TO THE EDITOR:
Introduction
Interferon gamma (IFN-γ) is a proinflammatory cytokine produced primarily by T cells and antigen presenting cells that enhances antigen presentation, induces expression of antiviral and microbicidal genes, and alters cell cycle proteins.1 IFN-γ has a fundamental role in pathologic inflammation in cytokine storm syndromes, including hemophagocytic lymphohistiocytosis (HLH) and cytokine release syndrome (CRS) secondary to chimeric antigen receptor T cells (CART).2,3 In 2018, the US Food and Drug Administration–approved emapalumab, a monoclonal antibody against IFN-γ, for use in refractory, recurrent, or progressive primary HLH or intolerance to conventional HLH therapy in children or adults.4 Based on the clinical efficacy of emapalumab against HLH,5 it has been used in anecdotal reports in other inflammatory conditions with elevated IFN-γ, including CRS induced by CART,6,7 macrophage activation syndrome secondary to systemic juvenile idiopathic arthritis,8 and transplant-associated thrombotic microangiopathy concurrent with HLH.9 We report the our center’s experience on the use of emapalumab in pediatric patients with CRS because of CART, compared with primary and secondary HLH and other inflammatory disorders.
Methods
A retrospective chart review was performed of all patients treated with emapalumab at the Children’s Hospital of Philadelphia (CHOP) between 20 November 2018 (Food and Drug Administration approval of emapalumab) and 8 March 2023. The CHOP Institutional Review Board determined that the study protocol met exemption criteria per 45 CFR 46.104(d) 4(iii). Study data were collected and managed using research electronic data capture (REDCap) electronic data capture tools hosted at CHOP.10,11 Data were subsequently exported into GraphPad Prism 9 for analysis. Two patients, 1 with HLH and 1 with CRS, have been reported previously.6,12
Results
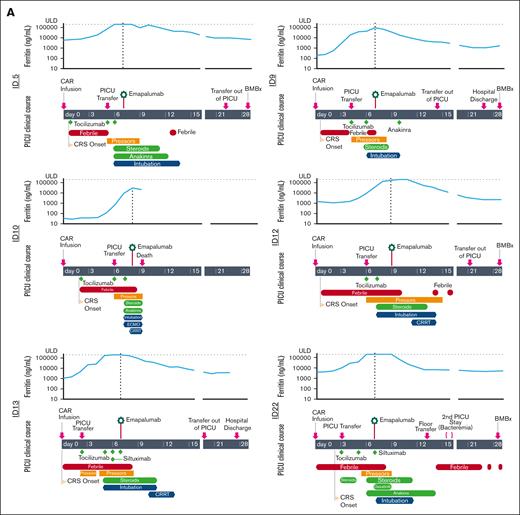
Of the 21 patients in our case series, 6 received emapalumab for the treatment of CRS refractory to standard therapies (Table 1; supplemental Table 1). All patients with CRS were diagnosed with relapsed B-cell acute lymphoblastic leukemia and received CART directed against CD19 (CART19). They subsequently developed severe CRS and immune effector cell–associated neurotoxicity syndrome and received escalation of care to the intensive care unit followed by intubation and usage of multiple vasoactive medications (Figure 1A). Unlike non-CRS indications that received a median of 3.5 doses of emapalumab (range 1-21) with up to 2 dose escalations, all patients with CRS received a single 1 mg/kg dose (supplemental Table 2). After administration of emapalumab, 5 of 6 patients subsequently had a pronounced decrease in inflammatory markers, including ferritin, and systemic cytokines, including IFN-γ, interleukin 2 (IL-2), IL-1β, and tumor necrosis factor (Figure 1A-B; supplemental Figure 1). All patients received acyclovir prophylaxis after emapalumab without development of subsequent infection, unless noted below. The individual courses of the patients were as follows:
Cohort characteristics
| CRS . | ||||||
|---|---|---|---|---|---|---|
| ID . | Sex . | Age (y) . | Primary diagnosis . | Location of emapalumab administration . | Day 28 disease status . | Survival at 6 mo . |
| 5 | M | 16 | B-ALL | ICU | MRD negative | Y |
| 9 | F | 5 | B-ALL | ICU | MRD negative | Y |
