Key Points
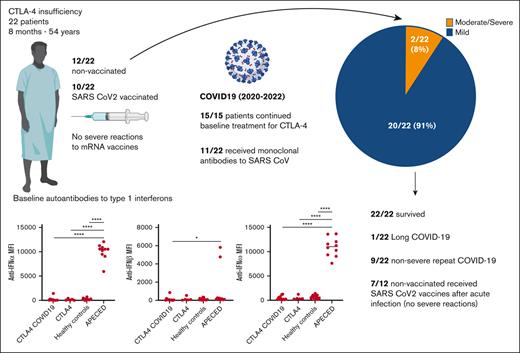
Despite defective T regulatory function and immune dysregulation, most patients with CTLA-4 insufficiency developed mild COVID-19.
Patients with CTLA-4 tolerated SARS CoV-2 messenger RNA vaccines with no serious adverse effects.
Abstract
Despite the high incidence of COVID-19 worldwide, clinical experience with severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) in inborn errors of immunity remains limited. Recent studies have shown that patients with defects in type 1 interferon (IFN)-related pathways or those with autoantibodies against type 1 IFNs develop severe COVID-19. We reported the clinical course of 22 patients with CTLA-4 insufficiency and COVID-19 and retrospectively examined autoantibodies against type 1 IFNs at baseline. Data were obtained from the patient interviews and chart reviews. Screening for anti-IFN autoantibodies was performed using a multiplex particle-based assay. Student t test, Mann Whitney, analysis of variance, or χ2 tests were used where appropriate. Twenty-two patients aged from 8 months to 54 years, with genetically confirmed CLTA-4 insufficiency, developed COVID-19 from 2020 to 2022. The most common symptoms were fever, cough, and nasal congestion, and the median duration of illness was 7.5 days. Twenty patients (91%) developed mild COVID-19 and were treated as outpatients. Two patients were hospitalized because of COVID-19 pneumonia but did not require mechanical ventilation. Ten (45%) patients were vaccinated at the time of their first COVID-19 infection. Eleven patients received outpatient treatment with monoclonal antibodies against the SARS-CoV-2 spike protein. During the study period, 17 patients were vaccinated against SARS-CoV-2, with no severe vaccine-related adverse effects. Although median anti-S titers following vaccination or infection were lower in patients receiving immunoglobulin replacement therapy (IGRT) (349 IU/dL) than in those not receiving IGRT (2594 IU/dL; P = .15); 3 of 9 patients on IGRT developed titers >2000 IU/dL. All patients tested negative for autoantibodies against IFN-α, IFN-β, and IFN-ω at baseline. Most patients with CTLA-4 insufficiency and COVID-19 had nonsevere disease, lacked autoantibodies against type 1 IFNs, and tolerated messenger RNA vaccines with few adverse effects. Whether our findings can be extrapolated to patients receiving CTLA-4-targeting checkpoint inhibitors requires further studies.
Introduction
With more than 6 million deaths and 700 000 000 cases worldwide, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has affected patients across all disease states, including those with rare inborn errors of immunity (IEIs), a heterogenous group of >470 genetic disorders characterized by susceptibility to infection and/or immune dysregulation. Clinicians caring for patients with IEIs face the challenge of making recommendations based on limited data. One multicenter study reported the clinical outcomes of 94 patients with IEI and COVID-19 but included a highly heterogenous group of genetic etiologies, limiting conclusions that can be made for specific genetic defects.1 One of the largest cohorts examining the effects of COVID-19 on a single gene disorder included 22 patients with the immune dysregulatory disorder autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) who harbor neutralizing autoantibodies against type 1 interferons (IFNs), which are known to be important for early viral immunity2-4; patients with APECED were found to have high rates of severe disease and mechanical ventilation. In patients with APECED and in the general population, autoantibodies against type 1 IFNs have been associated with severe COVID-19.2 However, for most monogenic IEI, including primary immunoregulatory disorders driven by T-cell and B-cell autoimmunity, the risk of severe COVID-19 or the presence of autoantibodies against type 1 IFNs have not been established.
