centrocytes with few intermingled centroblasts in the follicles characteristic of FL grade 1/2, (original magnification ×400; H&E stain). (C-D) BCL2-R–neg CD23+ FCL with diffuse growth pattern. (C) The H&E stain shows an inguinal lymph node with partial involvement of a diffuse lymphoid infiltration that leaves a rim of normal lymph node in the periphery. (D) The tumor cells are strongly CD23+. Note that in the periphery of the lymph node there are residual normal GCs and the CD23 stain highlights the follicular dendritic cells. (E) The tumor cells are also strongly positive for CD10. The residual normal GCs are CD10+. (Snapshot of scanned slides, ×10). (F-G) BCL2-R–neg CD23+ FCL with follicular growth pattern. (F) The H&E stain shows an axillary lymph node with involvement of a lymphoid proliferation with follicular growth pattern (original magnification ×40; H&E stain). (G) The tumor cells are CD23+ (original magnification ×40; immunohistochemistry). (H) The tumor cells are BCL2−. Note that the B cells in the attenuated mantle zone are BCL2+. (original magnification ×400; immunohistochemistry). (I) The neoplastic cells in the follicles are CD23+, (original magnification ×400; immunohistochemistry).
Morphological and immunophenotypic features of nodal t(14;18)-positive and t(14;18)-negative FLs. (A) t(14;18)-positive FL showing a nodular growth pattern, with follicles growing back to back and the lack of a defined mantle zone, (original magnification ×50; hematoxylin and eosin [H&E] stain). (B) Higher magnification demonstrates the presence of predominantly centrocytes with few intermingled centroblasts in the follicles characteristic of FL grade 1/2, (original magnification ×400; H&E stain). (C-D) BCL2-R–neg CD23+ FCL with diffuse growth pattern. (C) The H&E stain shows an inguinal lymph node with partial involvement of a diffuse lymphoid infiltration that leaves a rim of normal lymph node in the periphery. (D) The tumor cells are strongly CD23+. Note that in the periphery of the lymph node there are residual normal GCs and the CD23 stain highlights the follicular dendritic cells. (E) The tumor cells are also strongly positive for CD10. The residual normal GCs are CD10+. (Snapshot of scanned slides, ×10). (F-G) BCL2-R–neg CD23+ FCL with follicular growth pattern. (F) The H&E stain shows an axillary lymph node with involvement of a lymphoid proliferation with follicular growth pattern (original magnification ×40; H&E stain). (G) The tumor cells are CD23+ (original magnification ×40; immunohistochemistry). (H) The tumor cells are BCL2−. Note that the B cells in the attenuated mantle zone are BCL2+. (original magnification ×400; immunohistochemistry). (I) The neoplastic cells in the follicles are CD23+, (original magnification ×400; immunohistochemistry).
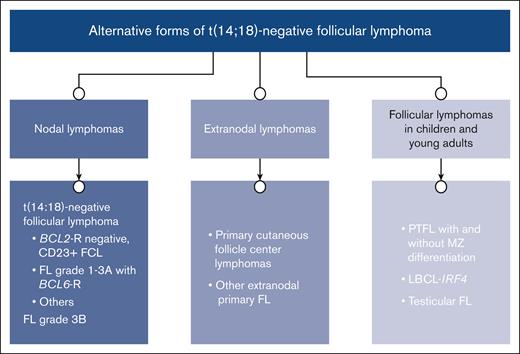
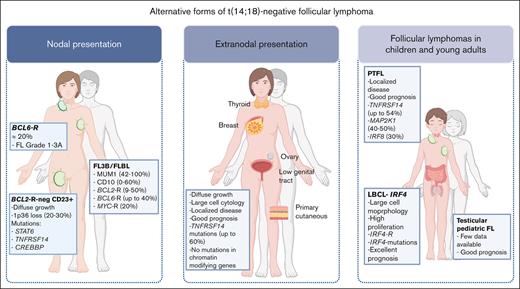
Alternative forms of t(14;18)-negative FL. The cases are classified per site of presentation; nodal vs extranodal, and those cases presenting mainly in children and young adults.
Alternative forms of t(14;18)-negative FL. The cases are classified per site of presentation; nodal vs extranodal, and those cases presenting mainly in children and young adults.
In this review, we focus on t(14;18)-negative FL, highlighting the similarities and differences to t(14;18)-positive FL. Despite the morphological and histogenetic similarities, these t(14;18)-negative variants have distinctive clinical and genetic features that will be discussed.
