Key Points
Chemotherapy–free biologic combination of romidepsin and lenalidomide is feasible and effective as initial therapy for PTCL.
Romidepsin and lenalidomide combination produces expected and acceptable toxicities in patients ineligible for intensive approach.
Abstract
Peripheral T-cell lymphomas (PTCLs) are associated with poor prognosis when treated with cytotoxic chemotherapy. We report the findings of a phase 2 study evaluating a chemotherapy-free combination of romidepsin plus lenalidomide as initial treatment for patients with PTCL who were aged >60 years or noncandidates for chemotherapy. Treatment was initiated with romidepsin 10 mg/m2 IV on days 1, 8, and 15 and lenalidomide 25 mg taken orally from days 1 to 21 of 28-day cycle for up to 1 year. The primary objective was overall response rate (ORR). Secondary objectives included safety and survival. The study enrolled 29 patients with a median age of 75 years, including 16 (55%) angioimmunoblastic T-cell lymphoma (AITL), 10 (34%) PTCL– not otherwise specified, 2 ATLL, and 1 EATL. Grade 3 to 4 hematologic toxicities included neutropenia (45%), thrombocytopenia (34%), and anemia (28%). Grade 3 to 4 nonhematologic toxicities included hyponatremia (45%), hypertension (38%), hypoalbuminemia (24%), fatigue (17%), hyperglycemia (14%), hypokalemia (14%), dehydration (10%), and infection (10%). At median follow-up of 15.7 months, 23 patients were evaluable and received a median treatment of 6 cycles. The ORR was 65.2% with complete response (CR) at 26.1%, including 78.6% ORR and 35.7% CR for AITL. Median duration of response was 10.7 months, with 27.1 months for patients achieving CR. The estimated 2-year progression-free survival was 31.5%, and 2-year overall survival was 49.5%. This study provides the first demonstration that the biologic combination of romidepsin and lenalidomide is feasible and effective as initial therapy for PTCL and warrants further evaluation. This trial was registered at www.clinicaltrials.gov as #NCT02232516.
Introduction
Peripheral T-cell lymphoma (PLTCL) is a group of aggressive non-Hodgkin lymphomas (NHL) often associated with poor prognosis when treated with conventional chemotherapy.1 Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) is the most commonly prescribed induction treatment for systemic PTCL.2 With the exception of ALK+ anaplastic large cell lymphoma (ALCL), CHOP is historically associated with overall response rate (ORR) ranging from 60% to 80%, complete response (CR) ranging from 30% to 40%, and long-term survival measured by 5-year overall survival (OS) ranging from 20% to 30% in PTCL.3-5 The toxicity associated with anthracycline-containing regimens prevents its safe deployment in a substantial portion of older patients with newly diagnosed PTCL because of associated comorbidities. In addition, dose-intensification or nonanthracycline based regimens5-7 do not clearly yield improved clinical outcomes. Therefore, developing new effective and well-tolerated frontline induction strategies is a significant unmet need, especially for the older patients and those with comorbidities in PTCL.
Romidepsin is a bicyclic class 1 selective histone deacetylases (HDAC) inhibitor approved for the treatment of patients with relapsed/refractory (R/R) PTCL, based on the results of a pivotal phase 2 trial that demonstrated an ORR of 25% (complete response or unconfirmed complete response [CR/CRu], 15%), with durable median duration of response (DOR) of 28 months in responders.8,9 The drug was well-tolerated with fatigue, infections, and cytopenia, including thrombocytopenia, as the main toxicities. In a real world multicenter retrospective study with 127 patients, romidepsin as a single agent or in combination was shown to have superior response rates in PTCL with T follicular helper phenotype, which includes angioimmunoblastic T-cell lymphoma (AITL).10 Lenalidomide is a well-established immunomodulatory agent in B-cell NHL and multiple myeloma, which has demonstrated single-agent activity in R/R PTCL,11,12 including 22% ORR with 11% CR/CRu in mixed PTCL subtypes, 31% ORR with 15% CR/CRu in AITL, and 42% ORR with 19% CR/CRu in adult T-cell leukemia/lymphoma (ATLL). Toxicities were manageable, with cytopenia and fatigue being most frequent.
Single-agent lenalidomide and romidepsin have been effective in PTCL, and the combination of immunomodulation and epigenetic manipulation has the potential for synergy by targeting both tumor microenvironment and tumor cells. The safety and efficacy of the romidepsin and lenalidomide combination has been evaluated in phase 1b/2a studies for R/R PTCL.13 The doublet study with romidepsin and lenalidomide included 27 patients with TCL, and the maximum tolerated dose (MTD) was romidepsin 14 mg/m2 IV on days 1, 8, and 15, plus lenalidomide 25 mg from days 1 to 21 of a 28-day cycle. The triplet study with romidepsin, lenalidomide, and carfilzomib included 16 patients with TCL, and the MTD was romidepsin 8 mg/m2 on days 1 and 8, lenalidomide 10 mg from days 1 to 14, plus carfilzomib 36 mg/m2 on days 1 and 8 of a 21-day cycle. The romidepsin and lenalidomide doublet showed an ORR of 50% with a CR of 13%, a median progression-free survival (PFS) of 4.8 months, and a median OS of 18.3 months. The triplet demonstrated a 50% ORR with 31% CR, which seemed to be enriched among patients with AITL (4/5 patients). The most common adverse events (AEs) of romidepsin combination therapies included cytopenia and electrolyte abnormalities, manageable by supportive care.