CTLA-4 insufficiency is an autosomal dominant, monogenic IEI characterized by multiorgan autoimmunity and immune dysregulation with variable penetrance.5-7 CTLA-4 is an inhibitory immune checkpoint receptor constitutively expressed by regulatory T cells (Tregs), competing with CD28 for its ligands CD80/CD86 on antigen-presenting cells, which play a pivotal role in establishing immune tolerance.8,9 Genetic defects in CTLA-4 lead to decreased Treg numbers and suppressor function, T-cell infiltration of target organs, hypogammaglobulinemia, lymphopenia, natural killer cell dysfunction, autoantibodies against several tissues, and increased susceptibility to bacterial, viral, and parasitic infections.5,6,10,11 The risk of severe COVID-19 in CTLA-4 is currently unknown. Furthermore, both patients and providers are often concerned about the possibility of disease exacerbations triggered by SARS CoV-2 vaccination, which has not yet been studied in this patient population. Given the susceptibility to autoimmunity and lymphopenia in CTLA-4 insufficiency, we hypothesized that our patients would develop higher rates of severe disease than the general population and that disease severity could be correlated with the numbers of autoantibodies against type 1 IFNs. Here we report COVID-19 severity, clinical presentation, management, reinfection rates, incidence of severe reactions to SARS-CoV-2 vaccination, and antibody responses of patients with CTLA-4 insufficiency. In addition, we evaluated the presence of autoantibodies against type I IFNs at baseline.
Methods
Patients were followed up in accordance with National Institute of Allergy and Infectious Disease Institutional Review Board–approved protocols. All patients were diagnosed with COVID-19 by their local providers using polymerase chain reaction or antigen testing. Data were obtained via patient interviews and chart reviews and prospectively collected under our natural history protocol. Additional data were retrospectively collected and analyzed for the purpose of this study. The severity of COVID-19 was assessed as asymptomatic, mild, moderate, severe, or critical illness based on published guidelines.12 Screening for anti-IFN autoantibodies in the plasma samples was performed using a particle-based assay. Briefly, we covalently coupled differentially fluorescent magnetic beads to recombinant human type 1 IFNs and incubated them for 30 minutes with 1:100 diluted serum or plasma samples. Subsequently, the samples were incubated with PE-labeled goat anti-human immunoglobulin G antibody for 30 minutes, analyzed using a BioPlex X200 instrument, and the mean fluorescence intensity was quantified. The methods have been previously described in detail by Shaw et al.13 Patients with CTLA-4 insufficiency who did not contract COVID-19 during the study period, healthy controls, and patients with APECED were used as controls. All plasma samples predated the SARS-CoV-2 infection and were retrieved from the National Institute of Allergy and Infectious Disease/National Institutes of Health biobank. One-way analysis of variance was conducted to compare 3 or more groups, followed by post hoc Tukey tests. Student t or Mann Whitney tests was used to compare the groups. Fischer exact test was used to compare categorical variables.
Results
Baseline characteristics, initial COVID-19 presentation, disease severity, and autoantibodies against type 1 IFNs
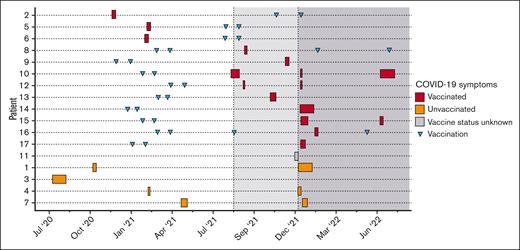
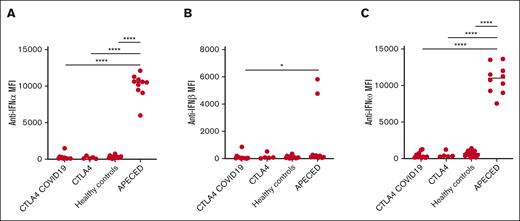
Twenty-two patients (17 residing in the United States [P1-P17], 4 in Germany [P18-P21], and 1 in Uruguay [P22]) developed COVID-19 from June 2020 to July 2022. The patients were aged from 8 months to 54 years (mean age, 31 years), 11 of 22 were female, and all had genotypically confirmed CTLA-4 insufficiency (Table 1). Twenty patients had 2 or more disease manifestations of CTLA-4 insufficiency. P14 and P17 were diagnosed based on an affected sibling but were asymptomatic. Baseline treatment for CLTA-4 insufficiency included mammalian target of rapamycin inhibitors (8/22), immunoglobulin replacement (12/22), abatacept (8/22), and/or steroids (4/22). Most patients were lymphopenic at baseline before contracting COVID-19 (median absolute lymphocyte count, 1080 cells/mL). Although variant strain identification was available for only 1 patient (P4, who tested positive for Omicron during the second COVID-19 infection), we plotted each COVID-19 episode in relation to the Center for Disease Control variance surveillance data for all patients who resided in the US at the time of infection (Figure 1). Approximately half of the patients (10/22) had received at least 1 dose of SARS CoV-2 vaccine at the time of their first COVID-19 episode (Figure 1). The most common symptoms were fever (11/22), nasal congestion (11/22), and cough (10/22) (Table 1). Most patients had mild disease (20/22) and were managed as outpatients. Two patients had moderate or severe disease: P3 was hospitalized for COVID-19 pneumonia requiring supplemental oxygen, and P19 reported dyspnea without requiring oxygen supplementation. No patient required critical care unit admission or mechanical ventilation. Baseline sera were available for 11 patients (including P3), and none had neutralizing autoantibodies against IFN-α, IFN-β, or IFN-ω before contracting SARS-CoV-2 (Figure 2).