t(14;18) positive FL
FL is an indolent, mature B-cell non-Hodgkin lymphoma characterized by a neoplastic proliferation of germinal center (GC) B cells and a complex, nonneoplastic microenvironment, imitating B-cell follicles.1,2 FL accounts for ∼20% of all lymphomas in Western countries. Conventional FL is characterized by constitutive expression of the antiapoptotic BCL2 protein as a result of the t(14;18)(q32;q21) translocation, which is the genetic hallmark of the disease, identified in between 85% and 90% of FL cases.3,4 The fifth edition of the World Health Organization (WHO) classification introduced the term “classic” FL (cFL) to separate cases that are t(14;18) positive from other rarer subtypes.5 This translocation occurs early in B-cell development resulting from a mistake in variable diversity joining recombination causing the juxtaposition of the BCL2 protooncogene with the immunoglobulin heavy chain (IGH) locus.3 The t(14;18) translocation is insufficient to cause manifestation of FL, and circulating t(14;18)+ B cells, so-called FL-like cells (FLLCs), are detected at low levels in ∼50% to 70% of healthy adult individuals.6-8 The number of circulating t(14;18)+ B cells rises with age, smoking, and exposure to pesticides.6,8 Although the presence of clonally expanded FLLCs at high frequencies predicts an increased risk, the majority of individuals never develop FL.9,10 Most BCL2 rearranged B-cell clones are long-lived memory B cells that have experienced the GC and can expand and persist for several years.7 The tissue equivalent of these FLLCs, also considered a precursor form of FL, is in situ follicular neoplasia (ISFN).11 Similar to FLLCs, ISFN carries the t(14;18) chromosomal translocation and expresses BCL2.12,13 By definition, ISFN involves the GC of normal reactive lymph nodes and the cells strongly express CD10 and BCL2 but show a very low proliferation rate.14 Another closely related lesion is duodenal-type FL, which is morphologically and immunophenotypically indistinguishable from cFL and is characterized by restricted involvement of intestinal mucosa and, usually, lack of extraintestinal manifestations, although rarely local lymph node involvement has been reported.15,16
Another feature of FL is the constitutive expression of activation-induced cytidine deaminase, which mediates the process of somatic hypermutation (SHM) and class switch recombination, leading to genomic instability and accumulation of genetic alterations. Accordingly, FL is characterized by frequent chromosomal number alterations and mutations targeting epigenetic regulator genes that occur early in FL pathogenesis, indicating that epigenetic dysregulation is a hallmark feature of FL.17-19 Mutations in histone acetyltransferases such as CREBBP and EP300, and mutations in histone methyltransferases such as KMT2D and EZH2 occur in up to 90% of FL cases.20,21 Moreover, alterations in genes involved in immune recognition (TNFRSF14 and CTSS), and in different signaling pathways such as B-cell receptor (BCR), NF-κB (CARD11 and TNFAIP3), JAK/STAT (STAT6) and mTOR (RRAGC, ATP6V1B2, ATP6AP1, and SESTRIN1) are frequently reported.17,19,22-27 High-grade transformation is associated with alterations in a distinct set of genes including TP53, CDKN2A/B, and MYC, as well as genes targeted by aberrant SHM.28
FL is the prototypical B-cell non-Hodgkin lymphoma with a strong dependence on its specialized tumor microenvironment (TME). Alterations in TNFRSF14 and in CTSS that seem to re-educate the microenvironment, increase the number of CD4+ T follicular helper (TFH) cells supporting lymphoma cell survival and promoting immune evasion through the exclusion of cytotoxic CD8+ T cells.23-25 Another peculiar finding is the introduction of new acquired N-glycosylation sites into the IGV regions during SHM that substitutes conventional antigen binding, favoring the generation of long-lived clones.29,30 FLLC, ISFN, and duodenal-type FL share key features with cFL, including secondary mutations of epigenetic regulator genes, as well as antigen–independent BCR signaling because of the presence of N-glycosylation motifs in the rearranged immunoglobulin genes.13,15,21,31
Alternative forms of t(14;18)-negative FL
Although the majority of FL carry the t(14;18) chromosomal translocation, a number of associated histopathological variants without this translocation are recognized. Several series of t(14;18)-negative FL have been published recently.32-35 These studies demonstrate genetic differences between t(14;18)-positive FL and other closely related histological entities but also some similarities among t(14;18)-negative FL groups (Figure 3).
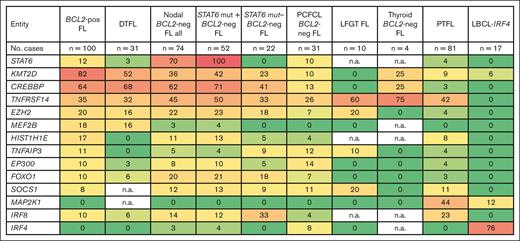
Comparison of mutational landscape between t(14;18)-positive and t(14;18)-negative FL entities. Frequencies of recurrently mutated genes in 100 t(14;18)-positive FL (BCL2-pos FL),17 31 duodenal-type FL (DTFL),15 74 nodal t(14;18)-negative cases,32,36-38 31 PCFCL,34,35 10 lower female genital tract (LFGT) FL,70 7 thyroid BCL2-neg FL,71 81 PTFL,40,82,84 and 17 LBCL-IRF4.91 Color gradient depicted per the percentage of mutated cases for each gene: dark red, 100%: dark green, 0%. neg, negative; mut, mutated; n.a.: not available.
Comparison of mutational landscape between t(14;18)-positive and t(14;18)-negative FL entities. Frequencies of recurrently mutated genes in 100 t(14;18)-positive FL (BCL2-pos FL),17 31 duodenal-type FL (DTFL),15 74 nodal t(14;18)-negative cases,32,36-38 31 PCFCL,34,35 10 lower female genital tract (LFGT) FL,70 7 thyroid BCL2-neg FL,71 81 PTFL,40,82,84 and 17 LBCL-IRF4.91 Color gradient depicted per the percentage of mutated cases for each gene: dark red, 100%: dark green, 0%. neg, negative; mut, mutated; n.a.: not available.
Nodal t(14;18)-negative FL
Nodal t(14;18)-negative FL represents 10% to 15% of FL cases and is heterogeneous both genetically and clinically.32,33,45 FL t(14;18)-negative is genetically less well characterized than the t(14;18)-positive counterpart and its pathogenesis is not well understood. A gene expression profiling study comparing t(14;18)-positive and t(14;18)-negative FLs showed signatures indicating subtle differences in the developmental stages of the neoplastic B cells, the use of divergent oncogenic programs, and the composition of the microenvironment.46 t(14;18)-positive FL is enriched in GC B-cell signatures, whereas in t(14;18)-negative FL, the gene expression and microRNA profiles resemble that of a late GC B cell that has not yet exited the GC stage of differentiation.46,47 Moreover, t(14;18)-negative FL might use different mechanisms of BCR stimulation, compared with the lectin-mediated binding characteristic of t(14;18)-positive FL, including responsiveness to autoantigens as indicated by biased IGHV4-34 use and enrichment of new acquired N-glycosylation sites in framework 3.48 A recent study demonstrated that t(14;18)-negative FL shows copy number alterations and gene mutations similar to t(14;18)-positive FL but with different frequencies.32 In general, the most frequently mutated genes were STAT6 (57%), CREBBP (49%), TNFRSF14 (39%), and KMT2D (27%). Three groups were recognized based on their molecular profile: (1) BCL2-rearrangement (BCL2-R)-negative CD23+ follicle center lymphoma (BCL2-R–neg CD23+ FCL); (2) FL with BCL6-R; and (3) “others.”
