Key Points
Mosunetuzumab-CHOP shows acceptable tolerability and preliminary activity in patients with previously untreated DLBCL.
These phase 2 findings support further evaluation of novel combinations with mosunetuzumab for first-line treatment of DLBCL.
Abstract
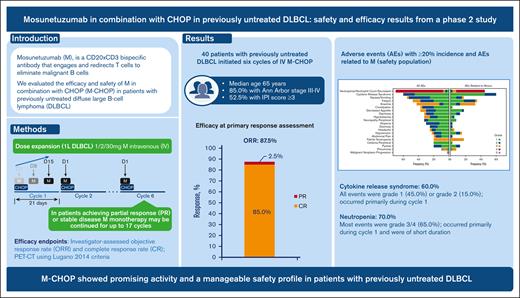
Up to 40% of patients with diffuse large B-cell lymphoma (DLBCL) are refractory to or relapse after first-line therapy, highlighting the need for better treatments. Mosunetuzumab is a CD20 × CD3 bispecific antibody that engages and redirects T cells to eliminate malignant B cells. In this phase 2, open-label study (NCT03677141), 40 patients (52.5% with international prognostic index ≥3) with previously untreated DLBCL initiated 6 cycles of IV mosunetuzumab with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. Mosunetuzumab was administered in cycle 1 as step-up doses to mitigate cytokine release syndrome [CRS], and a dose of 30 mg was given on day 1 of cycles 2-6. Efficacy end points included objective and complete response rates, as determined by the investigator, via positron emission tomography–computed tomography, using Lugano 2014 criteria (87.5% and 85.0%, respectively). At a median follow-up of 32.0 months, the estimated 2-year progression-free survival and event-free survival rates were 65.4% (95% confidence interval [CI], 49.5-81.4) and 60.4% (95% CI, 44.7-76.1), respectively. CRS occurred in 60.0% of patients; all events were grade 1 (45.0%) or grade 2 (15.0%) and occurred primarily in cycle 1. Mosunetuzumab-related grade ≥3 neurologic adverse events (AEs) potentially consistent with immune effector cell–associated neurotoxicity syndrome occurred in 1 patient (2.5%). Grade 5 AEs were reported in 2 patients. Neutropenia occurred in 70.0% of patients, mostly during cycle 1 and was of short duration. These findings demonstrate promising activity and a manageable safety profile for mosunetuzumab-CHOP and warrant further investigation of mosunetuzumab in first-line combination regimens for DLBCL.
Introduction
Despite numerous randomized trials designed to improve treatment outcomes over the last 20 years,1-9 the current standard of care for newly diagnosed patients with advanced-stage diffuse large B-cell lymphoma (DLBCL) has remained largely unchanged until recently, consisting primarily of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).1-10 Although most patients with DLBCL who receive R-CHOP are cured, up to 40% are refractory to such treatment or relapse and have poor outcomes.10,11 Rituximab, a monoclonal antibody directed against the CD20 antigen present on malignant B cells, mediates cytotoxicity via antibody-dependent and complement-dependent mechanisms.12,13 As an immunotherapy component, it contributes substantially to the overall treatment effect of R-CHOP, since R-CHOP confers significantly improved response rates, progression-free survival (PFS), and overall survival compared with CHOP alone.14,15
Recently developed bispecific antibodies directed against CD20 and CD3 constitute a promising novel class of immunotherapy for B-cell non-Hodgkin lymphomas (B-NHLs), such as DLBCL, offering the potential for further enhanced immune effector activity against malignant B cells via targeted T-cell recruitment, engagement, and activation.16 Mosunetuzumab is one such bispecific antibody that has demonstrated manageable safety with notable levels of durable activity as a single agent in patients with relapsed or refractory (R/R) B-NHL, including DLBCL.17 Mosunetuzumab was recently approved by the European Medicines Agency and the US Food and Drug Administration for the treatment of R/R follicular lymphoma in patients who have received ≥2 prior lines of therapy.18,19 Based on its distinct and highly specific mechanism of action, wherein T cells are directly recruited to CD20-expressing lymphoma cells, mosunetuzumab holds potential for replacing rituximab as a more potent and targeted component of immunochemotherapy against DLBCL.
As a follow-up to the clinical study of mosunetuzumab monotherapy in patients with R/R B-NHL, a phase 1b dose-escalation study was recently completed to establish the recommended phase 2 dose of mosunetuzumab in combination with CHOP (M-CHOP) in a similar patient population. This was followed by a phase 2, dose-expansion study in patients with previously untreated DLBCL to establish the initial safety, tolerability, and activity of M-CHOP as a potential alternative first-line regimen, the results of which are presented in this study.
Methods
Study design
This open-label study (NCT03677141) was conducted at 16 sites in Austria, South Korea, Spain, and the United States. The study consisted of phase 1b dose-finding cohorts in patients with R/R B-NHL and a dose-expansion component in patients with newly diagnosed DLBCL. Here, we focused on a single-arm, phase 2 safety and dose-expansion cohort, investigating M-CHOP in patients with previously untreated DLBCL.
All patients provided written informed consent. An institutional review board or ethics committee approved the protocol at each study site. The study was performed in accordance with the principles of the Declaration of Helsinki, applicable local laws and regulations, and the International Conference of Harmonization E6 Guideline for Good Clinical Practice.
Study population
Patients with previously untreated, newly diagnosed, histologically confirmed DLBCL with an International Prognostic Index (IPI) score between 2 and 5 were enrolled. Other eligibility criteria included: presence of at least 1 bidimensionally measurable nodal lesion >1.5 cm (or 1 bidimensionally measurable extranodal lesion >1.0 cm), Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, adequate cardiac, hematologic, and renal function, and life expectancy ≥24 weeks. Complete details of the eligibility criteria are provided in supplemental Appendix.
Study treatments
In cycle 1, mosunetuzumab was administered in step-up doses on day 1 (1 mg), day 8 (2 mg), and day 15 (30 mg) to mitigate cytokine release syndrome (CRS; supplemental Figure 1). The dose of 30 mg was given on day 1 of cycles 2 to 6 (and thereafter for patients who continued to receive mosunetuzumab monotherapy). CHOP was administered on day 1 of each 21-day cycle for 6 cycles. After treatment with M-CHOP, patients with a partial response (PR) or stable disease at the end of cycle 6 could continue mosunetuzumab monotherapy for up to 11 additional cycles (total of 17 cycles) unless they experienced progressive disease (PD) or unacceptable toxicity. Corticosteroid premedication with either dexamethasone 20 mg intravenous (IV) or methylprednisolone 80 mg IV was administered at least 1 hour before mosunetuzumab but was omitted on days when prednisone 100 mg was administered orally as part of CHOP. Patients were allowed, but not required, to receive anti-infection prophylaxis per institutional guidelines. Patients were required to receive prophylactic granulocyte colony-stimulating factor (G-CSF) during the 6 cycles of M-CHOP. Complete details on the administration of study treatment are provided in supplemental Appendix.