Herein, we report on the efficacy and safety of romidepsin plus lenalidomide in a multicenter phase 2 study in patients with previously untreated PTCL. The treatment population included patients aged ≥60 years, as well as those aged ≥18 years and <60 years with a cumulative illness rating scale (CIRS) score ≥614 or deemed not candidates for anthracycline-based or other multiagent chemotherapy by the treating investigator.
Methods
Patient eligibility
Patients with ALCL (ALK–), AITL, EATL, hepatosplenic T-cell lymphoma, PTCL–not otherwise specified (PTCL-NOS), subcutaneous panniculitis-like T-cell lymphoma, transformed mycosis fungoides, or ATLL subtypes were eligible for the study. Key eligibility criteria included histologically confirmed PTCL with measurable disease; age >60 years or noncandidates for cytotoxic chemotherapy because of presence of various comorbidities (CIRS score ≥6), cardiac disease, previous anthracyclines use, or deemed ineligible for cytotoxic chemotherapy by the treating investigator; Eastern Cooperative Oncology Group performance status ≤2; and absolute neutrophil count ≥750 cells/μL, platelets ≥50 000 cells/μL, total bilirubin ≤2× upper limit of normal, aspartate aminotransferase/alanine aminotransferase ≤3× of upper limit of normal, QT interval ≤480 millisecond, and creatinine clearance ≥30 mL/min. Key exclusion criteria included central nervous system lymphoma, known HIV, active hepatitis B or C, and any previous malignancies for <1 year.
Study treatment
Treatment was initiated with romidepsin 10 mg/m2 IV on days 1, 8, and 15 and lenalidomide 25 mg taken orally from days 1 to 21 of 28-day cycle. Treatment was continued for up to 1 year or until disease progression, development of unacceptable toxicity, or study withdrawal, whichever came earlier (Figure 1A). Romidepsin dose was allowed to escalate to 14 mg/m2 after 2 cycles as tolerated. The starting dose of lenalidomide was 10 mg daily for patients with reduced creatinine clearance ranging from 30 to 60 mL/min. Dose adjustment was recommended to either romidepsin or lenalidomide in the event of grade 3 to 4 cytopenias. Use of growth factors and prophylaxis against opportunistic infection were permitted at the discretion of physicians and per individual institutional guidelines. Patients received thromboprophylaxis with aspirin or low molecular weight heparin, unless requiring treatment for known thrombosis. Asymptomatic hepatitis B carriers received antiviral therapy.
Study treatment schema and patient disposition flowchart. (A) The study treatment schema. Treatment consisted of romidepsin 10 mg/m2 (with dose escalation to 14 mg/m2 as tolerated) IV on days 1, 8, and 15 and lenalidomide 25 mg taken orally from days 1 to 21 of 28-day cycle for up to 1 year. (B) The flowchart for patient disposition. Of the 29 patients enrolled, 20 completed at least 3 cycles of study treatment and were evaluable. Fifteen participants achieved responses.
Study treatment schema and patient disposition flowchart. (A) The study treatment schema. Treatment consisted of romidepsin 10 mg/m2 (with dose escalation to 14 mg/m2 as tolerated) IV on days 1, 8, and 15 and lenalidomide 25 mg taken orally from days 1 to 21 of 28-day cycle for up to 1 year. (B) The flowchart for patient disposition. Of the 29 patients enrolled, 20 completed at least 3 cycles of study treatment and were evaluable. Fifteen participants achieved responses.
Efficacy and safety assessment
Response and progression were evaluated using the 2007 Revised Response Criteria by Cheson et al.15 Computerized tomography (CT) or positron emission tomography–CT (PET-CT) scans were performed at study entry, after cycles 3 and 6, and every 6 months thereafter. PET-CT was optional at baseline. Bone marrow biopsy and PET-CT were done to confirm CR. AE monitoring was continuous throughout the study treatment period. Toxicities were graded according to National Cancer Institute Common Terminology Criteria for AEs version 4.03. Data from all participants receiving any therapy were included in the safety analyses.