| 10 | M | 5 | B-ALL | ICU | N/A | N |
| 12 | M | 19 | B-ALL | ICU | MRD negative | Y |
| 13 | F | 12 | B-ALL | ICU | N/A | Y |
| 22 | M | 20 | B-ALL | ICU | CRi | N/A |
| Primary hemophagocytic lymphohistiocytosis | ||||||
| ID | Sex | Age (y) | Causative mutation | Location of emapalumab administration | Subsequent HSCT? | Survival at 6 mo |
| 1 | M | 0.3 | STXBP2 | ICU | Y | Y |
| 14 | M | 0.2 | STXBP2 | ICU | Y | Y |
| 2 | F | 0.8 | PRF (homozygous) | Ward | Y | Y |
| 16 | M | 0.4 | PRF (complex heterogeneous) | Clinic | Y | Y |
| 17 | M | 1.1 | PRF (homozygous) | Clinic | Y | Y |
| Secondary hemophagocytic lymphohistiocytosis | ||||||
| ID | Sex | Age (y) | Causative disease | Location of emapalumab administration | Subsequent HSCT? | Survival at 6 mo |
| 4 | F | 15 | MAS due to unknown primary disease | ICU | N/A | Y |
| 15 | F | 11 | MAS due to sJIA | Clinic | N/A | Y |
| 6 | F | 12 | MAS due to sJIA | Ward | N/A | Y |
| 3 | M | 0.5 | SAA, iPALF | Ward | N/A | Y |
| 7 | M | 9 | SAA, monosomy 7 | ICU | N/A | N |
| 8 | F | 16 | NK lymphoma, EBV viremia | ICU | N/A | N |
| 11.1 | M | 26 | CAEBV | ICU | Y | Y |
| 11.2 | M | 27 | CAEBV, PTCL | Ward | Y | Y |
| 20 | F | 12 | EBV viremia | ICU | N/A | N |
| Other indication | ||||||
| ID | Sex | Age (y) | Primary diagnosis | Location of emapalumab administration | Indication for emapalumab | Survival at 6 mo |
| 18 | F | 21 | SAA, HSCT | ICU | Inflammatory pulmonary disease | N |
| 21 | F | 13 | Beta thalassemia major, HSCT | Ward | Crohn’s disease/GVHD | N/A |
| CRS . | ||||||
|---|---|---|---|---|---|---|
| ID . | Sex . | Age (y) . | Primary diagnosis . | Location of emapalumab administration . | Day 28 disease status . | Survival at 6 mo . |
| 5 | M | 16 | B-ALL | ICU | MRD negative | Y |
| 9 | F | 5 | B-ALL | ICU | MRD negative | Y |
| 10 | M | 5 | B-ALL | ICU | N/A | N |
| 12 | M | 19 | B-ALL | ICU | MRD negative | Y |
| 13 | F | 12 | B-ALL | ICU | N/A | Y |
| 22 | M | 20 | B-ALL | ICU | CRi | N/A |
| Primary hemophagocytic lymphohistiocytosis | ||||||
| ID | Sex | Age (y) | Causative mutation | Location of emapalumab administration | Subsequent HSCT? | Survival at 6 mo |
| 1 | M | 0.3 | STXBP2 | ICU | Y | Y |
| 14 | M | 0.2 | STXBP2 | ICU | Y | Y |
| 2 | F | 0.8 | PRF (homozygous) | Ward | Y | Y |
| 16 | M | 0.4 | PRF (complex heterogeneous) | Clinic | Y | Y |
| 17 | M | 1.1 | PRF (homozygous) | Clinic | Y | Y |
| Secondary hemophagocytic lymphohistiocytosis | ||||||
| ID | Sex | Age (y) | Causative disease | Location of emapalumab administration | Subsequent HSCT? | Survival at 6 mo |
| 4 | F | 15 | MAS due to unknown primary disease | ICU | N/A | Y |
| 15 | F | 11 | MAS due to sJIA | Clinic | N/A | Y |
| 6 | F | 12 | MAS due to sJIA | Ward | N/A | Y |
| 3 | M | 0.5 | SAA, iPALF | Ward | N/A | Y |
| 7 | M | 9 | SAA, monosomy 7 | ICU | N/A | N |
| 8 | F | 16 | NK lymphoma, EBV viremia | ICU | N/A | N |
| 11.1 | M | 26 | CAEBV | ICU | Y | Y |
| 11.2 | M | 27 | CAEBV, PTCL | Ward | Y | Y |
| 20 | F | 12 | EBV viremia | ICU | N/A | N |
| Other indication | ||||||
| ID | Sex | Age (y) | Primary diagnosis | Location of emapalumab administration | Indication for emapalumab | Survival at 6 mo |
| 18 | F | 21 | SAA, HSCT | ICU | Inflammatory pulmonary disease | N |
| 21 | F | 13 | Beta thalassemia major, HSCT | Ward | Crohn’s disease/GVHD | N/A |
Patient 11 received emapalumab at 2 separate times for different indications.