Study assessments
Interim and primary response assessments were obtained after cycle 4 and cycle 6, respectively. Response was assessed by the investigator, according to physical examination findings, positron emission tomography–computed tomography (PET-CT), and diagnostic CT (or magnetic resonance imaging). Response rates were based on Lugano criteria.20
Efficacy and safety analysis
All prespecified efficacy end points were determined by the investigator, including the complete response (CR) rate at the time of primary response assessment (6-8 weeks after cycle 6 day 1 of study treatment or early treatment discontinuation) based on the PET-CT. Other efficacy end points included overall response rate (ORR) at the end of induction, best ORR based on PET-CT, duration of response (DOR), PFS, and event-free survival (EFS). PFS was defined as the time from randomization to the first occurrence of PD or relapse, as determined by the investigator, or death from any cause, whichever occurred first. EFS was defined as the time from enrollment to the first occurrence of PD or relapse, as determined by the investigator, initiation of new antilymphoma therapy, or death from any cause, whichever occurred first. Complete definitions of the efficacy end points are provided in supplemental Appendix.
Safety and tolerability were assessed based on the occurrence and severity of adverse events (AEs), with severity determined according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. For CRS, severity was determined according to American Society for Transplantation and Cellular Therapy CRS Consensus Grading criteria.21 In addition, changes from screening assessments in laboratory test results, electrocardiograms, and vital signs were summarized.
Statistical analysis
The safety population included all patients who received treatment. Efficacy analyses were conducted in all enrolled patients. A sample size of 40 patients was calculated to obtain confidence intervals (CIs) of exactly 95% for the estimation of the true complete response (CR) rate, with a margin of error not exceeding ±17%. No formal hypothesis testing was performed. Demographic and baseline characteristics were summarized descriptively. CR rates were estimated, and corresponding Clopper-Pearson exact 95% CIs were constructed. DOR, PFS, and EFS were estimated using Kaplan-Meier methods, with the Brookmeyer-Crowley method used to construct the 95% CI for medians. Greenwood's formula was used to provide standard errors and corresponding 95% CIs for 1-year PFS.
Results
Before initiating the phase 2 portion of the study, 7 patients with R/R B-NHL were enrolled in a phase 1b dose-escalation portion; each received 6 cycles of M-CHOP to determine the recommended phase 2 IV dose of mosunetuzumab. The target dose was 13.5 mg for the first 3 patients and 30 mg for the ensuing 4 patients. No dose-limiting toxicities were observed, and 30 mg of mosunetuzumab was selected as the target dose to continue into the phase 2 portion. See supplemental Table 1 for additional details pertaining to the phase 1b cohort. The remainder of this report will focus on patients with previously untreated DLBCL who were enrolled in the phase 2, dose-expansion portion of the study.
Patient characteristics
The phase 2 portion of the study was conducted globally at 16 sites (supplemental Table 2). Between 22 November, 2019, and 13 July, 2020, 40 patients with previously untreated DLBCL were enrolled and initiated treatment with M-CHOP (Table 1). The median age was 65 years (range, 39-79). Most patients had an ECOG performance status of 0 or 1 (95.0%) and Ann Arbor stage III to IV disease (85.0%). The proportions of patients with IPI ≥3, bulky disease (≥7.5 cm), and extranodal involvement at study entry were 52.5%, 32.5%, and 75.0%, respectively. Available cell of origin and other laboratory characterization data (per local laboratory) are listed in detail in Table 1. At the clinical cutoff date (12 October, 2022), the median duration of follow-up was 32.0 months (range, 2.0-35.0).
Baseline patient demographic and disease characteristics
| Characteristic . | Patients (n = 40) . |
|---|---|
| Age, median (range), y | 65 (39-79) |
| 18–65 y, n (%) | 21 (52.5) |
| >65 y, n (%) | 19 (47.5) |
| Male, n (%) | 22 (55.0) |
| ECOG PS, n (%) | |
| 0 | 16 (40.0) |
| 1 | 22 (55.0) |
| 2 | 2 (5.0) |
| Ann Arbor stage at study entry, n (%) | |
| I to II | 6 (15.0) |
| III to IV | 34 (85.0) |
| IPI score at study entry, n (%) | |
| 2 | 19 (47.5) |
| 3 | 15 (37.5) |
| 4 | 6 (15.0) |
| Cell of origin by local laboratory, n (%) | |
| Germinal center B cell | 17 (42.5)∗ |
| Nongerminal center B cell | 21 (52.5)† |
| Unknown | 2 (5.0) |
| Additional characterization by local laboratory, n (%) | |
| Rearrangements of MYC, BCL2, and/or BCL6 | 3 (7.5) |
| Double expressor (MYC and BCL2 overexpression without translocation) | 6 (15.0)‡ |
| None of the above | 28 (70.0) |
| Not determined/available | 3 (7.5) |
| Characteristic . | Patients (n = 40) . |
|---|---|
| Age, median (range), y | 65 (39-79) |
| 18–65 y, n (%) | 21 (52.5) |
| >65 y, n (%) | 19 (47.5) |
| Male, n (%) | 22 (55.0) |
| ECOG PS, n (%) | |
| 0 | 16 (40.0) |
| 1 | 22 (55.0) |
| 2 | 2 (5.0) |
| Ann Arbor stage at study entry, n (%) | |
| I to II | 6 (15.0) |
| III to IV | 34 (85.0) |
| IPI score at study entry, n (%) | |
| 2 | 19 (47.5) |
| 3 | 15 (37.5) |
| 4 | 6 (15.0) |
| Cell of origin by local laboratory, n (%) | |
| Germinal center B cell | 17 (42.5)∗ |
| Nongerminal center B cell | 21 (52.5)† |
| Unknown | 2 (5.0) |
| Additional characterization by local laboratory, n (%) | |
| Rearrangements of MYC, BCL2, and/or BCL6 | 3 (7.5) |
| Double expressor (MYC and BCL2 overexpression without translocation) | 6 (15.0)‡ |
| None of the above | 28 (70.0) |
| Not determined/available | 3 (7.5) |
ECOG PS, Eastern Cooperative Oncology Group performance status; GEP, gene expression profiling; IHC, immunohistochemistry.