Statistical analysis
The primary objective of this phase 2 trial was to evaluate the efficacy of the combination of romidepsin plus lenalidomide in patients with previously untreated PTCL. Patients receiving at least 1 dose of study drug were evaluable for toxicity, and all patients completing at least 2 cycles of therapy were evaluable for response. The end point was objective response rate (ORR), with 95% confidence interval (CI) estimated via Clopper-Pearson exact method. Single-agent romidepsin or lenalidomide have response rates of ∼25% in patients with R/R PTCL. The combination of romidepsin and lenalidomide demonstrated an ORR of 50% in R/R PTCL. The trial has a Simon 2-stage design with an early assessment of futility after the treatment of the first 12 patients with an early stopping point if ≤6 patients had a complete or partial response. Sample size was calculated assuming the true ORR value of 0.5. PFS, DOR, and OS were estimated using the product-limit method of Kaplan-Meier.
Study oversight
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Institutional Review Boards approved the study protocol at the respective sites, and all participants provided written informed consent. An independent data and safety monitoring board at Robert H. Lurie Comprehensive Cancer Center of Northwestern University conducted safety reviews.
Results
Patient characteristics and disposition
The study enrolled 29 patients, including 16 (55%) with AITL, 10 (34%) with PTCL-NOS, 2 ATLL, and 1 EATCL, at 3 US centers (Table 1). Most patients were older with extensive disease. The median age was 75 (range, 49-84) years, and male to female ratio was 1:1. Twenty-seven (93%) had stage ¾ of the disease, 23 (79%) had elevated lactate dehydrogenase, and 22 (76%) had an International Prognostic Index (IPI) score ranging from 3 to 5.
Patient and disease characteristics
| Characteristic . | Overall, n (%) . | Evaluable, n (%) . |
|---|---|---|
| Number of patients (%) | 29 (100) | 23 (100) |
| Sex | ||
| Male | 14 (48) | 11 (48) |
| Female | 15 (52) | 12 (52) |
| Age, y | ||
| Median | 75 | 75 |
| Range | 49-84 | 49-84 |
| ECOG Performance Status | ||
| 0-1 | 22 (76) | 17 (74) |
| >1 | 7 (24) | 6 (26) |
| Ann Arbor stage | ||
| 1-2 | 2 (7) | 2 (9) |
| 3-4 | 27 (93) | 21 (91) |
| LDH | ||
| Normal | 6 (21) | 5 (22) |
| Elevated | 23 (79) | 18 (78) |
| Bone marrow involvement | ||
| Yes | 7 (24) | 6 (26) |
| No | 5 (17) | 4 (17) |
| Unknown | 17 (59) | 13 (57) |
| PTCL subtypes | ||
| AITL | 16 (55) | 14 (61) |
| PTCL-NOS | 10 (34) | 6 (26) |
| ATLL | 2 (7) | 2 (9) |
| EATL | 1 (3) | 1 (4) |
| IPI risk category | ||
| 0-1 | 1 (3) | 1 (4) |
| 2 | 6 (21) | 6 (26) |
| 3-5 | 22 (76) | 16 (70) |
| Characteristic . | Overall, n (%) . | Evaluable, n (%) . |
|---|---|---|
| Number of patients (%) | 29 (100) | 23 (100) |
| Sex | ||
| Male | 14 (48) | 11 (48) |
| Female | 15 (52) | 12 (52) |
| Age, y | ||
| Median | 75 | 75 |
| Range | 49-84 | 49-84 |
| ECOG Performance Status | ||
| 0-1 | 22 (76) | 17 (74) |
| >1 | 7 (24) | 6 (26) |
| Ann Arbor stage | ||
| 1-2 | 2 (7) | 2 (9) |
| 3-4 | 27 (93) | 21 (91) |
| LDH | ||
| Normal | 6 (21) | 5 (22) |
| Elevated | 23 (79) | 18 (78) |
| Bone marrow involvement | ||
| Yes | 7 (24) | 6 (26) |
| No | 5 (17) | 4 (17) |
| Unknown | 17 (59) | 13 (57) |
| PTCL subtypes | ||
| AITL | 16 (55) | 14 (61) |
| PTCL-NOS | 10 (34) | 6 (26) |
| ATLL | 2 (7) | 2 (9) |
| EATL | 1 (3) | 1 (4) |
| IPI risk category | ||
| 0-1 | 1 (3) | 1 (4) |
| 2 | 6 (21) | 6 (26) |
| 3-5 | 22 (76) | 16 (70) |
EATL, enteropathy-associated T-cell lymphoma; ECOG, Eastern Clinical Oncology Group; IPI, international prognostic index; LDH, lactate dehydrogenase.