Patient characteristics and initial COVID-19 disease course
| Patient . | Sex and age . | CTLA4 variant . | CTLA4 manifestations . | Baseline ALC (cells per mL) . | Auto-Abs to type 1 IFNs . | Immunomo-dulatory treatment . | Initial SARS-CoV-2 testing . | Manifestations of COVID-19 . | COVID-19 severity . | Treatment start, d∗ . | COVID-19 treatment . | Symptom resolution . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M 20 y | c.223C>T (R75W) | AIHA, ITP CNS infiltrate CVID Enteritis GH deficiency HSM ILD LAD | 810 | Neg | Everolimus Abatacept IVIG | PCR | Nasal congestion Anosmia | Mild | D 6 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose Azithromycin 250 mg daily for 7 d | D 7 |
| 2 | F 34 y | c.151C>T (R51X) | AI CVID Lymphopenia Neutropenia PA | 410 | Neg | Sirolimus IVIG | Antigen | Nasal congestion Anosmia Nausea Sore throat Cough Muscle pain Joint pain | Mild | D 4 | Bamlavinimab 700 mg 1 dose | D 7 |
| 3 | M 27 y | c.436G>A (G146R) | AI Arthritis Bronchiectasis CVID Enteritis HSM HT ILD Neutropenia, ITP PA | 690 | Neg | Sirolimus Abatacept IVIG | Antigen | Nasal congestion Anosmia Sore throat Cough SOB Fever Night sweats Fatigue Pulmonary infiltrates Hypoxemia | Severe | D 7 | Remdesevir 200 mg 1 dose followed by 100 mg daily for 2 d Meropenem 1g q 8 h for 3 d Azithromycin 250 mg daily for 7 d Dexamethasone ASA 81 daily | D 30 |
| 4 | M 20 y | c.410C>T (P137L) | ILD LAD Neutropenia Subclinical HT | 1520 | Neg | Everolimus | PCR | Fever Sore throat Headache | Mild | D 2 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 4 |
| 5 | F 19 y | c.436G>A (G146R) | AIHA, ITP Neutropenia Chronic Norovirus enteritis CNS infiltrate CVID HSM ILD LAD | 540 | Neg | IVIG | PCR | Fatigue Sore throat Cough Fever | Mild | D 3 | Bamlavinimab 700mg 1 dose | D 7 |
| 6 | F 54 y | c.436G>A (G146R) | Hypogammaglobulinemia | 2710 | Neg | None | PCR | Anosmia Fatigue Fever Headache Nasal congestion Sore throat | Mild | NA | None | D 7 |
| 7 | M 36 y | c.151C>T (p.R51X) | Arthritis CVID ITP T1DM | 2740 | Neg | IVIG | PCR | Chest pain Cough Fever Muscle pain | Mild | D 5 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 13 |
| 8 | M 38 y | c.151C>T p.Arg51Ter | AIHA, ITP CNS infiltrates CVID | 1211 | Unk | Abatacept SQIG | PCR | Cough Diarrhea Nasal congestion Nausea | Mild | None | None | D 5 |
| 9 | M 27 y | c.1A>G p.Met1 | Aplastic anemia CVID Enteropathy | 570 | Neg | Sirolimus IVIG | PCR | Cough Diarrhea Headache Nasal congestion | Mild | D 13 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | Unk |
| 10 | M 24 y | c.223C>T p.Arg75Trp | AIHA, ITP CNS infiltrates CVID Enteropathy HT ILD LAD | 350 | Neg | Abatacept IVIG | PCR | Fever Headache Nasal congestion | Mild | D 2 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 18‡ |
| 11 | M 44 y | 75delG (L28FfS†44) | AIHA, ITP CNS infiltrates CVID Enteropathy HSM ILD LN | 760 | Neg | Abatacept | PCR | Fever Muscle pain Sore throat | Mild | D 5 | Sotrovimab 500 mg Remdesevir 200 mg 1 dose followed by 100 mg daily for 2 d | D 7 |
| 12 | M 20 y | 410C>T (P137L) | CNS infiltrates Enteropathy ITP Neutropenia LN | 1310 | Neg | Abatacept Sirolimus | PCR | Fever Headache Sore throat | D 1 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 2 | |
| 13 | F 5 y | 410C>T (P137L) | HT | 1500 | Unk | None | PCR | Fatigue | Mild | D 11 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 13† |
| 14 | F 48 y | 410C>T (P137L) | Asymptomatic | 1960 | Unk | None | PCR | Fatigue Cough Nasal congestions | Mild | 1 | Remdesevir 200 mg 1 dose followed by 100 mg daily for 4 d | D 30 |