BCL2-R–negative CD23+ FCL
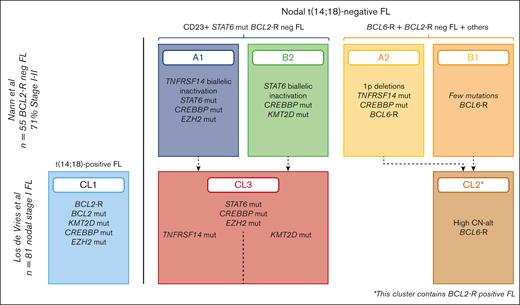
This subtype was proposed as a provisional new entity in the 2022 International Consensus Classification (ICC).11 This entity is not recognized in the fifth edition of the WHO classification,5 although there is some overlap with the “diffuse variant” included in the new subgroup of FL with uncommon features. This subtype was proposed as a provisional entity based on the distinguishing phenotype (CD23+), genotype (STAT6 mutations), and characteristic clinical presentation.32,36,37,49 The name FCL is to emphasize the parallelism to primary cutaneous follicle center lymphoma (PCFCL), in which a predominantly diffuse growth pattern is observed despite the follicle center derivation of the tumor cells. BCL2-R–neg CD23+ FCL has a female predominance (male to female ratio, 2:1), low-stage disease at presentation (stage I/II), and often inguinal involvement (40%-83%) but also cervical and axillary presentation occurs.32,49 Morphologically, the tumor growth might be diffuse, follicular and diffuse, or purely follicular. Previous studies suggesting the predominantly diffuse growth pattern suffered the bias that diffuse growth pattern was part of the inclusion criteria.36-38,49 Nevertheless, cases with predominantly inguinal presentation had higher frequency of diffuse growth pattern, CD23 expression, STAT6 mutations, and a lower number of copy number alterations (Figure 1C-E). Diagnostic criteria include the lack of t(14;18) translocation, expression of CD23 in the tumor cells, and at least 1 GC marker.36,50 BCL2 is usually negative.37 In the original description by Katzenberger et al,49 1p36 loss was reported in 93% of cases; however, in following studies this chromosomal alteration is reported to occur in 20% to 30% of cases.32,36,38 Activating STAT6 mutations in the DNA binding domain that cooccurred with CREBBP and/or TNFRSF14 mutations reveal alternative oncogenic pathways. One pathway (cluster A1) is distinguished by biallelic inactivation of TNFRSF14 (mutation and/or 1p36 loss/loss of heterozygosity; 100%), and CREBBP and EZH2 mutations with recurrent loss of 6q21-q24, sharing many features with t(14;18)-positive FL. The second pathway (cluster B2) is characterized by CREBBP mutations/16p alterations (85%) and lack of TNFRSF14 and EZH2 mutations. These findings were corroborated by 2 recent studies providing further evidence for this provisional entity as a distinct molecular subtype of FL. The first study identified 3 genotypic subgroups within FL, including a cluster with frequent cooccurrence of STAT6 and CREBBP mutations.51 The second study took the approach of analyzing nodal FL in stage I disease.33 This analysis revealed that the majority of these cases corresponded to t(14;18)-negative FL, and their molecular cluster 3 (CL3) mimicked the clusters A1 and B2,32 although the expression of CD23 was not reported. (Figure 4).
Different genetic clusters of nodal t(14;18)-negative FL. Comparison of 2 studies that identified a distinct molecular cluster characterized by STAT6 mutations.32,33 The 2 studies highlight the frequent cooccurrence of STAT6 and CREBBP mutations.
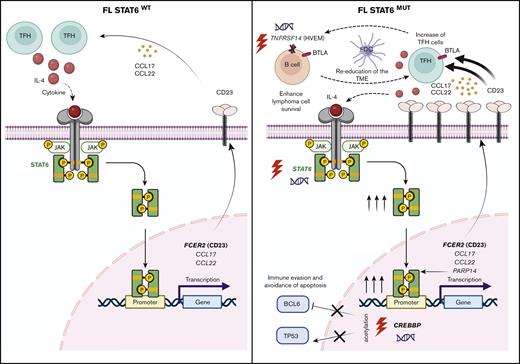
STAT6 mutations showed a strong positive correlation with CD23 expression, indicating that the expression of CD23 may be secondary to activating STAT6 mutations and a good surrogate marker in routine diagnosis (Figure 1).32 Accordingly, recent gene expression profile studies in FL with STAT6 mutation showed upregulation of interleukin-4 (IL-4)/STAT6 target genes including FCER2 (CD23), CCL17, and CCL22 (Figure 5).52,53STAT6 mutations amplify IL-4–induced STAT6-dependent gene activation and, therefore, seem to intensify the IL-4–driven reeducation process of the FL TME.52 Of note, CD23 can also be upregulated in t(14;18)-positive FL without STAT6 mutations, highlighting the key role of IL-4/STAT6 pathway in the pathogenesis of FL. Accordingly, previous studies have demonstrated that in t(14;18)-positive FL, TFH cells are expanded and are the major IL-4–producing cells creating an IL-4–dependent TFH–B-cell axis, important for the communication of FL cells with its TME.55 Moreover, the activated IL4/STAT6 pathway seems to favor malignant B-cell survival and proliferation.53BCL2-R–neg CD23+ FCL that lacks STAT6 mutations often carry SOCS1 mutations, which are upstream of STAT6, contributing to STAT6 activation.32 The question remains why t(14;18)-negative FL select for activating STAT6 mutations. Recent findings indicate that activating STAT6 mutations are not enough to initiate FL lymphomagenesis.52 An important observation is that all STAT6-mutated t(14;18)-negative FL carry CREBBP and/or TNFRSF14 mutations, strongly suggesting that these mutations cooperate (Figure 5).16,36,38 Notably, the impact of TNFRSF14 mutations is manifested by an increase in TFH cells and activation of the tumor stroma,23 whereas CREBBP mutations in the HAT domain induce downregulation of major histocompatibility complex class II and the ability of T cells to recognize B cells.56CREBBP mutations impair the acetylation activity of CREBBP, interfering with terminal B-cell differentiation, leaving the regulation of BCL6-repressed targets unrestrained.54CREBBP loss is also associated with decreased p53 activity, abrogating optimal cellular response to DNA damage, facilitating transformation, and improving the “clonal fitness” of the cells.20,21 Further studies are warranted to understand the pathogenesis of STAT6-mutated FL cases.