Thirteen patients assigned based on IHC and 4 assigned based on GEP.
Seventeen patients assigned based on IHC and 4 patients based on GEP (3 had activated B cell, and 1 was unclassified).
Includes 1 patient whose double expressor status was not confirmed until after the clinical cutoff date.
Treatment exposure
The median number of mosunetuzumab doses received was 8 (range, 1-13; including the first 2 step-up doses). The median number of cycles of chemotherapy received was 6 (range, 1-6); 34 patients (85.0%) received 6 cycles of M-CHOP. One patient continued to receive mosunetuzumab monotherapy for an additional 5 cycles based on a PR assessed after 6 cycles of M-CHOP (the patient had PD at the end of study treatment). The proportions of patients who received ≥90% dose intensity of the components of M-CHOP were 97.5% (mosunetuzumab), 85.0% (doxorubicin, prednisone, and vincristine), and 82.5% (cyclophosphamide); chemotherapy dose reductions occurred primarily in response to toxicity.
Efficacy
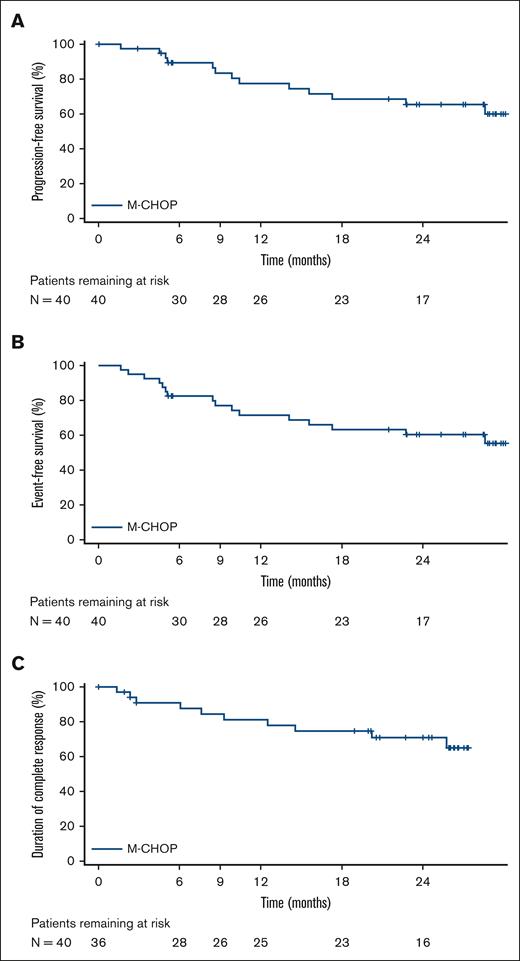
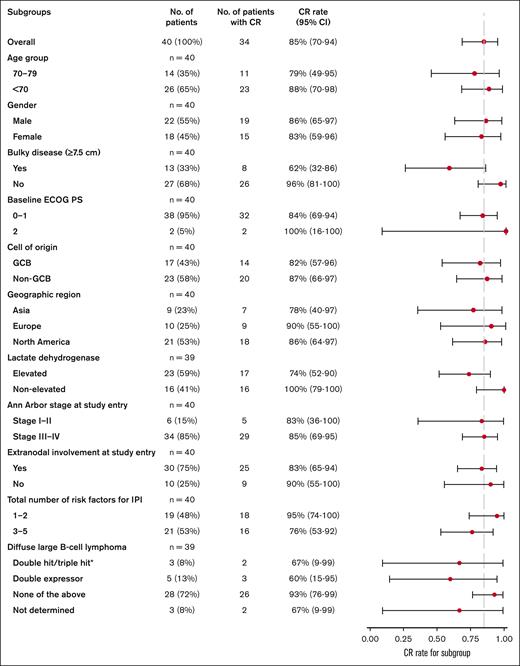
All reported efficacy assessments were determined by the investigator per Lugano criteria.20 Best ORR, defined as the occurrence of CR or PR at any point during the study, including at the interim response assessment, was 95.0% (95% CI, 83.1-99.4; CR rate, 90.0% [95% CI, 76.3-97.2]; PR rate, 5.0% [95% CI, 0.6-16.9]; Table 2). At the time of the primary response assessment, the ORR was 87.5% (95% CI, 73.2-95.8; CR rate, 85.0% [95% CI, 70.2-94.3]; PR rate, 2.5% [95% CI, 0.1-13.2]). Median values for DOR, duration of CR, PFS, and EFS were not reached (Figure 1). Estimated 2-year DOR, duration of CR, PFS, and EFS rates were 66.8% (95% CI, 50.7-83.0), 70.9% (95% CI, 54.8-87.1), 65.4% (95% CI, 49.5-81.4), and 60.4% (95% CI, 44.7-76.1), respectively. Results of an exploratory subgroup analysis of CR rate are shown in Figure 2; for each subgroup analyzed, the 95% CI included the overall point estimate of CR rate (85.0%) of the entire study cohort. The subgroup of patients with bulky disease at diagnosis (at least 1 lesion ≥7.5 cm; n = 13) had the lowest point estimate for CR rate (62%; 95% CI, 32-86).
Investigator-assessed objective response rates
| Response, % (95% CI) . | Best overall response (n = 40) . | Overall response at primary response assessment (n = 40) . |
|---|---|---|
| Overall response | 95.0 (83.1-99.4) | 87.5 (73.2-95.8) |
| CR | 90.0 (76.3-97.2) | 85.0 (70.2-94.3) |
| PR | 5.0 (0.6-16.9) | 2.5 (0.1-13.2) |
| Stable disease | 0 (0-8.8) | 0 (0-8.8) |
| PD | 0 (0-8.8) | 7.5 (1.6-20.4) |
| Data missing∗, % | 5.0 | 5.0 |
| Response, % (95% CI) . | Best overall response (n = 40) . | Overall response at primary response assessment (n = 40) . |
|---|---|---|
| Overall response | 95.0 (83.1-99.4) | 87.5 (73.2-95.8) |
| CR | 90.0 (76.3-97.2) | 85.0 (70.2-94.3) |
| PR | 5.0 (0.6-16.9) | 2.5 (0.1-13.2) |
| Stable disease | 0 (0-8.8) | 0 (0-8.8) |
| PD | 0 (0-8.8) | 7.5 (1.6-20.4) |
| Data missing∗, % | 5.0 | 5.0 |
CR, complete response; PR, partial response; PD, progressive disease
Two patients discontinued study treatment before having any response assessment performed.