B-ALL, B-cell acute lymphoblastic leukemia; CAEBV, chronic active Epstein Barr virus; CRi, complete remission with incomplete count recovery; EBV, Epstein Barr virus; F, female; GVHD, graft versus host disease; HSTCT, hematopoietic stem cell transplant; ICU, intensive care unit; iPALF, indeterminate pediatric acute liver failure; M, male; MAS, macrophage activation syndrome; N, no; N/A. not available; NK, natural killer; PRF, perforin; PTCL, peripheral T-cell lymphoma; sJIA, systemic juvenile idiopathic arthritis; SAA, severe aplastic anemia; Y, yes.
Clinical course and cytokine levels for patients with CRS after CART19 who received emapalumab. (A) Clinical course of patients who underwent emapalumab treatment for CRS after CART19. Upper graph for each patient shows serial ferritin levels, with horizontal dotted line representing the upper limit of detection and the vertical line delineating the date of emapalumab treatment. (B) Cytokine levels the day before and within 48 hours after emapalumab administration. Upper and lower dotted lines on graphs represent the upper and lower limits of detection, respectively. Patient ID12 did not receive a post-emapalumab cytokine panel, so was not included in this figure.
Clinical course and cytokine levels for patients with CRS after CART19 who received emapalumab. (A) Clinical course of patients who underwent emapalumab treatment for CRS after CART19. Upper graph for each patient shows serial ferritin levels, with horizontal dotted line representing the upper limit of detection and the vertical line delineating the date of emapalumab treatment. (B) Cytokine levels the day before and within 48 hours after emapalumab administration. Upper and lower dotted lines on graphs represent the upper and lower limits of detection, respectively. Patient ID12 did not receive a post-emapalumab cytokine panel, so was not included in this figure.
Patient 5 developed CRS on day +1 with initiation of vasoactive medications on day +5 and intubation on day +6. They received tocilizumab (IL6 receptor antibody) on days +1, +5, and +6 and started dexamethasone and anakinra (IL-1 receptor antagonist) on day +6. They received emapalumab on day +7, discontinued vasoactive medications on day +9, and were extubated on day +16. Day 28 bone marrow biopsy (BMBx) demonstrated a measurable residual disease (MRD)–negative remission.
Patient 9 developed CRS on day +1 with initiation of vasoactive medications on day +4 and intubation on day +6. They received tocilizumab on days +4 and +6, started methylprednisolone on day +6, and started anakinra on day +10. They received emapalumab on day +7, discontinued vasoactive medications on day +8, and were extubated on day +10. Day 28 BMBx demonstrated MRD-negative remission.
Patient 10 developed CRS on day +1 with initiation of vasoactive medications on day +6 and intubation on day +7. They received tocilizumab on days +2, +6, and +7 and started methylprednisolone and anakinra on day +7. Owing to clinical decompensation, they started extracorporeal membrane oxygenation (ECMO) on day +7. They received emapalumab on day +8, had progressive CRS requiring initiation of dialysis, and died of cerebral edema <24 hours after receiving emapalumab.
Patient 12 developed CRS on day +1 with initiation of vasoactive medications on day +6 and intubation on day +7. They received tocilizumab on days +6 and +7 and started methylprednisolone on day +7. They received emapalumab on day +9, were extubated on day +14, and discontinued vasoactive medications on day +15. They underwent dialysis from days +11 to 14 with subsequent resumption of native kidney function without chronic kidney disease. Day 28 BMBx demonstrated MRD-negative remission.
Patient 13 developed CRS on day 0 with initiation of vasoactive medications on day +2 and intubation on day +5. They received tocilizumab on days +2, +5, +6, and +7, started methylprednisolone on day +5, and received siltuximab (IL-6 antibody) on day +6. They received emapalumab on day +7, discontinued vasoactive medications on day +8, and were extubated on day +11. They underwent dialysis from days +10 to 13 with subsequent resumption of native kidney function without chronic kidney disease. They did not undergo BMBx at day 28 and were not evaluable for response.