Model of mutated STAT6 (STAT6mut)–mediated pathogenesis in FL. Left panel: FL with wild-type STAT6. FL is characterized by increased numbers of TFH cells, which are the main source of IL-4 production that initiates the cascade of IL-4–STAT6 pathway with activation of STAT6 and induction of STAT6-dependent genes including FCER2 (CD23), CCL17, and CCL22. In some t(14;18)-positive FL cases this might induce the expression of CD23 in the absence of STAT6 mutation. Right panel: FL with activating mutations of STAT6. STAT6 mutations amplify IL-4–induced STAT6-dependent gene activation via an intracellular self-reinforcing regulatory microcircuit that involves aberrantly increased PARP14 levels.52 The increased expression of cytokines CCL17 and CCL22 contribute to re-educate the TME, with further recruitment of IL-4–producing TFH cells. STAT6mut in FL cells cooccurred either with CREBBP mutations and/or TNFRSF14 mutations.32,33,51CREBBP mutations favor immune evasion and precludes apoptosis by inhibiting the acetylation of CREBBP target genes, BCL6 and TP53.53,54TNFRSF14 mutations help to re-educate the TME and enhance lymphoma cell survival by disrupting the BTLA–TNFRSF14 pathway.23 Figure created with BioRender.com.
Model of mutated STAT6 (STAT6mut)–mediated pathogenesis in FL. Left panel: FL with wild-type STAT6. FL is characterized by increased numbers of TFH cells, which are the main source of IL-4 production that initiates the cascade of IL-4–STAT6 pathway with activation of STAT6 and induction of STAT6-dependent genes including FCER2 (CD23), CCL17, and CCL22. In some t(14;18)-positive FL cases this might induce the expression of CD23 in the absence of STAT6 mutation. Right panel: FL with activating mutations of STAT6. STAT6 mutations amplify IL-4–induced STAT6-dependent gene activation via an intracellular self-reinforcing regulatory microcircuit that involves aberrantly increased PARP14 levels.52 The increased expression of cytokines CCL17 and CCL22 contribute to re-educate the TME, with further recruitment of IL-4–producing TFH cells. STAT6mut in FL cells cooccurred either with CREBBP mutations and/or TNFRSF14 mutations.32,33,51CREBBP mutations favor immune evasion and precludes apoptosis by inhibiting the acetylation of CREBBP target genes, BCL6 and TP53.53,54TNFRSF14 mutations help to re-educate the TME and enhance lymphoma cell survival by disrupting the BTLA–TNFRSF14 pathway.23 Figure created with BioRender.com.
FL with BCL6-rearrangement
Another interesting genetic group within nodal t(14;18)-negative FL, is the group harboring BCL6-R (3q27), which results in the deregulation of BCL6 expression.57-59 Although BCL6-R has been identified predominantly in FL grade 3B (FL3B, 40%, see later discussion), it has also been reported to occur in ∼20% of t(14;18)-negative FL grade 1/2 and 3A. These studies suggested that BCL6 deregulation can play an important role in the initiation and development of FL that is indistinguishable morphologically from its BCL2-R counterpart. Nevertheless, BCL6 rearrangements are the product of class switch recombination in the GC and the breakpoints occur in the switch regions.60 A recent study confirmed the important role of BCL6-R in a subgroup of t(14;18)-negative FL grade 1/2 and 3A, representing 22% of cases.32 Patients with BCL6-R presented at more advanced clinical stages (stage III/IV), all cases had a follicular growth pattern and showed more complex genetic profile, similar to t(14;18)-positive FL. Comparable findings were reported in a study in which BCL6-R cases represented 64% of t(14;18)-negative FL in stage III/IV, with fewer CREBBP (45%) and STAT6 (9%) mutations.33 These 2 recent studies revealed that t(14;18)-negative FL in clinical stage I/II is predominantly STAT6 driven whereas stage III/IV appear to be more BCL6 driven and closer resemble t(14;18)-positive FL. More studies are needed to confirm these findings.
Other nodal t(14;18)-negative FL
Within the group of nodal t(14;18)-negative FL there is a group without BCL6-R, STAT6 mutations, or other genetic alterations, mainly noninguinal presentation and with follicular growth pattern.32 The differential diagnosis with reactive hyperplasia and nodal marginal zone lymphoma (NMZL) can be challenging. However, these cases are monoclonal, express ≥1 GC markers and lack the typical mutations of NMZL. This group with low genomic complexity warrants further analysis.
FL grade 3B
The term FL3B has been retained in the ICC,11 whereas the fifth edition of the WHO classification has renamed this subgroup as follicular large B-cell lymphoma (FLBL) to stress that biologically it is more closely related to diffuse large B-cell lymphoma (DLBCL).5 In FL3B/FLBL, by definition, the follicles are composed entirely of centroblasts. FL3/FLBL needs to be distinguished from FL3A, a task that is not always easy, as reflected in the heterogeneous results of published studies.61-68 The ICC recommends that in difficult cases the absence of BCL2-R and/or CD10 expression favors the diagnosis of FL3B/FLBL.11 In FL3B/FLBL, BCL2-R is reported in 9% to 50% of cases, whereas BCL6-R is reported in up to 40% and MYC-R in ∼20% of cases. The tumor cells are often negative for CD10 (40%-100%) and BCL6 (0%-50%) with frequent IRF4/MUM1 (42%-100%) expression. Cases expressing IRF4/MUM1 should be evaluated for IRF4 alterations, to exclude the diagnosis of large B-cell lymphoma with IRF4-R (discussed hereafter). Interestingly, 2 gene expression profiling studies concluded that FL3B/FLBL has a distinct signature closer to that of FL3A and different from DLBCL and FL grades 1/2.67,68 These data support that FL3B/FLBL belongs to the group of FL rather than DLBCL. More studies are warranted to clarify this issue; for the moment, FL3B/FLBL remains within FL.