Kaplan-Meier curves for PFS, EFS∗, and DOCR in efficacy-evaluable patients. ∗Events consisted of 8 instances of PD, 5 deaths, and 3 instances of nonstudy next antilymphoma therapy (NALT) being introduced. Of the NALT events, 2 occurred because of AEs leading to early study treatment discontinuation (and subsequent transition to R-CHOP therapy), and 1 NALT event occurred when prophylactic systemic methotrexate was administered in violation of the protocol.
Kaplan-Meier curves for PFS, EFS∗, and DOCR in efficacy-evaluable patients. ∗Events consisted of 8 instances of PD, 5 deaths, and 3 instances of nonstudy next antilymphoma therapy (NALT) being introduced. Of the NALT events, 2 occurred because of AEs leading to early study treatment discontinuation (and subsequent transition to R-CHOP therapy), and 1 NALT event occurred when prophylactic systemic methotrexate was administered in violation of the protocol.
Forest plot of exploratory subgroup analyses of CR rates in patients at the primary response assessment by PET-CT. Response assessments, according to Lugano criteria,20 are displayed for subgroups of interest within the efficacy-evaluable population (n = 40). Data for each group are presented as the proportion of patients with CR and 95% CI (based on the Clopper-Pearson method). Dashed vertical line represents the point estimate of the overall study cohort. ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal center B cell.
Forest plot of exploratory subgroup analyses of CR rates in patients at the primary response assessment by PET-CT. Response assessments, according to Lugano criteria,20 are displayed for subgroups of interest within the efficacy-evaluable population (n = 40). Data for each group are presented as the proportion of patients with CR and 95% CI (based on the Clopper-Pearson method). Dashed vertical line represents the point estimate of the overall study cohort. ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal center B cell.
Pharmacokinetics
The pharmacokinetics of mosunetuzumab in combination with CHOP were comparable with those of mosunetuzumab as monotherapy,17,19 suggesting no significant impact of CHOP on mosunetuzumab pharmacokinetics and similar exposures between patients with R/R NHL and those with previously untreated DLBCL (supplemental Figure 2). No antidrug antibodies against mosunetuzumab were detected.
Safety
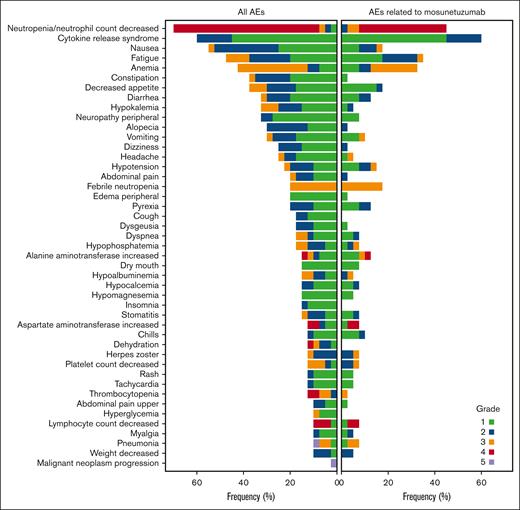
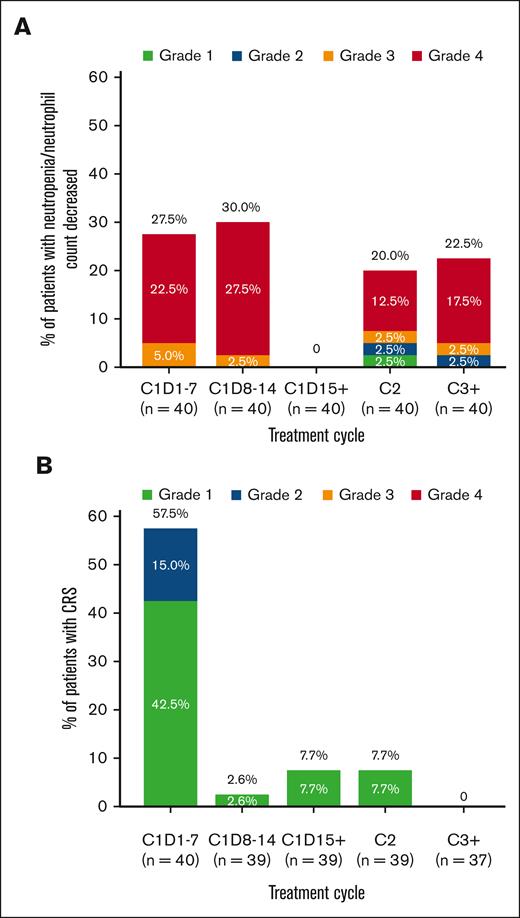
Treatment-emergent AEs are summarized in Table 3. The most common any-grade AEs (≥40% of patients) were neutropenia or decrease in neutrophil count (70.0%), CRS (60.0%), nausea (55.0%), fatigue (47.5%), and anemia (42.5%) (Figure 3). Grade 3 to 4 AEs occurred in 35 patients (87.5%), with 25 patients (62.5%) experiencing grade 3 to 4 AEs considered related to mosunetuzumab. The most frequent grade 3 to 4 events were neutropenia or decrease in neutrophil count (65.0%), anemia (30.0%), and febrile neutropenia (20.0%); most of these events were assessed as related to the study treatment. The median onset of neutropenia or decrease in neutrophil count was 22 days after the start of study treatment (range, 7-237), and the median duration was 8 days (range, 2-265). Neutropenia-related events were mostly reported during cycle 1 (Figure 4). All patients received G-CSF; 15 patients (37.5%) received at least 1 dose for treatment of neutropenia. Serious infections with reported concurrent neutropenia were rare; 1 grade 3 pneumonia occurred with concurrent grade 4 neutropenia.