Six patients received <2 cycles of study treatment and were unevaluable for efficacy, with 2 withdrawing consent and 4 stopping treatment because of toxicities (Figure 1B; supplemental Table 1). Of the 23 evaluable patients, 8 patients discontinued therapy, including 3 receiving <2 cycles of treatment and 5 after interim assessment after cycle 3, owing to lack of clinical benefit (progression of disease and stable disease). Of the 15 patients achieving responses on study, 1 patient completed the 1-year study treatment program, whereas 12 patients discontinued study treatment before reaching 1-year mark owing to either progression of disease (n = 4), toxicity (n = 6), or a wish to stop chronic treatment (n = 2). Two patients aged <65 years, 1 previously received anthracycline-based chemotherapy for remote history of B-cell lymphoma and the other considered noncandidate for anthracycline-based cytotoxic therapy because of comorbidities, underwent consolidative autologous stem cell transplantation after demonstrating significant response to treatment and improvement in performance status.
Efficacy
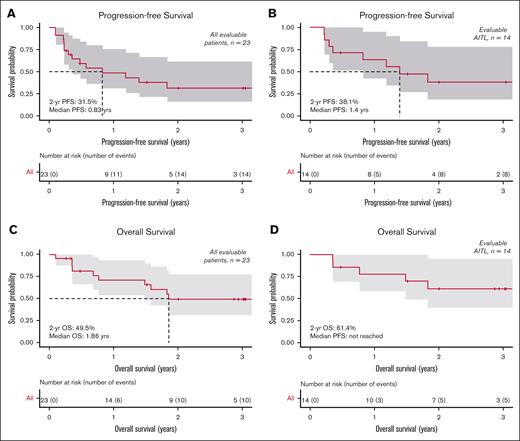
The ORR was 65.2% (95% CI, 42.7-83.6) with CR at 26.1% (95% CI, 10.2-48.4) for all evaluable patients (n = 23) (Table 2). For evaluable AITL (n = 14), the ORR was 78.6% (95% CI, 49.2-95.3) with CR at 35.7% (95% CI, 12.8-64.9). For evaluable PTCL-NOS (n = 6), the ORR was 50% (95% CI, 11.8-88.2) with CR at 16.7% (95% CI, 0.4-64.1). The intent-to-treat ORR for all 29 patients was 51.7% (supplemental Table 2). The median time to best responses was 86 days, including median time to CR at 82 days. Median DOR was 10.7 months for all responders and 27.1 months for patients with CR. For evaluable patients (n = 23) at a median follow-up of 15.7 months, the median PFS was 0.83 years, the estimated 1-year PFS was 48.6% (95% CI, 31.1-75.9) with 2-year PFS at 31.5% (95% CI, 16.1-61.5), and the estimated 1-year OS was 71.1% (95% CI, 54.0-93.7) with 2-year OS at 49.5% (95% CI, 31.6-77.8). For evaluable AITL subtype (n = 14), the median PFS was 1.4 years, the estimated 1-year PFS was 63.5% (95% CI, 42.4-95.1) with 2-year PFS at 38.1% (95% CI, 18.6-78.1), and the estimated 1-year OS was 77.9% (95% CI, 58.7-100) with 2-year OS at 61.4% (95% CI, 39.6-95.1) (Figure 2).
Efficacy
| Best response . | Evaluable patients (n = 23) . | AITL (n = 14) . | PTCL-NOS (n = 6) . | |||
|---|---|---|---|---|---|---|
| Number . | Response rate, n (%) . | Number . | Response rate, n (%) . | Number . | Response rate, n (%) . | |
| ORR | 15 | 65.2 | 11 | 78.6 | 3 | 50 |
| CR | 6 | 26.1 | 5 | 35.7 | 1 | 16.7 |
| PR | 9 | 39.1 | 6 | 42.9 | 2 | 33.3 |
| SD | 2 | 8.7 | 1 | 7.1 | 1 | 16.7 |
| PD | 6 | 26.1 | 2 | 14.3 | 2 | 33.3 |
| Median Follow-up | 15.7 mo | |||||
| Median time to BR | 86 d | |||||
| 2-y PFS (n = 23) (%) | 31.5 (95% CI, 16.1-61.5) | |||||
| 2-y OS (n = 23) (%) | 49.5 (95% CI, 31.6-77.8) | |||||
| Best response . | Evaluable patients (n = 23) . | AITL (n = 14) . | PTCL-NOS (n = 6) . | |||
|---|---|---|---|---|---|---|
| Number . | Response rate, n (%) . | Number . | Response rate, n (%) . | Number . | Response rate, n (%) . | |
| ORR | 15 | 65.2 | 11 | 78.6 | 3 | 50 |
| CR | 6 | 26.1 | 5 | 35.7 | 1 | 16.7 |
| PR | 9 | 39.1 | 6 | 42.9 | 2 | 33.3 |
| SD | 2 | 8.7 | 1 | 7.1 | 1 | 16.7 |
| PD | 6 | 26.1 | 2 | 14.3 | 2 | 33.3 |
| Median Follow-up | 15.7 mo | |||||
| Median time to BR | 86 d | |||||
| 2-y PFS (n = 23) (%) | 31.5 (95% CI, 16.1-61.5) | |||||
| 2-y OS (n = 23) (%) | 49.5 (95% CI, 31.6-77.8) | |||||
BR, best response; PD, progression of disease; PR, partial response; SD, stable disease.