Patient 22 developed CRS on day +2 with initiation of vasoactive medications on day +5 and intubation on day +7. They received tocilizumab on days +3 and +5, started dexamethasone on day +3, started anakinra on day +6, received siltuximab on day +7, and dasatinib on days +7 to 9. They received emapalumab on day +7, discontinued vasoactive medication on day +9, and were extubated on day +11. They developed enterococcus faecium bacteremia 9 days after emapalumab administration but did not require hemodynamic support. Day 28 BMBx showed a morphologic complete response with incomplete count recovery.
Discussion
In this report, we describe the targeted use of emapalumab for CRS after CART19. Our group and others have previously reported single cases of the use of emapalumab in the treatment of severe, refractory, secondary HLH12 and CRS.6,7 Furthermore, recent studies in mice suggest that CART efficacy is preserved in the setting of IFN-γ blockade in hematologic malignancies but not in solid tumors.13 In our report, all 4 evaluable patients had no morphologic or MRD-level evidence of leukemia on day 28, suggesting that emapalumab does not impair the short-term efficacy of CART19. The impact of emapalumab on long-term efficacy of CART should be investigated in therapeutic trials. In our institutional experience, emapalumab was used after the development of severe CRS when patients had severe organ dysfunction (respiratory, renal, and cardiac dysfunction). Future studies should prospectively evaluate the potential of emapalumab for the prophylaxis of severe CRS in those at highest risk to avoid severe organ toxicity or mortality. Importantly, all patients with CRS also received other immunomodulatory agents. Although we present exclusively retrospective data in this case series, the ability to safely combine emapalumab with multiple immunomodulatory agents will serve as a critical foundation for future prospective trials.
Future studies should examine optimal dosing regimens. Given the prolonged half-life of emapalumab of up to 17.5 days in pediatric patients,14 we used a single-dose regimen to minimize impact on CART efficacy. However, future pharmacokinetic studies can establish whether dose escalation or multiple administrations may be more effective, as the levels of IFN-γ and CXCL9 are very high in severe CRS, often exceeding those seen in primary HLH.
In summary, emapalumab was safe and effective in our cohort of patients with refractory CRS. These results warrant future study in prospective trials.
Acknowledgments: The authors thank Meghan Rys for assistance with preparation and submission of the report for the institutional review board approval. C.D. was supported by an American Society of Clinical Oncology (ASCO) Conquer Cancer YI Award, a Canadian Institues of Health Reseasrch (CIHR) Fellowship Award, and the Abramson Cancer Center K12.
Contribution: M.R.S., D.T.T., and C.D. designed the study; C.C., A.L., C.D., and M.R.S. collected the data; M.R.S., H.B., E.M.B., S.C., A.D., S.G., M.L., R.M., S.L.M., K.E.S., D.T.T., and C.D. prepared and revised the manuscript; and all authors made significant intellectual contributions to this manuscript, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: E.M.B. receives research funding from AB2Bio and provides consultancy to Sobi and Genzyme. S.G. receives research funding from Novartis, Kite, Vertex, and Servier; provides consultancy to Novartis, Jazz pharmaceuticals, Roche, GlaxoSmithKline, Humanigen, Cellular Biomedicine Group, Eureka, and Janssen/Johnson & Johnson; and serves on scientific advisory boards or steering committees for Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene and Cabaletta. S.L.M. reports clinical trial support from Novartis, Wugen; provides consulting or is on the advisory boards or study steering committee of Novartis; and has the following patent pending and licensed to Novartis Pharmaceuticals for PCT/US2017/044425: Combination Therapies of CAR and PD-1 Inhibitors. D.T.T. receives research funding from Jazz, Servier, Beam Therapeutics, and NeoImmune Tech; serves on the advisory boards for Sobi and Jazz; and holds multiple patents on CAR T-cell therapy. The remaining authors declare no competing financial interests.
Correspondence: Caroline Diorio, Division of Oncology, Department of Pediatrics, The Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, 3401 Civic Center Blvd, Philadelphia, PA 19104; e-mail: diorioc@chop.edu.
References
Author notes
The data that support the findings of this study are available on request from the corresponding author, Caroline Diorio (diorioc@chop.edu).
The full-text version of this article contains a data supplement.