Summary of AEs
| n (%) . | Patients (n = 40) . |
|---|---|
| Patients with at least one AE | 40 (100.0) |
| Mosunetuzumab-related AE | 40 (100.0) |
| SAE, not including grade 5 disease progression | 20 (50.0) |
| Mosunetuzumab-related SAE | 12 (30.0) |
| Grade 5 AE | 2 (5.0)∗ |
| AE leading to mosunetuzumab discontinuation | 4 (10.0) |
| Treatment-related AE leading to mosunetuzumab discontinuation | 1 (2.5) |
| n (%) . | Patients (n = 40) . |
|---|---|
| Patients with at least one AE | 40 (100.0) |
| Mosunetuzumab-related AE | 40 (100.0) |
| SAE, not including grade 5 disease progression | 20 (50.0) |
| Mosunetuzumab-related SAE | 12 (30.0) |
| Grade 5 AE | 2 (5.0)∗ |
| AE leading to mosunetuzumab discontinuation | 4 (10.0) |
| Treatment-related AE leading to mosunetuzumab discontinuation | 1 (2.5) |
| Any-grade AEs (≥20% of patients) or grade ≥3 AEs (≥5% of patients) based on preferred term . | Any grade . | Grade ≥3 . |
|---|---|---|
| Neutropenia/neutrophil count decreased | 28 (70.0) | 26 (65.0) |
| CRS | 24 (60.0) | 0 |
| Nausea | 22 (55.0) | 1 (2.5) |
| Fatigue | 19 (47.5) | 4 (10.0) |
| Anemia/hemoglobin decreased | 17 (42.5) | 12 (30.0) |
| Decreased appetite | 15 (37.5) | 3 (7.5) |
| Constipation | 15 (37.5) | 1 (2.5) |
| Hypokalemia | 13 (32.5) | 3 (7.5) |
| Peripheral neuropathy | 13 (32.5) | 1 (2.5) |
| Diarrhea | 13 (32.5) | 1 (2.5) |
| Alopecia | 12 (30.0) | 0 |
| Vomiting | 12 (30.0) | 1 (2.5) |
| Thrombocytopenia/platelet count decreased | 10 (25.0) | 7 (17.5) |
| Headache | 10 (25.0) | 1 (2.5) |
| Dizziness | 10 (25.0) | 0 |
| Hypotension | 9 (22.5) | 1 (2.5) |
| Febrile neutropenia | 8 (20.0) | 8 (20.0) |
| Abdominal pain | 8 (20.0) | 1 (2.5) |
| Peripheral edema | 8 (20.0) | 0 |
| Pyrexia | 8 (20.0) | 0 |
| Dyspnea | 7 (17.5) | 2 (5.0) |
| Hypophosphatemia | 7 (17.5) | 2 (5.0) |
| Hypoalbuminemia | 6 (15.0) | 2 (5.0) |
| Alanine aminotransferase increased | 6 (15.0) | 2 (5.0) |
| Aspartate transaminase increased | 5 (12.5) | 2 (5.0) |
| Lymphopenia/lymphocyte count decreased | 5 (12.5) | 4 (10.0) |
| Dehydration | 5 (12.5) | 2 (5.0) |
| Leukopenia/white blood cell decreased | 4 (10.0) | 3 (7.5) |
| Pneumonia | 4 (10.0) | 3 (7.5) |
| Colitis | 3 (7.5) | 2 (5.0) |
| Syncope | 2 (5.0) | 2 (5.0) |
| Any-grade AEs (≥20% of patients) or grade ≥3 AEs (≥5% of patients) based on preferred term . | Any grade . | Grade ≥3 . |
|---|---|---|
| Neutropenia/neutrophil count decreased | 28 (70.0) | 26 (65.0) |
| CRS | 24 (60.0) | 0 |
| Nausea | 22 (55.0) | 1 (2.5) |
| Fatigue | 19 (47.5) | 4 (10.0) |
| Anemia/hemoglobin decreased | 17 (42.5) | 12 (30.0) |
| Decreased appetite | 15 (37.5) | 3 (7.5) |
| Constipation | 15 (37.5) | 1 (2.5) |
| Hypokalemia | 13 (32.5) | 3 (7.5) |
| Peripheral neuropathy | 13 (32.5) | 1 (2.5) |
| Diarrhea | 13 (32.5) | 1 (2.5) |
| Alopecia | 12 (30.0) | 0 |
| Vomiting | 12 (30.0) | 1 (2.5) |
| Thrombocytopenia/platelet count decreased | 10 (25.0) | 7 (17.5) |
| Headache | 10 (25.0) | 1 (2.5) |
| Dizziness | 10 (25.0) | 0 |
| Hypotension | 9 (22.5) | 1 (2.5) |
| Febrile neutropenia | 8 (20.0) | 8 (20.0) |
| Abdominal pain | 8 (20.0) | 1 (2.5) |
| Peripheral edema | 8 (20.0) | 0 |
| Pyrexia | 8 (20.0) | 0 |
| Dyspnea | 7 (17.5) | 2 (5.0) |
| Hypophosphatemia | 7 (17.5) | 2 (5.0) |
| Hypoalbuminemia | 6 (15.0) | 2 (5.0) |
| Alanine aminotransferase increased | 6 (15.0) | 2 (5.0) |
| Aspartate transaminase increased | 5 (12.5) | 2 (5.0) |
| Lymphopenia/lymphocyte count decreased | 5 (12.5) | 4 (10.0) |
| Dehydration | 5 (12.5) | 2 (5.0) |
| Leukopenia/white blood cell decreased | 4 (10.0) | 3 (7.5) |
| Pneumonia | 4 (10.0) | 3 (7.5) |
| Colitis | 3 (7.5) | 2 (5.0) |
| Syncope | 2 (5.0) | 2 (5.0) |
| SAEs (≥5% of patients) based on preferred term . | Any grade . | Grade ≥3 . |
|---|---|---|
| Febrile neutropenia | 6 (15.0) | 6 (15.0) |
| Neutropenia/neutrophil count decreased | 3 (7.5) | 3 (7.5) |
| Pneumonia | 3 (7.5) | 3 (7.5) |
| Colitis | 2 (5.0) | 2 (5.0) |
| Nausea | 2 (5.0) | 1 (2.5) |
| SAEs (≥5% of patients) based on preferred term . | Any grade . | Grade ≥3 . |
|---|---|---|
| Febrile neutropenia | 6 (15.0) | 6 (15.0) |
| Neutropenia/neutrophil count decreased | 3 (7.5) | 3 (7.5) |
| Pneumonia | 3 (7.5) | 3 (7.5) |
| Colitis | 2 (5.0) | 2 (5.0) |
| Nausea | 2 (5.0) | 1 (2.5) |
AE, adverse event; SAE, serious adverse event
One case of pneumonia unrelated to mosunetuzumab but related to chemotherapy and 1 case of malignant disease progression.