Kaplan-Meier survival curves. PFS for all patients (A) and AITL subset (B). OS for all patients (C) and AITL subset (D).
Kaplan-Meier survival curves. PFS for all patients (A) and AITL subset (B). OS for all patients (C) and AITL subset (D).
The median treatment length was 6 cycles for evaluable patients who had at least 1 response assessment. A total of 10 of 29 patients (34%) received additional cytotoxic chemotherapy after study treatment discontinuation. Of the 23 evaluable patients, 2 underwent consolidative autologous stem cell transplantation while in remission and 7 additional patients received CHOP-based chemotherapy in subsequent treatment for progressive disease, with 1 of them proceeding to allogeneic transplant. Of the 6 unevaluable patients, 1 patient received CHOP-based salvage chemotherapy after study treatment discontinuation. (Figure 3; supplemental Table 1).
Swimmer plot of time on study and duration of responses. Solid bar length indicates time on study, with red solid bar denoting CR, orange denoting partial response, purple denoting stable disease, and blue denoting progression of disease. Blue shaded bar denotes response duration after study treatment discontinuation. Green diamond denotes stem cell transplant, yellow cross denotes subsequent cytotoxic chemotherapy, green asterisk denotes time to best response, black cross denotes disease progression, gray circle denotes study withdrawal or lost to follow-up, and black diamond denotes death. “//” stands for axis break, which denotes noncontinuous timeline.
Swimmer plot of time on study and duration of responses. Solid bar length indicates time on study, with red solid bar denoting CR, orange denoting partial response, purple denoting stable disease, and blue denoting progression of disease. Blue shaded bar denotes response duration after study treatment discontinuation. Green diamond denotes stem cell transplant, yellow cross denotes subsequent cytotoxic chemotherapy, green asterisk denotes time to best response, black cross denotes disease progression, gray circle denotes study withdrawal or lost to follow-up, and black diamond denotes death. “//” stands for axis break, which denotes noncontinuous timeline.
Safety
Treatment–related side effects were generally expected in this older and high-risk population (Table 3). Grade 3 to 4 hematologic toxicities included neutropenia (45%), thrombocytopenia (34%), and anemia (28%). Significant grade 3 to 4 nonhematologic toxicities occurring in >10% patients included hyponatremia (45%), hypertension (38%), hypoalbuminemia (24%), fatigue (17%), hyperglycemia (14%), hypokalemia (14%), and dehydration (10%). Grade 3 hyponatremia (13 incidences, 45%) appeared to be multifactorial, occurring mostly in patients with baseline fluctuating grade 1 to 2 hyponatremia because of lymphoma, which was exacerbated to grade 3 during study treatment from nausea, dehydration, and sometimes infections. Infections at grade 3 and above included lung infection (10%), sepsis (10%), upper respiratory infection (3%), urinary track infection (3%), and cellulitis (3%), which were managed with supportive care. One patient died from complication of sepsis. One patient with AITL developed acute myeloid leukemia after achieved partial response after the completion of 6 cycles of study treatment, and they subsequently received acute myeloid leukemia–specific therapy with azacitidine plus venetoclax (supplemental Table 1). For all patients, the median dose of romidepsin was 10 mg/m2; 5 (17%) patients were able to dose escalate to 14 mg/m2, whereas 6 (21%) required dose reduction to 8 mg/m2. The median dose of lenalidomide was 15 mg, with 3 patients (10%) requiring dose reduction from 25 mg to 20 mg, 2 (7%) to 15 mg, 11 (38%) to 10 mg, and 2 (7%) to 5 mg. Seven of 29 patients (24%) received growth factor support while on study. Four patients, with ages ranging from 62 to 80 years and IPI scores ranging from 3 to 4, developed acute toxicities, including sepsis, tumor lysis, abdominal pain, and hyponatremia during cycle 1, which led to treatment discontinuation.