| 15 | M 27 y | 223C>T (R75W) | CNS infiltrates CVID Enteropathy HSM ILD | 2760 | Unk | IVIG Prednisone | PCR | Nasal congestion | Mild | NA | None | D 15 |
| 16 | M 19 y | 567+5G>C | AIHA CNS infiltrates CVID Eczema Enteropathy HSM ILD | 890 | Unk | IVIG Sirolimus | PCR | Nasal congestion | Mild | 4 | Sotrovimab 500 mg 1 dose | D 6 |
| 17 | M 51 y | 223C>T (R75W) | Asymptomatic | 2610 | Unk | None | PCR | Asymptomatic | NA | None | D 11 | |
| 18 | F 45 y | c.371_372insG (L126TfsTer28) | AI Arthritis Colitis CVID Eczema HT ITP LN PA Vitiligo | 7960 | Unk | IVIG Abatacept Prednisolone Budesonide | PCR | Cough Fever | Mild | D 4 | Remdesevir 200 mg 1 dose followed by 100 mg daily for 4 d | D 7 |
| 19 | F 32 y | c.416A>G (Y139C) | Alopecia Eczema HT | Unk | Unk | None | PCR | Anosmia Cough SOB | Moderate | NA | None | D 30 |
| 20 | F 8 mo | c.416A>G (Y139C) | Arthritis | Unk | Unk | Prednisone | PCR | Fever Nasal congestion | Mild | NA | None | D 8 |
| 21 | F 19 y | c.487delC (L163SfsTer24) | AIHA Colitis CVID ITP Psoriasis | 410 | Unk | IVIG Prednisolone | PCR | Anosmia Cough Fatigue Fever Headache Muscle Ache | Mild | D 2 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 8 |
| 22 | F 34 y | c.280 G>T (p.Glu94X) | Arthritis CVID Enteropathy HT | 950 | Unk | Sirolimus Abatacept IVIG | Antigen | Fatigue | Mild | None | None | D 20 |
| Patient . | Sex and age . | CTLA4 variant . | CTLA4 manifestations . | Baseline ALC (cells per mL) . | Auto-Abs to type 1 IFNs . | Immunomo-dulatory treatment . | Initial SARS-CoV-2 testing . | Manifestations of COVID-19 . | COVID-19 severity . | Treatment start, d∗ . | COVID-19 treatment . | Symptom resolution . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M 20 y | c.223C>T (R75W) | AIHA, ITP CNS infiltrate CVID Enteritis GH deficiency HSM ILD LAD | 810 | Neg | Everolimus Abatacept IVIG | PCR | Nasal congestion Anosmia | Mild | D 6 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose Azithromycin 250 mg daily for 7 d | D 7 |
| 2 | F 34 y | c.151C>T (R51X) | AI CVID Lymphopenia Neutropenia PA | 410 | Neg | Sirolimus IVIG | Antigen | Nasal congestion Anosmia Nausea Sore throat Cough Muscle pain Joint pain | Mild | D 4 | Bamlavinimab 700 mg 1 dose | D 7 |
| 3 | M 27 y | c.436G>A (G146R) | AI Arthritis Bronchiectasis CVID Enteritis HSM HT ILD Neutropenia, ITP PA | 690 | Neg | Sirolimus Abatacept IVIG | Antigen | Nasal congestion Anosmia Sore throat Cough SOB Fever Night sweats Fatigue Pulmonary infiltrates Hypoxemia | Severe | D 7 | Remdesevir 200 mg 1 dose followed by 100 mg daily for 2 d Meropenem 1g q 8 h for 3 d Azithromycin 250 mg daily for 7 d Dexamethasone ASA 81 daily | D 30 |
| 4 | M 20 y | c.410C>T (P137L) | ILD LAD Neutropenia Subclinical HT | 1520 | Neg | Everolimus | PCR | Fever Sore throat Headache | Mild | D 2 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 4 |
| 5 | F 19 y | c.436G>A (G146R) | AIHA, ITP Neutropenia Chronic Norovirus enteritis CNS infiltrate CVID HSM ILD LAD | 540 | Neg | IVIG | PCR | Fatigue Sore throat Cough Fever | Mild | D 3 | Bamlavinimab 700mg 1 dose | D 7 |
| 6 | F 54 y | c.436G>A (G146R) | Hypogammaglobulinemia | 2710 | Neg | None | PCR | Anosmia Fatigue Fever Headache Nasal congestion Sore throat | Mild | NA | None | D 7 |
| 7 | M 36 y | c.151C>T (p.R51X) | Arthritis CVID ITP T1DM | 2740 | Neg | IVIG | PCR | Chest pain Cough Fever Muscle pain | Mild | D 5 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 13 |
| 8 | M 38 y | c.151C>T p.Arg51Ter | AIHA, ITP CNS infiltrates CVID | 1211 | Unk | Abatacept SQIG | PCR | Cough Diarrhea Nasal congestion Nausea | Mild | None | None | D 5 |