Extranodal t(14;18)-negative FLs
PCFCL
PCFCL is a neoplasm composed predominantly of large centrocytes, often with diffuse growth pattern and expression of at least 1 GC marker. CD10 is positive in cases with follicular pattern but usually negative in cases with diffuse growth pattern.69 The t(14;18) chromosomal alteration is usually negative but up to 10% of cases show BCL2-R. The most frequent mutation reported in PCFCL is TNFRSF14 (40%) often with 1p36 deletion (10%-22%).34,35 However, the mutational profile differs from that of t(14;18)-positive FL with rare mutations in chromatin modifying genes (CREBBP, EZH2, and KMT2D), and frequent mutations in TNFAIP3 (25%). A scoring system to predict secondary skin involvement of a systemic FL has been proposed and includes: (1) the presence of mutations in chromatin modifier genes, (2) BCL2-R, and (3) low Ki-67 proliferation index.35 Two of these features support systemic disease. Interestingly, the mutational profile of PCFCL is similar to other extranodal t(14;18)-negative FLs,41,69-71 suggesting that inactivation of TNFRSF14 might play a role in the development of t(14;18)-negative lymphomas. The exact mechanism of TNFRSF14 mutations promoting FL lymphomagenesis remains to be elucidated. TNFRSF14 delivers both costimulatory (via tumor necrosis factor superfamily member 14, [TNFSF14] also named as LIGHT, and lymphotoxin-α) and inhibitory signals (via B- and T-lymphocyte attenuator [BTLA] and CD160) to T cells.72 It has been proposed that the disruption of the BTLA–TNFRSF14 pathway might be involved in the activation of GC B cells and that there is some pressure to inactivate TNFRSF14 to prevent interactions with BTLA-expressing TFH cells.23,73
Other extranodal primary t(14;18)-negative FL
With the exception of PCFCL, the genetics of extranodal t(14;18)-negative FL are not well defined. Recently, a group of FCLs of the lower female genital tract presenting in the uterine cervix and vagina was reported.70 These cases shared many features with PCFCL and was proposed as a novel variant of PCFCL. The most frequent mutated gene was TNFRSF14 (60%), with no mutations in chromatin modifying genes. The prognosis is excellent after systemic treatment, with complete remission in 83% of the cases.
Primary thyroid t(14;18)-negative FL is another rare but well-recognized variant.41 A recent study with relatively few cases demonstrated the lack of chromatin modifying gene mutations but no special mutations were identified.71 Similar cases have been reported in the ovary39 and other extranodal sites; however, no genetic information is available. Nevertheless, all these extranodal lymphomas present with localized disease (stage IE), predominantly large cell cytology, often diffuse growth areas, diminished CD10 expression, and excellent prognosis compared with their t(14:18)-positive counterpart.
FL in children and young adults
FL in children, adolescent, and young adults comprises a group of biologically and clinically distinct indolent lymphomas characterized by lack of t(14;18), high-grade cytology, high proliferation index but localized disease, and excellent prognosis. These lymphomas rarely affect adults. Three main defined entities are recognized: (1) pediatric-type FL (PTFL) with and without marginal zone differentiation, (2) large B-cell lymphoma with IRF4-R (LBCL-IRF4), and (3) testicular FL (TFL) (Table 1).
Comparison of clinicopathological and genetic features of PTFL, LBCL-IRF4, and TFL
| . | PTFL∗ . | LBCL-IRF4† . | TFL‡ . |
|---|---|---|---|
| Median age, y (range) | 15 (5-31) | 12 (3-28) | 4 (3-16) |
| M:F | 20:1 | 1.5:1 | --- |
| Stage at presentation | 100% localized Stages I-II | 89% localized Stages I-II | Mostly stage IE |
| Site of presentation | Head and neck LN Rare cases reported in conjunctiva | Waldeyer ring, mainly tonsils GI tract | Testis |
| Recommended treatment | Surgical excision, watch-and-wait 100% CR | 25% surgical excision only; 75% systemic treatment§ 100% CR | Orchiectomy only||; watch-and-wait 100% CR |
| Morphology | Large serpiginous, ill-defined follicles with attenuated lymphoid cuffs, and a prominent starry-sky pattern. Monotonous medium- to large-sized blastoid cells. Some cases with centroblastic morphology. | Large, closely packed expansile follicles predominantly composed of medium-sized blasts with fine chromatin, inconspicuous nucleoli, and scant cytoplasm. Some cases with centroblastic morphology¶ | Back-to-back atypical small follicles composed predominantly of centroblasts admixed with centrocytes associated with well-formed FDC meshworks |
| Diffuse areas | Negative | 41% purely diffuse 28% follicular/diffuse 31% purely follicular | Often observed, usually focal74 |
| Starry-sky pattern | Characteristic finding | Negative | Negative |
| CD10+ | 100% (strong) | 55% | Some negative cases |
| BCL2+ | 18% | 60% | Usually negative |
| BCL6+ | 100% (strong) | 100% | 100% |
| IRF4/MUM1+ | Negative | 100% | Negative |
| BCL2-R | Negative | Negative | Negative |
| BCL6-R | Negative | 11% | One case reported74 |
| IRF4-R | Negative | 100% ∼10% cryptic | Negative |