Tornado plot of AEs with ≥10% incidence and AEs related to mosunetuzumab in the safety population.
Tornado plot of AEs with ≥10% incidence and AEs related to mosunetuzumab in the safety population.
Reported events of neutropenia/decreased neutrophil count and CRS events based on the cycle and grade.
Reported events of neutropenia/decreased neutrophil count and CRS events based on the cycle and grade.
Infections occurred in 21 patients (52.5%). The median onset of infection was 50 days after the start of study treatment (range, 1-190), and the median duration was 14 days (range, 1-76). The most common infections were herpes zoster, reported in 5 patients (12.5%), pneumonia (cause unknown, 10.0%), oral candidiasis (7.5%), and upper respiratory tract infection (5.0%). Grade 3 to 5 infections occurred in 9 patients (22.5%), including 1 fatal event. Serious infections, consisting of pneumonia (1 grade 5 event and 2 grade 3 events), grade 3 aspiration pneumonia, grade 3 herpes zoster, grade 3 vascular device infection, grade 3 infectious enterocolitis, grade 3 influenza, and grade 3 soft tissue infection, occurred in 7 patients (17.5%). The fatal case of pneumonia occurred after 2 cycles of treatment, with presenting symptoms of fever, oral mucositis, persistent dyspnea, and anorexia. The patient was given multiple courses of antibiotics, steroids, and high-flow oxygen but did not receive a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test. Despite treatment, the patient died on study day 50. The event was considered related to chemotherapy but unrelated to mosunetuzumab. Grade 1 COVID-19 was reported in 1 other patient.
Serious AEs (SAEs) occurred in 20 patients (50.0%), with 12 patients (30.0%) experiencing mosunetuzumab-related SAEs. The most common SAEs were febrile neutropenia (15.0%), neutropenia (7.5%), and pneumonia (7.5%), most of which were considered related to study treatment. Four patients (10.0%) had AEs leading to discontinuation of mosunetuzumab and CHOP: grade 5 pneumonia, grade 2 anastomotic leak, grade 4 fistula, and grade 3 pneumonitis. Of these, only grade 3 pneumonitis was considered related to mosunetuzumab, and the event resolved after 17 days. Grade 5 (fatal) AEs occurred in 2 patients (1 case each of pneumonia and malignant PD).
All CRS events were grade 1 (18 patients, 45.0%) or grade 2 (6 patients, 15.0%), and all resolved. None of the CRS events required new hospitalization or prolongation of existing hospitalization, intensive care unit admission, vasopressor support, or high-flow oxygen. The median time to onset of CRS was 1 day after the first dose of mosunetuzumab (range, 1-21), and the median duration was 2 days (range, 1-21). Most CRS events occurred in cycle 1 during step-up dosing of mosunetuzumab, primarily after the first dose (Figure 4). Among 24 patients who experienced CRS, the most common manifestations were pyrexia (20 patients; 83.3%), hypotension (7 patients; 29.2%), nausea (4 patients; 16.7%), chills (3 patients; 12.5%), and hypoxia (2 patients; 8.3%). Treatment for CRS management included IV fluids (5 patients; 20.8%), tocilizumab (2 patients; 8.3%), and steroids (1 patient; 4.2%). Most CRS occurrences were single events; 4 patients had multiple CRS events (3 with 2 events and 1 with 4 events, all grade 1).
Five patients (12.5%) experienced mosunetuzumab-related neurologic events potentially consistent with immune effector cell–associated neurotoxicity syndrome (ICANS), comprising grade 3 syncope, grade 2 cognitive disorder, grade 2 ICANS, grade 2 lethargy, and grade 1 memory impairment. The grade 3 syncope event occurred on day 6 of cycle 3 with diarrhea, severe fatigue, and leukopenia. All events resolved apart from the grade 2 cognitive disorder. The most common neurologic AEs (≥10% of patients) were peripheral neuropathy, reported in 13 patients (32.5%), headache (25%), dizziness (25%), dysgeusia (17.5%), and insomnia (15%); most were assessed as unrelated to mosunetuzumab. Most neurologic AEs were grade 1 or 2. Grade 3 neurologic AEs occurred in 4 patients (10%), reported as headache (n = 1), peripheral motor neuropathy (n = 1), and syncope (n = 2). The median onset of neurologic AEs was study day 25 (range, 1-203), with a median duration of 8 days (range, 1-837). No grade 4 or 5 neurologic AEs were reported.
Discussion
In this phase 2 study of 40 patients with previously untreated DLBCL (52.5% with IPI ≥3), M-CHOP induced a high proportion of objective responses, the vast majority of which were CRs. Although analyses were limited by small sample sizes, CR rates were consistent across subgroups. Although the PFS estimate (65.4% at 2 years) was lower than that in some recent phase 3 trials in DLBCL (eg, 67% with R-CHOP in GOYA and 70% in POLARIX),8,22 the 95% CI was wide (49.5-81.4) for this secondary end point, and the study was not powered to draw conclusions regarding the efficacy based on this estimate. A larger, randomized study would be needed to fully assess the survival rates of a mosunetuzumab-containing combination regimen.
The ORR and CR rate from this study (87.5% and 85.0%, respectively, at the end of treatment) compare favorably with those observed in larger benchmark trials of alternative regimens used for first-line treatment of DLBCL. Acknowledging the limitations of cross-study comparisons, the final analysis of the GOYA study reported end-of-treatment ORRs and CR rates (using PET-CT criteria) of 77.6% and 59.1%, respectively, in patients who received R-CHOP.23 In the POLARIX trial, patients randomly assigned to receive R-CHP-Pola (n = 440), which substitutes the use of the CD79b-directed antibody-drug conjugate polatuzumab vedotin for vincristine, exhibited a 27% reduction in risk of PD, relapse, or death at 2 years compared with that in patients randomly assigned to receive R-CHOP (n = 439),22 marking a significant advancement in first-line treatment for DLBCL. Response rates observed with M-CHOP compare favorably with the end-of-treatment ORRs and CR rates for both arms reported in POLARIX: 83.8% and 74.0%, respectively, with R-CHOP and 85.5% and 78.0%, respectively, with R-CHP-Pola.22 Furthermore, response rates in this study also compare favorably with early results from recent studies of other anti-CD20/CD3 bispecific antibodies used in combination with R-CHOP.24,25 Although there are pros and cons to either strategy, using the CD20/CD3 bispecific antibody as the only CD20-directed agent may help maximize receptor occupancy and prevent competitive binding using doses already established as effective for mosunetuzumab monotherapy. Our findings support the chosen therapeutic strategy of using mosunetuzumab alone (rather than being added to rituximab) in combination with CHOP, but definitive conclusions await further study. Finally, although this phase 2 study was not designed or powered to compare results among patient subgroups, most subgroups exhibited a CR rate similar to that seen in the overall cohort (with the possible exception of patients with bulky disease; Figure 2).