| 9 | M 27 y | c.1A>G p.Met1 | Aplastic anemia CVID Enteropathy | 570 | Neg | Sirolimus IVIG | PCR | Cough Diarrhea Headache Nasal congestion | Mild | D 13 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | Unk |
| 10 | M 24 y | c.223C>T p.Arg75Trp | AIHA, ITP CNS infiltrates CVID Enteropathy HT ILD LAD | 350 | Neg | Abatacept IVIG | PCR | Fever Headache Nasal congestion | Mild | D 2 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 18‡ |
| 11 | M 44 y | 75delG (L28FfS†44) | AIHA, ITP CNS infiltrates CVID Enteropathy HSM ILD LN | 760 | Neg | Abatacept | PCR | Fever Muscle pain Sore throat | Mild | D 5 | Sotrovimab 500 mg Remdesevir 200 mg 1 dose followed by 100 mg daily for 2 d | D 7 |
| 12 | M 20 y | 410C>T (P137L) | CNS infiltrates Enteropathy ITP Neutropenia LN | 1310 | Neg | Abatacept Sirolimus | PCR | Fever Headache Sore throat | D 1 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 2 | |
| 13 | F 5 y | 410C>T (P137L) | HT | 1500 | Unk | None | PCR | Fatigue | Mild | D 11 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 13† |
| 14 | F 48 y | 410C>T (P137L) | Asymptomatic | 1960 | Unk | None | PCR | Fatigue Cough Nasal congestions | Mild | 1 | Remdesevir 200 mg 1 dose followed by 100 mg daily for 4 d | D 30 |
| 15 | M 27 y | 223C>T (R75W) | CNS infiltrates CVID Enteropathy HSM ILD | 2760 | Unk | IVIG Prednisone | PCR | Nasal congestion | Mild | NA | None | D 15 |
| 16 | M 19 y | 567+5G>C | AIHA CNS infiltrates CVID Eczema Enteropathy HSM ILD | 890 | Unk | IVIG Sirolimus | PCR | Nasal congestion | Mild | 4 | Sotrovimab 500 mg 1 dose | D 6 |
| 17 | M 51 y | 223C>T (R75W) | Asymptomatic | 2610 | Unk | None | PCR | Asymptomatic | NA | None | D 11 | |
| 18 | F 45 y | c.371_372insG (L126TfsTer28) | AI Arthritis Colitis CVID Eczema HT ITP LN PA Vitiligo | 7960 | Unk | IVIG Abatacept Prednisolone Budesonide | PCR | Cough Fever | Mild | D 4 | Remdesevir 200 mg 1 dose followed by 100 mg daily for 4 d | D 7 |
| 19 | F 32 y | c.416A>G (Y139C) | Alopecia Eczema HT | Unk | Unk | None | PCR | Anosmia Cough SOB | Moderate | NA | None | D 30 |
| 20 | F 8 mo | c.416A>G (Y139C) | Arthritis | Unk | Unk | Prednisone | PCR | Fever Nasal congestion | Mild | NA | None | D 8 |
| 21 | F 19 y | c.487delC (L163SfsTer24) | AIHA Colitis CVID ITP Psoriasis | 410 | Unk | IVIG Prednisolone | PCR | Anosmia Cough Fatigue Fever Headache Muscle Ache | Mild | D 2 | Casirivimab 1200 mg Imdevimab 1200 mg 1 dose | D 8 |
| 22 | F 34 y | c.280 G>T (p.Glu94X) | Arthritis CVID Enteropathy HT | 950 | Unk | Sirolimus Abatacept IVIG | Antigen | Fatigue | Mild | None | None | D 20 |
Abs, antibodies; AI, adrenal insufficiency; AIHA, autoimmune hemolytic anemia; ALC, absolute lymphocyte count; CNS, central nervous system; CVID, common variable immunodeficiency; F, female; GH, growth hormone; HSM, hepatosplenomegaly; HT, hypothyroidism; ILD, interstitial lung disease; ITP, immune thrombocytopenic purpura; LAD, lymphadenopathy; M, male; N/A, not applicable; Neg, negative; PA, pernicious anemia; PCR, polymerase chain reaction; SOB, shortness of breath; SQIG, subcutaneous immunoglobulin; Tx, treatment; Unk, unknown; q, daily.
Day 0 was defined as the first day of symptoms.
Based on a previously described ordinal scale: 1, not hospitalized and no limitations of activities; 2, not hospitalized, with limitation of activities, home oxygen requirement, or both; 3, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalization was extended for infection-control or other nonmedical reasons); 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related to COVID-19 or to other medical conditions); 5, hospitalized, requiring any supplemental oxygen; 6, hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices; 7, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; and 8, death.14