Adverse events
| Toxicities∗ . | Any grade . | Grade ≥3 . | ||
|---|---|---|---|---|
| No . | Percentage (%) . | No . | Percentage (%) . | |
| Hematologic | ||||
| Thrombocytopenia | 27 | 93 | 10 | 34 |
| Anemia | 26 | 90 | 8 | 28 |
| Neutropenia | 20 | 69 | 13 | 45 |
| Infectious | ||||
| URI | 5 | 17 | 1 | 3 |
| Lung infection | 3 | 10 | 3 | 10 |
| Sepsis | 3 | 10 | 3 | 10 |
| UTI | 3 | 10 | 1 | 3 |
| Cellulitis | 3 | 10 | 1 | 3 |
| Other | ||||
| Hypoalbuminemia | 27 | 93 | 7 | 24 |
| Fatigue | 27 | 93 | 5 | 17 |
| Hyponatremia | 26 | 90 | 13 | 45 |
| Hypertension | 25 | 86 | 11 | 38 |
| Nausea | 23 | 79 | 0 | 0 |
| Hypocalcemia | 22 | 76 | 1 | 3 |
| Constipation | 21 | 72 | 0 | 0 |
| Hypomagnesemia | 20 | 69 | 1 | 3 |
| Hypokalemia | 18 | 62 | 4 | 14 |
| Anorexia | 16 | 55 | 1 | 3 |
| Cough | 16 | 55 | 0 | 0 |
| Hyperglycemia | 15 | 52 | 4 | 14 |
| Fever | 15 | 52 | 2 | 7 |
| Blood bilirubin increased | 14 | 48 | 4 | 14 |
| Diarrhea | 14 | 48 | 1 | 3 |
| Edema | 14 | 48 | 1 | 3 |
| Dyspnea | 13 | 45 | 2 | 7 |
| Weight loss | 13 | 45 | 1 | 3 |
| AST increased | 12 | 41 | 2 | 7 |
| Dysgeusia | 12 | 41 | 0 | 0 |
| Creatinine increased | 12 | 41 | 1 | 3 |
| Pruritis | 11 | 38 | 2 | 7 |
| Abdominal pain | 10 | 34 | 1 | 3 |
| Reflux | 10 | 34 | 0 | 0 |
| Dizziness | 9 | 31 | 0 | 0 |
| Headache | 9 | 31 | 0 | 0 |
| Vomiting | 9 | 31 | 0 | 0 |
| ALT increased | 8 | 28 | 1 | 3 |
| Hypotension | 7 | 24 | 1 | 3 |
| Rash | 7 | 24 | 1 | 3 |
| Hypothyroidism | 5 | 17 | 0 | 0 |
| Dehydration | 4 | 14 | 3 | 10 |
| Hypoglycemia | 4 | 14 | 0 | 0 |
| Mucositis | 4 | 14 | 0 | 0 |
| Sinus bradycardia | 4 | 14 | 0 | 0 |
| Colitis | 3 | 10 | 2 | 7 |
| Arthralgia | 3 | 10 | 1 | 3 |
| Atrial fibrillation | 3 | 10 | 1 | 3 |
| Confusion | 3 | 10 | 1 | 3 |
| Hypercalcemia | 3 | 10 | 0 | 0 |
| Hyperkalemia | 3 | 10 | 0 | 0 |
| Hypermagnesemia | 3 | 10 | 0 | 0 |
| Hypernatremia | 3 | 10 | 0 | 0 |
| Toxicities∗ . | Any grade . | Grade ≥3 . | ||
|---|---|---|---|---|
| No . | Percentage (%) . | No . | Percentage (%) . | |
| Hematologic | ||||
| Thrombocytopenia | 27 | 93 | 10 | 34 |
| Anemia | 26 | 90 | 8 | 28 |
| Neutropenia | 20 | 69 | 13 | 45 |
| Infectious | ||||
| URI | 5 | 17 | 1 | 3 |
| Lung infection | 3 | 10 | 3 | 10 |
| Sepsis | 3 | 10 | 3 | 10 |
| UTI | 3 | 10 | 1 | 3 |
| Cellulitis | 3 | 10 | 1 | 3 |
| Other | ||||
| Hypoalbuminemia | 27 | 93 | 7 | 24 |
| Fatigue | 27 | 93 | 5 | 17 |
| Hyponatremia | 26 | 90 | 13 | 45 |
| Hypertension | 25 | 86 | 11 | 38 |
| Nausea | 23 | 79 | 0 | 0 |
| Hypocalcemia | 22 | 76 | 1 | 3 |
| Constipation | 21 | 72 | 0 | 0 |
| Hypomagnesemia | 20 | 69 | 1 | 3 |
| Hypokalemia | 18 | 62 | 4 | 14 |
| Anorexia | 16 | 55 | 1 | 3 |
| Cough | 16 | 55 | 0 | 0 |
| Hyperglycemia | 15 | 52 | 4 | 14 |
| Fever | 15 | 52 | 2 | 7 |
| Blood bilirubin increased | 14 | 48 | 4 | 14 |
| Diarrhea | 14 | 48 | 1 | 3 |
| Edema | 14 | 48 | 1 | 3 |
| Dyspnea | 13 | 45 | 2 | 7 |
| Weight loss | 13 | 45 | 1 | 3 |
| AST increased | 12 | 41 | 2 | 7 |
| Dysgeusia | 12 | 41 | 0 | 0 |
| Creatinine increased | 12 | 41 | 1 | 3 |
| Pruritis | 11 | 38 | 2 | 7 |
| Abdominal pain | 10 | 34 | 1 | 3 |
| Reflux | 10 | 34 | 0 | 0 |
| Dizziness | 9 | 31 | 0 | 0 |
| Headache | 9 | 31 | 0 | 0 |
| Vomiting | 9 | 31 | 0 | 0 |
| ALT increased | 8 | 28 | 1 | 3 |
| Hypotension | 7 | 24 | 1 | 3 |
| Rash | 7 | 24 | 1 | 3 |
| Hypothyroidism | 5 | 17 | 0 | 0 |
| Dehydration | 4 | 14 | 3 | 10 |
| Hypoglycemia | 4 | 14 | 0 | 0 |
| Mucositis | 4 | 14 | 0 | 0 |
| Sinus bradycardia | 4 | 14 | 0 | 0 |
| Colitis | 3 | 10 | 2 | 7 |
| Arthralgia | 3 | 10 | 1 | 3 |
| Atrial fibrillation | 3 | 10 | 1 | 3 |
| Confusion | 3 | 10 | 1 | 3 |
| Hypercalcemia | 3 | 10 | 0 | 0 |
| Hyperkalemia | 3 | 10 | 0 | 0 |
| Hypermagnesemia | 3 | 10 | 0 | 0 |
| Hypernatremia | 3 | 10 | 0 | 0 |
AST, aspartate transaminase; URI, upper respiratory infection; UTI, urinary track infection.