| Genetic features | Mutations: TNFRSF14 (54%) MAP2K1 (40%-50%) IRF8 (30%) | Mutations: IRF4 (85%), BCL6, and NF-κB pathway genes | Limited data available |
| . | PTFL∗ . | LBCL-IRF4† . | TFL‡ . |
|---|---|---|---|
| Median age, y (range) | 15 (5-31) | 12 (3-28) | 4 (3-16) |
| M:F | 20:1 | 1.5:1 | --- |
| Stage at presentation | 100% localized Stages I-II | 89% localized Stages I-II | Mostly stage IE |
| Site of presentation | Head and neck LN Rare cases reported in conjunctiva | Waldeyer ring, mainly tonsils GI tract | Testis |
| Recommended treatment | Surgical excision, watch-and-wait 100% CR | 25% surgical excision only; 75% systemic treatment§ 100% CR | Orchiectomy only||; watch-and-wait 100% CR |
| Morphology | Large serpiginous, ill-defined follicles with attenuated lymphoid cuffs, and a prominent starry-sky pattern. Monotonous medium- to large-sized blastoid cells. Some cases with centroblastic morphology. | Large, closely packed expansile follicles predominantly composed of medium-sized blasts with fine chromatin, inconspicuous nucleoli, and scant cytoplasm. Some cases with centroblastic morphology¶ | Back-to-back atypical small follicles composed predominantly of centroblasts admixed with centrocytes associated with well-formed FDC meshworks |
| Diffuse areas | Negative | 41% purely diffuse 28% follicular/diffuse 31% purely follicular | Often observed, usually focal74 |
| Starry-sky pattern | Characteristic finding | Negative | Negative |
| CD10+ | 100% (strong) | 55% | Some negative cases |
| BCL2+ | 18% | 60% | Usually negative |
| BCL6+ | 100% (strong) | 100% | 100% |
| IRF4/MUM1+ | Negative | 100% | Negative |
| BCL2-R | Negative | Negative | Negative |
| BCL6-R | Negative | 11% | One case reported74 |
| IRF4-R | Negative | 100% ∼10% cryptic | Negative |
| Genetic features | Mutations: TNFRSF14 (54%) MAP2K1 (40%-50%) IRF8 (30%) | Mutations: IRF4 (85%), BCL6, and NF-κB pathway genes | Limited data available |
CR: complete remission; GI: gastrointestinal; FDC, follicular dendritic cells; M:F, male to female ratio; LN, lymph nodes; R: rearranged.
Data obtained from references 42, 78 and 93; 75 patients in total.
Data obtained from references 89, 91, 92, 93 and 94; 61 patients in total.
Data obtained from references 93, 74, 95, 96, 97, and 98; 20 cases in total.
Cases with purely follicular growth pattern and stage I disease do well only with surgical excision, most patients still receive systemic treatment.
Patients were previously treated with systemic therapy after orchiectomy. This is no longer recommended.
Aberrant expression of CD5 is well recognized.
PTFL with and without marginal zone differentiation
PTFL is a neoplastic condition that is distinct from adult-type FL. PTFL predominantly affects lymph nodes, presents with limited-stage disease, and has an invariably indolent behavior after a conservative watch-and-wait approach.40,75 Ocular/conjunctiva presentation is rare but well documented.76,77 Morphologically, PTFL is characterized by expanded and serpiginous follicles, composed of medium- to large-size blastoid cells,42,78 with strong expression of CD10 and BCL6. The demonstration of a monoclonal B-cell population is required for the diagnosis.79 Some PTFL have been reported to display marginal zone differentiation, and some pediatric NMZL to have GC markers, suggesting that PTFL and pediatric NMZL may represent a morphological spectrum within the same biological entity.80 Because of the shared indolent clinical behavior and similar morphological and genetic features, we have recently suggested to rename these entities under the name of PTFL with and without marginal zone differentiation. These findings have been confirmed by 2 subsequent studies, justifying the unification of these entities.43,81 PTFL displays low genomic complexity when compared with both t(14;18)-positive and t(14;18)-negative FL. In terms of mutational profile, PTFL lacks mutations in epigenetic regulators such as KMT2D, CREBBP, or EZH2, but shares the presence of biallelic inactivation of TNFRSF14 with other t(14;18)-negative FLs. Furthermore, 2 characteristic alterations that distinguish PTFL from other types of FL are mutations targeting IRF8 and MAP2K1 genes.40,78,82-84IRF8 and TNFRSF14 are critical regulators of immune system development and function and both genes regulate GC B-cell activation.23,85 Deficiency of IRF8 reportedly induce a hyperproliferative phenotype in pre-B cells.85IRF8 mutations at DNA binding motif hot spots K66R and Y23H seem to be specific to PTFL, in contrast to the IRF8 variants described in adult FL and DLBCL, which are frequently indel and missense mutations predominantly located in the C-terminal domain.17IRF8 mutations in PTFL are identified in ∼30% of cases and often cooccur with TNFRSF14 mutations, suggesting possible cooperation between these 2 genes.80,83,84MAP2K1 mutations have been identified in 40% to 50% of the cases and are associated with constitutive expression of phospho-ERK. The presence of MAP2K1 mutations supports the diagnosis of PTFL. TNFRSF14 and MAP2K1 mutations are mutually exclusive in most cases, suggesting that both mutations might play an important role in PTFL lymphomagenesis.