Persistent fatigue after symptom resolution.
Timeline of COVID-19 infection in 17 patients residing in the United States. All patients tested positive for COVID-19 via polymerase chain reaction or antigen testing. The duration of symptoms is indicated using rectangles, which are depending on whether they received COVID-19 vaccination. The timing of each SARS-CoV-2 vaccine dose is depicted using triangles. The dotted lines indicate when >95% of COVID-19 cases were caused by Delta (light gray) or Omicron (dark gray) variants, based on the Center for Disease Control variant surveillance data. Patients were grouped based on their vaccination status.
Timeline of COVID-19 infection in 17 patients residing in the United States. All patients tested positive for COVID-19 via polymerase chain reaction or antigen testing. The duration of symptoms is indicated using rectangles, which are depending on whether they received COVID-19 vaccination. The timing of each SARS-CoV-2 vaccine dose is depicted using triangles. The dotted lines indicate when >95% of COVID-19 cases were caused by Delta (light gray) or Omicron (dark gray) variants, based on the Center for Disease Control variant surveillance data. Patients were grouped based on their vaccination status.
Autoantibodies against type 1 IFNs. Autoantibodies against IFN-α (A), IFN-β (B), and IFN-ω (C) in patients with CTLA-4 insufficiency and COVID-19 (n = 6), CTLA-4 insufficiency with no COVID-19 (n = 10), healthy controls (n = 18), and patients with APECED (n = 10). One-way analysis of variance and post hoc Tukey tests were used for all comparisons. Nonnormally distributed data were transformed for normality. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Autoantibodies against type 1 IFNs. Autoantibodies against IFN-α (A), IFN-β (B), and IFN-ω (C) in patients with CTLA-4 insufficiency and COVID-19 (n = 6), CTLA-4 insufficiency with no COVID-19 (n = 10), healthy controls (n = 18), and patients with APECED (n = 10). One-way analysis of variance and post hoc Tukey tests were used for all comparisons. Nonnormally distributed data were transformed for normality. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Initial COVID-19 management and outcomes
Baseline treatment for CTLA-4 insufficiency was continued in all patients, except for P10, in whom ustekinumab was held during COVID-19 infection. Eleven patients received 1 of the 3 monoclonal antibodies against the SARS-CoV-2 spike protein (anti-S): casivirimab + endevimab (7), bamlavinimab (2), or sotrovimab (2). Four patients received remdesivir, and 1 was treated with dexamethasone (P3) (Table 1). Seven patients received no treatment other than supportive care. The number of baseline clinical manifestations related to CLTA-4 insufficiency was lower in patients who received supportive care only (2.5 vs 6; P = .005). Initiation of SARS-CoV-2–directed treatment after symptom onset was 5 days for any agent and 4.2 days for anti-S monoclonal antibodies. Most patients with interstitial lung disease (7/8) received SARS-CoV-2–directed treatment and had a mild course, despite the presence of chronic lung disease. All patients showed complete resolution of symptoms (median duration, 7.5 days; range, 2-30). P13 had prolonged fatigue, which resolved several months after the initial infection. There were no cases of multisystem inflammatory syndrome. Three patients in whom serial SARS-CoV-2 polymerase chain reaction testing was performed (P1, P2, and P3) showed prolonged viral shedding beyond 30 days.