Nonhematologic AEs occurring in >10% patients.
Discussion
To our knowledge, this study provides the first demonstration that chemotherapy-free biologic combination of romidepsin and lenalidomide is feasible and effective as initial therapy for patients with PTCL who are not candidates for cytotoxic chemotherapy.
Treatment decision for patients with PTCL with aggressive disease and significant comorbidities has been challenging. Anthracycline-based regimen such as CHOP has been the most common regimen for PTCL induction because of paucity of data on better alternatives, even though CHOP-based regimen produces suboptimal outcome and does not deliver cure for most of the non-ALCL PTCL subtypes. Although poor performance status alone was not a contraindication for anthracycline-based regimen, disease factors such as PTCL subtypes, as well as patient factors such as previous medical and treatment history, comorbidities, and patient preferences, play important roles in decision making. CIRS is a rigorous tool designed to evaluate the impact of comorbidities in geriatric patients who are hospitalized, although it has not become part of routine clinical practice in oncology owing to complexity in scoring. CIRS has been used extensively in major clinical trials as part of inclusion criteria and for baseline demographic assessment in lymphoma clinical research, particularly in chronic lymphocytic leukemia, in which CIRS score of >6 predicts survival with either chemoimmunotherapy or novel agents.16,17
This study was designed based on the knowledge that PTCLs have a unique sensitivity to HDAC inhibitors and both romidepsin and lenalidomide have clinical activity in the R/R setting. Furthermore, preclinical studies have suggested that romidepsin and lenalidomide may be synergistic.18 The experience with single-agent romidepsin in the pivotal trial demonstrated that extended treatment (beyond 6 cycles) for responders was feasible without added toxicity.9
The treatment of romidepsin and lenalidomide had expected side-effect profile in most patients, manageable with supportive care. The median doses were 10 mg/m2 for romidepsin and 15 mg for lenalidomide, on par with that reported for R/R disease (romidepsin 8 mg/m2 and lenalidomide 10 mg). The frontline study adopted a lower starting dose of romidepsin at 10 mg/m2, with a ramp-up schedule to accommodate for older patients (median age, 75 years) and those with comorbidities who may not tolerate the MTD identified in the prior study for R/R PTCL that enrolled a younger patient cohort (median age, 61 years). Electrolyte abnormalities and hypoalbuminemia were common and expected with these agents and other treatment in PTCL as previously reported.19 The causality of grade 3 hyponatremia (45%) in this study appears to be multifactorial in this high-risk patient population, possibly attributable to active TCL and comorbidities such as low appetite and infection, made worse by medication-related side effects. Most of the grade 3 incidences responded to a combination of supportive care such as antiemetics, IV hydration, and treating underlying infection, underscoring the importance of supportive care optimization during therapy.
The romidepsin and lenalidomide combination was highly effective. The ORR of 65.2%, CR of 26.1%, 2-year PFS of 31.5%, 2-year OS of 49.5% in all evaluable patients approached historical data with CHOP-based chemotherapy. Those with AITL (n = 14) seemed to have more favorable response and survival, with 78.6% ORR and 35.7% CR, than those with PTCL-NOS. DOR of 27.1 months was superior in patients achieving CR and durable in patients who discontinued study medications without further consolidation, suggesting subtype and quality of response impact outcome. Notably, 3 patients were able to move onto consolidative stem cell transplant while in remission with improved performance status, speaking to the overall efficacy of the induction regimen. These data justify further evaluation of such novel agents as a frontline strategy in a larger population, particularly in older patients.