The overall safety profile of mosunetuzumab was comparable with that of previous experience with this agent in the R/R B-NHL setting.17,26 No new safety signals emerged from treating patients who were newly diagnosed with DLBCL, but some AEs were observed at higher frequencies in this study than in larger studies of this patient population.8,22 Four patients experienced AEs leading to treatment discontinuation; only 1 of these was attributed to mosunetuzumab. SAEs were reported in 50.0% of patients, compared with SAE frequencies of 37.6% and 30.6% in the R-CHOP arms of the larger phase 3 GOYA8 and POLARIX22 trials, respectively. Grade ≥3 AEs were reported in 87.5% of patients in this study, compared with 64.7% and 59.8% of patients in the R-CHOP arms of GOYA8 and POLARIX,22 respectively. However, some AEs were observed at a higher frequency than in larger studies of this patient population. These differences were largely driven by events related to neutropenia and anemia, which were reported more frequently in this study than in the R-CHOP arms of GOYA8 and POLARIX22 (neutropenia, 65.0% compared with 38.1% and 30.8%, respectively; anemia, 30.0% compared with 7.5% and 8.4%, respectively). Most cases of neutropenia in this study were grade 3 or 4 but of relatively short duration, and they occurred early during treatment. The higher prevalence of neutropenia and anemia might have resulted from the greater frequency of protocol-mandated study visits and laboratory tests (which were required on days 8 and 15 of the first 2 treatment cycles, coinciding with hematologic nadirs expected from chemotherapy). We observed a higher incidence of febrile neutropenia (20.0%) than reported in POLARIX and other recent studies of R-CHOP (8.0%-15.2%).8,22 Higher rates of grade ≥3 infections were observed in this study (22.5%) than those reported in the R-CHOP arms of GOYA (15.5%) and POLARIX (12.6%; only grade 3/4 infections), which could potentially be related to neutropenia.8,22 However, serious infection concurrent with reported neutropenia occurred in only 1 patient. Patients in this phase 2 study were allowed, but not required, to receive anti-infection prophylaxis per institutional guidelines. Furthermore, although only a single COVID-19–related event was reported, the study enrolled patients during the early phases of the COVID-19 pandemic, before the wide availability of SARS-CoV-2 testing, prophylaxis, vaccination, and treatment measures, such that undiagnosed COVID-19–related illnesses might have contributed to the higher rates of severe infections, for example, the case of grade 5 pneumonia which occurred in February 2020, and SAEs in this study. Incidentally, although the fatal case of pneumonia was assessed by the investigator as unrelated to mosunetuzumab, the significant COVID-19–related morbidity and mortality risks observed to date in patients who have received CD20-directed bispecific antibody therapy for NHL must be carefully considered and managed.27
Given the mechanism of action, the risk of CRS is a primary concern while using bispecific antibodies, such as mosunetuzumab, which engage with and activate cytotoxic T cells. T-cell activation may lead to an excess of systemic cytokine release, which, in some cases, has resulted in serious and, even, fatal events, as observed with other T-cell engaging agents, such as blinatumomab28 and chimeric antigen receptor (CAR) T-cell therapy.29,30 Risk factors for severe CRS after CART-cell therapy and, therefore, potentially other T-cell engaging therapies are thought to include lymphoma bone marrow involvement, extranodal disease, B-cell lymphocytosis, and the presence of circulating peripheral malignant cells. With step-up dosing of mosunetuzumab during cycle 1, 60.0% of patients had CRS, which is higher than that previously described in patients receiving mosunetuzumab monotherapy.17,26 This increased prevalence might have been because of differences in the respective study populations (previously untreated vs R/R disease, DLBCL vs indolent B-NHL), treatment settings (in combination with chemotherapy vs as monotherapy) and/or steroid premedication regimen (oral prednisone as a component of CHOP vs dexamethasone/methylprednisolone in prior studies).17,26 Nevertheless, all CRS events were grade 1 or 2, without the need of intensive care unit or vasopressor support, transient, and predictable, with most cases occurring as isolated events. Although CRS events may require hospitalization in some cases, the overall safety profile of mosunetuzumab observed in this study supports delivery of M-CHOP in an outpatient setting, with any subsequent inpatient care dictated by the clinical course of events. Outpatient administration of mosunetuzumab26 is consistent with cumulative clinical experience with this agent and existing marketing authorizations.18,19 Finally, although higher-grade ICANS-like events were uncommon in this small phase 2 study, the risk of such types of neurotoxicity must be carefully considered, especially when comparing bispecific-containing to more conventional immunochemotherapy regimens.
In summary, the findings from this phase 2 study demonstrate that M-CHOP exhibits a manageable risk-benefit profile for patients with previously untreated DLBCL, eliciting high response rates and acceptable tolerance with no new safety signals detected compared with mosunetuzumab monotherapy and CHOP. Again, acknowledging the limitations of cross-study comparisons, the response rates in this study compare favorably with those reported in prior studies of standard, first-line immunochemotherapy regimens for DLBCL; however, PFS requires further evaluation in a comparative study. The response and survival rates observed suggest that novel combination regimens incorporating enhanced immune effector molecules, such as mosunetuzumab offer promise for newly diagnosed patients with DLBCL. Based partly on these findings as well as those from studies demonstrating promising outcomes with R-CHP-Pola,22,31 a randomized trial comparing mosunetuzumab in combination with CHP-Pola (cyclophosphamide, doxorubicin, prednisone and polatuzumab vedotin) against R-CHP-Pola as first-line treatment for DLBCL has been initiated, the results of which will be reported in the future.
Acknowledgments
Third party medical writing assistance, under the direction of the authors, was provided by Aisling Lynch, PhD, and Louise Profit, PhD, of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd. Statistical support in finalizing this manuscript was provided by Amy (Sisi) Kapp (employee of Genentech, Inc). NCT03677141 is sponsored by Genentech, Inc.
The sponsor was involved in the design and conduct of the study, collection, management, analysis, and interpretation of the data. All authors had full access to the data in the study.