Repeat COVID-19 infections and long-term sequelae
Nine patients developed a second COVID-19 episode (P1, P4, P7, P10, P12, P15, P18, P20, and P21) and 2 patients (P10 and P18) developed a third (Figure 1). The median symptom duration for repeat infection was 8.5 days (range, 3-30), and all cases were mild. P10 developed Long COVID-19, with persistent fatigue, headaches, brain fog, memory loss, leg and joint pain, dyspnea with exertion, and worsening of colitis after his third COVID-19 episode. P18 had an episode of immune thrombocytopenic purpura after the second COVID-19 episode, which responded to rituximab. Of the 9 patients who had repeated infections, 4 were not vaccinated (P1, P4, P7, and P20).
SARS-CoV-2 vaccination and anti-S serologies
Seventeen patients were vaccinated (BNT162 = 10, messenger RNA 1273 [mRNA-1273] = 4, ChAdOx1 in combination with BNT162 = 1, CoronaVac in combination with BNT162 = 1, unknown = 1), and 6 of these patients (P8, P11, P16, P19, P21, and P22) received 3 or more doses. For all vaccinated patients, the median number of doses was 2. There were no cases of vaccine-associated myocarditis, severe vaccine-associated adverse effects, or disease flares attributable to the mRNA vaccination. P21 had an autoimmune hemolytic anemia flare within 2 weeks of receiving the first dose of ChAdOx1. Serologies were available for 16 patients. The median time between anti-S serology and the most recent SARS-CoV-2 vaccination or COVID-19 episode was 130 days (interquartile range, 85-251), and the median anti-S titers were 2090 IU/dL (interquartile range, 46-7583 IU/dL) (Figure 3). In patients receiving immunoglobulin replacement therapy (IGRT) (n = 10), the median anti-S titer was 349 IU/dL, compared with 2594 IU/dL in patients not receiving IGRT (n = 6; P = 0.15) (Figure 3). In both groups, the median time between serologies and the most recent vaccination or COVID-19 was similar (118 days vs 132 days; P = 0.7). P16 had high anti-S titers after receiving tixagevimab/cilgavimab and was not included in the analysis. Pre- and postvaccination serologies were only available for 2 patients receiving IGRT. Both patients had increased anti-S titers after vaccination (6.8-6348 IU/dL 112 days after vaccination in P2 and 755-6632 IU/dL 118 days after vaccination in P8).
Anti-S serologies after vaccination or SARS-CoV-2 infection. Anti-S serologies in 16 patients who had available titers at several time points after SARS-CoV-2 infection or vaccination during the study period. The y-axis for P13 and P16 is shown using a different scale, given the high anti-S titers relative to that of other patients. P16 received tixagevimab/cilgavimab before serologies were drawn.
Anti-S serologies after vaccination or SARS-CoV-2 infection. Anti-S serologies in 16 patients who had available titers at several time points after SARS-CoV-2 infection or vaccination during the study period. The y-axis for P13 and P16 is shown using a different scale, given the high anti-S titers relative to that of other patients. P16 received tixagevimab/cilgavimab before serologies were drawn.