There has not been a dedicated prospective interventional study using chemotherapy–free alternative strategy for patients with PTCL who are not chemotherapy candidates in the first-line setting. In the Swedish lymphoma registry study, over 20% (163/757) patients with PTCL did not receive systemic chemotherapy, and age as a part of IPI is associated with adverse survival outcome.20 In a US multicenter retrospective study with over 300 patients, 7% patients who were older and with comorbidities received only palliative therapy and survived <3 months.21
Survival outcome remains suboptimal for most patients with PTCL receiving conventional cytotoxic chemotherapy. In the randomized phase 3 trial (ECHELON-2) evaluating the combination of brentuximab vedotin (BV) + CHP vs CHOP alone in patients with CD30+ (≥10%) newly diagnosed PTCL, survival benefit was largely driven by superior PFS and OS in patients with ALCL, whereas the estimated 5-year PFS for patients with non-ALCL, including those with PTCL-NOS and AITL, were ∼26%, underscoring the unmet need to explore novel combinations for these subtypes.22 Emerging application of novel agents alludes to the potential of chemotherapy-free combinations. For example, Falchi et al recently reported promising clinical activities with the combination of oral azacytidine and romidepsin in a phase 1 study that included both patients with R/R disease and those who were treatment naïve.23 High response rates (ORR, 80% and CR, 67%) were observed in PTCL with T follicular helper phenotype, although the sample size of patients who were treatment naïve was small at 11.
Although the indication of romidepsin for PTCL was withdrawn because of a lack of benefit of Ro-CHOP combination in a phase 3 frontline trial,24 romidepsin continues to be available for patients with cutaneous T-cell lymphoma under Food and Drug Administration label and for PTCL under National Comprehensive Cancer Network guidelines. This study was designed with the rationale that epigenetic agent, such as HDAC inhibitor, targets important epigenetic pathways in PTCL, and therefore HDAC inhibitor combinations remain important therapeutic strategies, including romidepsin combination in the frontline setting, which requires further study.
In summary, chemotherapy–free biologic combination of romidepsin and lenalidomide can be safely administered with supportive care as initial therapy for patients with PTCL who are not candidates for cytotoxic chemotherapy. The favorable efficacy in response rates and duration of responses support further evaluation in both patients who are chemotherapy eligible and those who are ineligible, particularly those with AITL, as induction treatment. Furthermore, studies to identify predictors of response and resistance including genetic mutational profile would be particularly important in understanding how best to select patients likely to benefit from this combination.
Acknowledgments
The authors thank all of the study investigators, patients, and their families.
The study was supported in part by Celgene, a Bristol Myers Squibb Company, and the National Cancer Institute. The authors acknowledge funding from Jane and Stuart Weitzman and Family (J.R.).
Authorship
Contribution: A.M.P. designed the study; J.R. and B. Pro wrote the manuscript, recruited study subjects, and contributed to the study concept; B. Pro, J.R., and J.Z. contributed to coordination of the logistics, interpretation of the data, and critically reviewed the manuscript; X.M. and B.J. performed biostatistical analysis of patient data; A.S. contributed to preparation and critical review of the manuscript; A.S., J.N.W., L.I.G., R.K., and J.M. contributed to patient care and critical review of the manuscript; and all investigators gathered the data, have full access to all data, were responsible for statistical analyses and interpretation, and approved the final manuscript.
Conflict-of-interest disclosure: J.R. has received research support, provided consultancy, and served on the advisory board meetings for Bristol Myers Squibb (BMS)/Celgene, AstraZeneca, Genentech, Daiichi Sankyo, Seattle Genetics, Secura Bio and Kite Pharma. B.P. is currently employed by Hutchmed. J.N.W. has received research support from Merck. L.I.G. has served on the data and safety monitoring board for Janssen; served on the advisory board for Kite Pharma and BMS; and provided consultancy for Ono Pharmaceutical. R.K. has served on the advisory boards for BMS, Gilead Sciences/Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, Epizyme, Genentech/Roche, EUSA, Calithera, and Janssen; has received research support from BMS, Takeda, BeiGene, Gilead Sciences/Kite Pharma; and has received honoraria from AstraZeneca, BeiGene, and MorphoSys. B.P. has received research support and honoraria from Seattle Genetics and Takeda, and has received research support from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Barbara Pro, Division of Hematology/Oncology, Columbia University Medical Center, 177 Fort Washington Ave, Suite 6GN-435, New York, NY 10032; e-mail: bp2655@cumc.columbia.edu; and Jia Ruan, Division of Hematology/Oncology, Meyer Cancer Center, Weill Cornell Medicine, 525 E 68th St, New York, NY 10065; e-mail: jruan@med.cornell.edu.
References
Author notes
Individual participant data will not be shared. Data are available on request from the corresponding author, Barbara Pro (bp2655@cumc.columbia.edu).
The full-text version of this article contains a data supplement.