Authorship
Contribution: J.S. conceptualized and designed the study; A.J.O., R.G., I.S.L., T.M.K., J.L.M., U.J., A.M., P.A., D.H.Y., J.W., E.P., and J.S. provided study materials or patients’ details; A.J.O., R.G., J.W., V.C., E.P., and J.S. collected and assembled data; A.J.O., I.S.L., T.M.K., J.L.M., M.C.W., D.L.Y., A.M., M.S.H., T.J.P., P.A., D.H.Y., J.W., A.Y., V.C., E.P., and J.S. analyzed and interpreted data; and all authors contributed to writing and final approval of manuscript and were accountable for all aspects of the work.
Conflict-of-interest disclosure: A.J.O. is a clinical research scholar of the Leukemia and Lymphoma Society; reports consultancy role with Genmab, Schrodinger, Blue Cross and Blue Shield of Rhode Island, TG Therapeutics; and receives research funding from Adaptive Biotechnologies; T.J.P. reports consultancy role with AbbVie, AstraZeneca, and ADC Therapeutics; research funding from AbbVie, Bayer, Bristol Myers Squibb, and Genentech, Inc.; and has advisory board membership with Epizyme; M.S.H. reports consultancy role with AbbVie, AstraZeneca, BeiGene, Eli Lilly, Janssen, Kite, Novartis, Pharmacyclics, and TG Therapeutics; P.A. reports consultancy roles with Merck, Bristol Myers Squibb, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech, Inc./F. Hoffmann-La Roche Ltd, Xencor, and Foresight; research funding from Kite, Merck, Bristol Myers Squibb, Affimed, Adaptive, Tensha, Otsuka, Sigma-Tau, Genentech, Inc./F. Hoffmann-La Roche Ltd, IGM Biosciences, and AstraZeneca; and honoraria from Merck and Bristol Myers Squibb; T.M.K. reports a consulting role with AstraZeneca, Janssen, Novartis, Takeda, Yuhan, Regeneron, and Samsung Bioepis; and speakers bureau role with Takeda, Janssen, and IMBDx Inc; D.H.Y. reports a consulting role with GI cell, AB clone, and Pharos iBio; research funding from Samyang, Boryung, BeiGene, Sanofi, Celltrion, and Janssen; and honoraria from F. Hoffmann-La Roche Ltd, Janssen, Amgen, Bristol Myers Squibb, Novartis, Celltrion, Samyang, Boryung, Kirin Pharm, Takeda, GlaxoSmithKline, and Janssen; A.M. reports a consulting role with Gilead, AstraZeneca, Seagen, Incyte/MorphoSys, BeiGen, F. Hoffmann-La Roche Ltd/Genentech, Inc., and ADC Therapeutics; honoraria from Gilead, AstraZeneca, Seagen, Incyte/MorphoSys, Kyowa Kirin, BeiGen, and F. Hoffmann-La Roche Ltd/Genentech, Inc.; and research funding from Incyte, Takeda, Gilead, Bristol Myers Squibb, Innate Pharma, Seagen, and ONO Pharmaceuticals; R.G. reports a consulting role with Celgene, Novartis, F. Hoffmann-La Roche Ltd, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, Merck, Sharp & Dohme, Merck, Gilead, Daiichi Sankyo, and Sanofi; honoraria fees from Celgene, F. Hoffmann-La Roche Ltd, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, Merck, Sharp & Dohme, Sandoz, AbbVie, Gilead, Daiichi Sankyo, and Sanofi; meeting attendance and/or travel support for F. Hoffmann-La Roche Ltd, Amgen, Janssen, AstraZeneca, Novartis, Merck, Sharp & Dohme, Celgene, Gilead, Bristol Myers Squibb, AbbVie, and Daiichi Sankyo; and advisory board participation with F. Hoffmann-La Roche Ltd, Janssen, AstraZeneca, Novartis, Merck, Sharp & Dohme, Celgene, Gilead, Bristol Myers Squibb, AbbVie, Daiichi Sankyo, Takeda, Merck, and Sanofi; J.W. reports a consulting role with F. Hoffmann-La Roche Ltd/Genentech, Inc., Kite, Bristol Myers Squibb, Novartis, MorphoSys/Incyte, AstraZeneca, Merck, ADC Therapeutics, Monte Rosa, and Iksuda; and research funding from F. Hoffmann-La Roche Ltd/Genentech, Inc., Kite, Bristol Myers Squibb, Novartis, MorphoSys/Incyte, AstraZeneca, and ADC Therapeutics; I.S.L. reports honoraria from Adaptive and BeiGene for participation in advisory boards; and advisory committee role with LRF; J.L.M. reports a consulting role with Pharmacyclics/AbbVie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa, Alexion, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, BeiGene, Servier, Novartis, MorphoSys/Incyte, MEIPharma, Zodiac, TG Therapeutics, and Lilly/Loxo; research funding from Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Inc., Pharmacyclics, Seattle Genetics, Janssen, and Millennium; honoraria from Targeted Oncology, OncView, Curio, Kyowa, Physicians' Education Resource, and Seattle Genetics; and speaker’s bureau fees from Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, BeiGene, Verastem, AstraZeneca, Celgene/Bristol Myers Squibb, and Genentech, Inc./F. Hoffmann-La Roche Ltd; J.S. reports employment with Genentech, Inc. and is an equity holder in F. Hoffmann-La Roche Ltd; M.C.W. reports meeting attendance and/or travel support for F. Hoffmann-La Roche Ltd/Genentech, Inc.; employment by Genentech, Inc.; patents planned, issued, or pending for Genentech, Inc.; and stock and stock options in F. Hoffmann-La Roche Ltd; A.Y. reports employment with Genentech, Inc. and has stock and stock options in F. Hoffmann-La Roche Ltd/Genentech, Inc.; V.C. reports employment with Genentech, Inc.; E.P. and D.L.Y. report employment with Genentech, Inc. and are equity holders in F. Hoffmann-La Roche Ltd; U.J. reports a consulting role, research funding, and honoraria from F. Hoffmann-La Roche Ltd; honoraria from AbbVie, Gilead, Novartis, Milteyi, Janssen, and Sanofi Aventis.
Correspondence: Adam J. Olszewski, Lifespan Cancer Institute, Warren Alpert Medical School of Brown University, 222 Richmond St, Providence, RI 02903; e-mail: adam_olszewski@brown.edu.
References
Author notes
Individual patient-level data are available to qualified researchers through the clinical study data request platform at https://vivli.org/.
Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers/.
For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/ research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The full-text version of this article contains a data supplement.