Discussion
Despite the high propensity for exuberant inflammatory responses in patients with CTLA-4 insufficiency, resulting from impaired Treg function and immune dysregulation, our findings indicate that most patients with CTLA-4 develop a mild course of COVID-19. Although a significant number of patients had potential risk factors for severe disease, such as having multiple CTLA-4-associated comorbid conditions, chronic lung disease (interstitial lung disease), unvaccinated status, and contracting SARS-CoV-2 when Alpha and Delta variants were predominant, only 2 patients required hospitalization, and both did not necessitate mechanical ventilation; 90% of the patients were treated as outpatients, with a time to symptom resolution similar to that of the general population. Although the outcomes in most patients with CTLA-4 insufficiency seem reassuring, it is important to highlight that most patients were young adults, had immediate access to care, were vaccinated at the time of infection, underwent testing quite early in their disease course, were closely followed up by their local providers and our CTLA-4 clinical team, and continued their baseline immunomodulatory treatment during their COVID-19 course. Furthermore, patients who received specific treatment for SARS-CoV-2 had twice the baseline comorbidities of those who received supportive care only, suggesting that the decision to start such treatment might have been influenced by the disease burden at baseline. Anti-S protein antibodies, when started early, have resulted in a reduction in hospitalization risk, especially in patients at high risk.15-18 Although there are no data on the use of these monoclonal antibodies or convalescent plasma in CTLA-4 insufficiency, we believe that our patients were at high risk and could benefit from treatment, and it is possible that treatment ameliorated the disease course in some patients. It is also important to note that although the disease course in most patients was mild, 3 patients had complications from their disease, including hypoxemia, immune thrombocytopenic purpura (responsive to rituximab), and prolonged COVID-19 with difficult-to-treat colitis.
Our observations also suggest that CTLA-4 insufficient patients can safely receive mRNA vaccines, with no severe adverse effects reported in our cohort. Although unvaccinated patients were more likely to develop COVID-19 and 2 unvaccinated patients developed COVID-19-associated complications (severe disease in P3 and long COVID-19 in P10), no conclusions regarding vaccine protection can be made because of possible confounders (eg, dominant SARS-CoV-2 variant at the time of infection, use of masks and other behavioral factors, etc). Although our study was not powered or designed to detect differences in anti-S titers between patients on immunoglobulin replacement therapy and those without, the median anti-S titers were 83% lower in patients receiving IGRT, with a similar time from COVID-19 vaccination or infection in both groups. Finally, in contrast to patients with autoantibodies against IFN-α, IFN-β, and IFN-ω and life-threatening COVID-19 infection,2,4 our cohort of CTLA-4 insufficient patients did not have neutralizing autoantibodies against type 1 IFNs. Whether there is a potential role for autoantibodies against type 1 IFNs for risk stratification in patients with IEI and propensity for autoimmunity warrants further studies.
Our report represents an observational retrospective study of a rare disorder of genetically determined immune dysregulation and autoimmunity. Our relatively small sample size, differences in underlying disease severity, and immunomodulatory treatment at the time of SARS-CoV-2 infection and vaccination represent challenges in establishing firm conclusions regarding the management of COVID-19.
With this study, we seek to add to the scarce literature on the outcomes and management of patients with underlying IEIs and SARS-CoV-2 infection and help providers caring for patients with CLTA-4 insufficiency to better understand COVID-19 disease outcomes. The management and outcomes of our CTLA-4 insufficient cohort could potentially be extrapolated to patients with cancer who are at risk of developing autoimmune complications while being treated with the CTLA4-blocking checkpoint inhibitor ipilimumab.19
Acknowledgments
The authors thank the families and patients who participated in this study. This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health Intramural funds, and National Institutes of Health Clinical Center Bench-to-Bedside Program grant awarded to G.U. for the project titled “Deciphering Immune Dysregulation due to Germ line CTLA4 mutations” (480707). This work was also supported by intramural research funds from German Research Society grant SFB1160/2_B5 and German Ministry of Education and Research grant GAIN01GM1910A.
Authorship
Contribution: S.O., M.S.A., and G.U. designed the research, performed the research, contributed vital new reagents or analytical tools, analyzed data, and wrote the manuscript; L.R. contributed vital new reagents or analytical tools; and A.R., K.H., J.A.L., B.L.W., D.S., M.K., B.G., and M.S.L. performed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.A.L. has received institutional funding from DBV Technologies, Aimmune, Regeneron, and Leadiant Biosciences. B.G. has received research funding and clinical trial funding from Bristol Myers Squibb, the manufacturer of Orencia (abatacept), however, not affecting any of the data presented in this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Gulbu Uzel, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 9000 Rockville Pike, Bldg 10, Room 12C103A, Bethesda, MD 20892; e-mail: guzel@niaid.nih.gov.
References
Author notes
All data sets and protocols are available on request from the corresponding author, Gulbu Uzel (guzel@niaid.nih.gov).