LBCL-IRF4
LBCL-IRF4 was introduced as a provisional entity in the 2017 WHO classification45,86 and is now recognized as a definitive entity in both the ICC11 and in the fifth edition of the WHO classification.5 LBCL-IRF4 is frequently seen in children and young adults and preferentially involves the head and neck region, including the Waldeyer ring, and the gastrointestinal tract; however, it can also occur in adults.44,87,88 It usually presents as a localized disease (stage I/II) with invariably excellent prognosis after systemic treatment.89-92 Morphologically, LBCL-IRF4 is often follicular and diffuse but it might be purely follicular or diffuse. It is composed predominantly of medium-sized blasts with high proliferation rate and sometimes a starry-sky pattern. The tumor cells express GC markers including CD10 and BCL6, together with IRF4/MUM1.93IGH::IRF4 rearrangements are detected in most of the cases, however, cryptic translocations occur.93 In the correct context, the demonstration of an IGH break in the absence of BCL2, BCL6, and MYC rearrangements, and/or presence of IRF4 mutations support the diagnosis.44,87,91,93,94MYC and BCL2 are not rearranged, but breaks affecting the BCL6 locus can be detected.89 The mutational profile shows recurrent somatic mutations of IRF4 and BCL6 in the context of aberrant SHM pattern (IRF4 mutations are likely a consequence of the juxtaposition of IRF4 to the IGH locus), and mutations in NF-κB pathway genes (CARD11, CD79B, and MYD88).89,91 Interestingly, mutations affecting the NF-κB pathway were restricted to cases with pure DLBCL morphology, whereas MAP2K1 mutations, characteristic of PTFL, were detected in 2 of 9 investigated cases with follicular component and/or predominantly follicular pattern (Figure 6).91 LBCL-IRF4 shows a GCB-type gene expression profile, despite the constitutive IRF4 overexpression.89
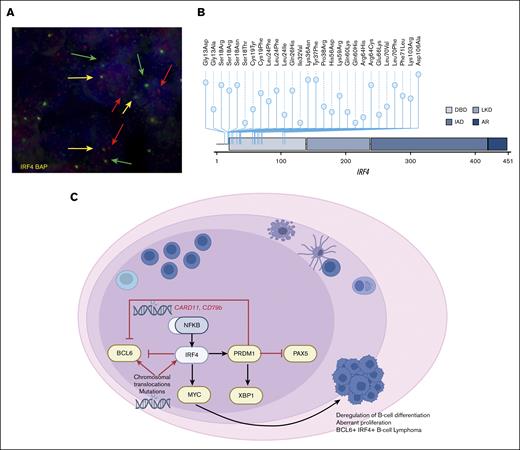
Genetic alterations and model of IRF4mut-mediated pathogenesis in LBCL-IRF4. (A) Fluorescence in situ hybridization with IRF4 break-apart probe shows a signal constellation of 1 colocalization (yellow arrow) and 1 split signal (red and green arrows) consistent with the gene rearrangement in a case with LBCL-IRF4 with pure FL morphology. (B) A diagram of the relative positions of IRF4 mutations in cases with LBCL-IRF444 (A Colmenero, I Salaverria, unpublished data, March 2023). x-axis indicates amino acid position. IRF4 domains: DBD, DNA binding domain; LKD, linker domain; IAD, IRF association domain; and AR, autoinhibitory region. (C) Schematic overview of B-cell differentiation deregulation by constitutive activation of IRF4 and/or NF-κB, which induces the oncogenic transcription program in LBCL-IRF4.
Genetic alterations and model of IRF4mut-mediated pathogenesis in LBCL-IRF4. (A) Fluorescence in situ hybridization with IRF4 break-apart probe shows a signal constellation of 1 colocalization (yellow arrow) and 1 split signal (red and green arrows) consistent with the gene rearrangement in a case with LBCL-IRF4 with pure FL morphology. (B) A diagram of the relative positions of IRF4 mutations in cases with LBCL-IRF444 (A Colmenero, I Salaverria, unpublished data, March 2023). x-axis indicates amino acid position. IRF4 domains: DBD, DNA binding domain; LKD, linker domain; IAD, IRF association domain; and AR, autoinhibitory region. (C) Schematic overview of B-cell differentiation deregulation by constitutive activation of IRF4 and/or NF-κB, which induces the oncogenic transcription program in LBCL-IRF4.
TFL
Primary TFL is a rare variant of FL that presents mainly in children, adolescent, and young adults, as stage IE disease.74,95-97 Morphologically, the atypical follicles are composed of a mixture of cells resembling classic centroblasts and centrocytes, usually associated with well-formed follicular dendritic cells (FDC) meshworks. In contrast to PTFL, TFL cells are negative for surface immunoglobulins, demonstrated by flow cytometry.98 The genetic features are poorly defined. Interestingly, in individual cases, similar to PTFL, mutations in TNFRSF14 and IRF8 have been reported, suggesting that these 2 disorders might be related.99 Future studies are needed to define the biology of this specific variant.
Conclusion and future perspectives
Although there is increasing recognition of distinct molecular differences between t(14;18)-positive and t(14;18)-negative FL, the clinical implications remain unclear. The revised classifications now acknowledge the t(14;18) status but the biggest challenge is to demonstrate clinical utility.
It is challenging to evaluate the clinical implications of t(14;18) negativity because of the paucity of cases and the high proportion of unknown translocation status in many cohorts because, in routine diagnosis and in most clinical trials, testing is not mandatory to make the diagnosis of FL. Moreover, t(14;18)-negative FL spans a wide spectrum of subtypes, which are often managed differently because of their distinct clinical presentation. For instance, PTFL is a highly indolent subtype that can be managed by complete surgical excision followed by observation in most cases.75 Likewise, PCFCL is a clinically indolent disease, confined to circumscribed skin areas and effectively treated with local radiotherapy or excision.100 In contrast, FL3B/FLBL is enriched for t(14;18)-negative cases and should be treated with regimens used for clinically aggressive lymphomas,101 because this subtype is clinically and molecularly more closely related to DLBCL.68 Thus, t(14;18)-negative FLs comprise both highly indolent as well as aggressive subtypes, and this makes it difficult to draw general conclusions.
Most data come from patients with nodal FL presenting with limited or advanced stage disease, who were enrolled in clinical trials, irrespective of the t(14;18) status. In retrospective analyses, no differences in clinical outcomes were observed for t(14;18)-positive and t(14;18)-negative FL with currently used cytotoxic treatment regimens. This included irradiation-based therapies for limited-stage disease (given with a curative intent), as well as anti-CD20–based immunochemotherapies for patients with advanced stage FL in need of treatment.102-104 However, this may change with the advent of novel molecular- and immune-targeting therapies that are increasingly available for clinical use, because biological differences may be unmasked and treatment outcomes may well differ depending on the molecular underpinnings of the disease. In fact, understanding the molecular mechanisms underlying t(14;18)-negative FL may inform individualized treatment decisions and pave the way to develop novel molecular- and/or immune-targeting therapies. Accordingly, nodal t(14;18)-negative FLs are enriched for STAT6 mutations, suggesting that targeting the IL-4/JAK/STAT pathway may be particularly beneficial for this patient subset.52 Furthermore, differences in the mutations that are involved in immune evasion (eg, CREBBP, EZH2, TNFRSF14, B2M, and FAS) and different immune landscapes may profoundly affect the efficacy of T-cell–based therapies, such as immune checkpoint blockade, bispecific antibodies, and chimeric antigen receptor T cells.105,106
Many questions remain unanswered, such as the initial oncogenic hit in t(14;18)-negative cases, whether t(14;18)-negative and t(14;18)-positive FLs share common precursor cells,17,28 and the role of lymphoid clonal hematopoiesis.107 It is also unclear whether the follicular phenotype in t(14;18)-negative FL, including its TME, is induced by alternative oncogenic programs that substitute for the BCL2 translocation/overexpression, or by BCL2-independent programs that are shared in FLs with and without the t(14;18) translocation. Finally, it remains to be determined to what extent the clinical differences are directly linked to the absence or presence of the BCL2 translocation itself, or rather the distinct associated molecular landscapes. Much remains to be learned about, but also from, rare subtypes like t(14;18)-negative FL, and a deeper understanding of the underlying molecular mechanisms will help to unravel the biological drivers responsible for divergent clinical behaviors, treatment susceptibilities, and resistance patterns. A more immediate use of comprehensive molecular analysis of FL subtypes is to resolve potentially challenging differential diagnosis.108
In conclusion, the new classifications5,11 that acknowledge the t(14;18) status are welcome, and knowing the translocation status (and the associated molecular landscape and TME alterations) will fill a critical knowledge gap, including diagnostic refinement and, potentially, patient stratification in the near future.
Acknowledgments
The authors thank Ivonne Montes-Mojarro for her help in designing the figures for this manuscript using the program BioRender.com. The authors also thank Snjezana Dotlic for providing 1 of the cases shown in Figure 1.
I.S. was supported by the Instituto de Salud Carlos III (Miguel Servet II Program CPII18/00015), Generalitat de Catalunya Suport Grups de Recerca (2017-SGR-1107 and 2021-SGR-01293), and the European Regional Development Fund “Una manera de fer Europa.” L.Q.-M. is funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany´s Excellence Strategy–EXC2180-390900677. O.W. is supported by the Else Kröner Excellence Fellowship (Else Kröner-Fresenius-Stiftung, 2021_EKES.13), the Lymphoma Research Foundation (Jaime Peykoff Follicular Lymphoma Initiative), the Wilhelm Sander-Stiftung (2022.093.1), and the German Research Foundation (WE 4679/2-1).
Authorship
Contribution: This review article was designed by L.Q.-M., I.S., and O.W.; all authors contributed to writing the manuscript; L.Q.-M. and I.S. designed and created all figures; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leticia Quintanilla-Martinez, Institute of Pathology and Neuropathology, Eberhard-Karls University of Tübingen and Comprehensive Cancer Center, University Hospital Tübingen, Liebermeisterstrasse 8, 72076 Tübingen, Germany; e-mail: leticia.quintanilla-fend@med.uni-tuebingen.de.

![Morphological and immunophenotypic features of nodal t(14;18)-positive and t(14;18)-negative FLs. (A) t(14;18)-positive FL showing a nodular growth pattern, with follicles growing back to back and the lack of a defined mantle zone, (original magnification ×50; hematoxylin and eosin [H&E] stain). (B) Higher magnification demonstrates the presence of predominantly centrocytes with few intermingled centroblasts in the follicles characteristic of FL grade 1/2, (original magnification ×400; H&E stain). (C-D) BCL2-R–neg CD23+ FCL with diffuse growth pattern. (C) The H&E stain shows an inguinal lymph node with partial involvement of a diffuse lymphoid infiltration that leaves a rim of normal lymph node in the periphery. (D) The tumor cells are strongly CD23+. Note that in the periphery of the lymph node there are residual normal GCs and the CD23 stain highlights the follicular dendritic cells. (E) The tumor cells are also strongly positive for CD10. The residual normal GCs are CD10+. (Snapshot of scanned slides, ×10). (F-G) BCL2-R–neg CD23+ FCL with follicular growth pattern. (F) The H&E stain shows an axillary lymph node with involvement of a lymphoid proliferation with follicular growth pattern (original magnification ×40; H&E stain). (G) The tumor cells are CD23+ (original magnification ×40; immunohistochemistry). (H) The tumor cells are BCL2−. Note that the B cells in the attenuated mantle zone are BCL2+. (original magnification ×400; immunohistochemistry). (I) The neoplastic cells in the follicles are CD23+, (original magnification ×400; immunohistochemistry).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/18/10.1182_bloodadvances.2022009456/2/m_blooda_adv-2022-009456-c-gr1.jpeg?Expires=1769087434&Signature=15zgNPHdP7t3rPq2FBusROu0KIu-KxwcP8rv1~MoxVsOpi3xSDigBR4tP7BDuv6n1NIjWC1as-50R0IjWyxfh-ZEJ-5GHOKIG3sHgT2C2pQdKVwnk1prriMowY0R0NXRF9hrtKJJz4qs8zGmNvlZ3CfONZElxZXdcTQ9LlD9glRYcG1C8tTxJqBs5JFHNIFg2gu54-TFuFxEnv~uHD~BJxw7PwrQ~UEcNnk7JcRm0B0k5~mKBGG~pg~WFh6SWuA0naqJ86OUTYC3Q-96Z5RG7KqXjB6VdSv7pZQm2jZaONWlpb4HYpryVm9k9wgRbynJHS2mkY8pErMHVMTbweLjew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)