TO THE EDITOR:
Primary mediastinal B-cell lymphoma (PMBCL) is an aggressive large B-cell lymphoma (LBCL) of thymic origin occurring predominantly in young patients and accounting for 2% to 4% of non-Hodgkin lymphomas.1,2 Anti-CD19 chimeric antigen receptor T cells (CARTs) have been adopted as standard of care for the treatment of patients with relapsed and/or refractory (r/r) LBCL, including PMBCL beyond second treatment line.3,4 Axicabtagene ciloleucel (axi-cel) has been the first CART therapy specifically approved for treatment of PMBCL in this setting. However, because of the rarity of the disease, information on safety and efficacy of CARTs in PMBCL is limited.5-7 Here, we compared course and outcome of axi-cel-treatment in patients with r/r PMBCL and those with r/r diffuse LBCL (DLBCL), not otherwise specified.
The patient cohort of the previous real-world analysis performed under the auspices of the Working Group Hematopoietic Cell Therapy of the German Lymphoma Alliance (GLA) and the German Stem Cell Transplantation Registry (DRST)8 formed the basis of this retrospective subset analysis. The primary study included adult (aged ≥18 years) patients with LBCL who had been treated with commercially available tisagenlecleucel or axi-cel from November 2018 to April 2021, as documented in the DRST database. For this analysis, investigators were approached to provide confirmation of diagnosis and follow-up information for each patient with PMBCL included in the original study. Because the primary cohort included only a single case of a patient with PMBCL who had received tisagenlecleucel, this study was restricted to axi-cel. Patients with DLBCL treated with axi-cel from the same data set served as comparators. The study was approved by the ethical committee of the University of Tuebingen (reference number 277/2020BO2) and performed in accordance with the Declaration of Helsinki.
In total, 144 patients met the eligibility criteria for this analysis, including 13 patients with PMBCL and 131 patients with DLBCL. Not unexpectedly, patients with PMBCL were significantly younger (median age, 39 years [range, 20-48 years]) and more often female when compared with patients with DLBCL (median age, 60 years [range, 20-83 years]). For other baseline parameters, patient cohorts were comparable, including performance status, prior treatment lines, prior transplantation, and International Prognostic Index score as well as lactate dehydrogenase levels and disease status at lymphodepletion (Table 1). In the PMBCL population, International Prognostic Index score was high/high-intermediate in 54% of patients. Because of omission of bridging in 38% of patients and lack of response to bridging in 54% of patients, 92% of patients had active disease at lymphodepletion (progressive disease, n = 10; stable disease, n = 2). Among the patients with PMBCL, 18% had received prior radiotherapy, and 18% had received treatment with checkpoint inhibitors.
Patient baseline characteristics
| . | PMBCL, n (%) . | DLBCL, NOS n (%) . | P . |
|---|---|---|---|
| No. of patients | 13 | 131 | — |
| Age, y median (range) | 39 (20-48) | 60 (20-83) | <.0001 |
| Sex, female | 6 of 13 (46) | 34 of 130 (26) | ns |
| Performance status ≥ 2 (ECOG) | 2 of 11 (18) | 16 of 102 (16) | ns |
| Prior therapy lines median (range) | 3 (2-4) | 3 (2-8) | ns |
| Prior auto-HSCT | 0 of 11 | 29 of 125 (23) | ns |
| Prior allo-HSCT | 0 of 11 | 6 of 125 (5) | ns |
| Prior checkpoint inhibition | 2 of 11 (18) | 2 of 122 (2) | .034 |
| Prior radiotherapy | 2 of 11 (18) | 29 of 122 (24) | ns |
| IPI unfavorable (high-intermediate or high) | 6 of 13 (46) | 61 of 123 (48) | ns |
| Refractory disease (refractory against any line of treatment) | 12 of 13 (92) | 108 of 131 (82) | ns |
| LDH elevated∗ | 7 of 13 (54) | 86 of 131 (66) | ns |
| Advanced Ann Arbor stage∗ (III/IV) | 9 of 12 (75) | 88 of 127 (69) | ns |
| Active disease∗ (SD/PD) | 12 of 13 (92) | 112 of 130 (86) | ns |
| Bridging† | ns | ||
| None | 5 of 13 (38) | 34 of 130 (26) | |
| Successful (CR/PR) | 1 of 13 (8) | 19 of 130 (15) | |
| Unsuccessful (SD/PD) | 7 of 13 (54) | 77 of 130 (59) |
| . | PMBCL, n (%) . | DLBCL, NOS n (%) . | P . |
|---|---|---|---|
| No. of patients | 13 | 131 | — |
| Age, y median (range) | 39 (20-48) | 60 (20-83) | <.0001 |
| Sex, female | 6 of 13 (46) | 34 of 130 (26) | ns |
| Performance status ≥ 2 (ECOG) | 2 of 11 (18) | 16 of 102 (16) | ns |
| Prior therapy lines median (range) | 3 (2-4) | 3 (2-8) | ns |
| Prior auto-HSCT | 0 of 11 | 29 of 125 (23) | ns |
| Prior allo-HSCT | 0 of 11 | 6 of 125 (5) | ns |
| Prior checkpoint inhibition | 2 of 11 (18) | 2 of 122 (2) | .034 |
| Prior radiotherapy | 2 of 11 (18) | 29 of 122 (24) | ns |
| IPI unfavorable (high-intermediate or high) | 6 of 13 (46) | 61 of 123 (48) | ns |
| Refractory disease (refractory against any line of treatment) | 12 of 13 (92) | 108 of 131 (82) | ns |
| LDH elevated∗ | 7 of 13 (54) | 86 of 131 (66) | ns |
| Advanced Ann Arbor stage∗ (III/IV) | 9 of 12 (75) | 88 of 127 (69) | ns |
| Active disease∗ (SD/PD) | 12 of 13 (92) | 112 of 130 (86) | ns |
| Bridging† | ns | ||
| None | 5 of 13 (38) | 34 of 130 (26) | |
| Successful (CR/PR) | 1 of 13 (8) | 19 of 130 (15) | |
| Unsuccessful (SD/PD) | 7 of 13 (54) | 77 of 130 (59) |
P < .05 indicates statistical significance.
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; auto-HSCT, autologous hematopoietic stem cell transplantation; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; LDH, lactate dehydrogenase; NOS, not otherwise specified; ns, not significant; PD, progressive disease; PR, partial remission; PS, performance status; SD, stable disease.
Assessed at lymphodepletion.
Bridging was immunotherapy based in the majority of patients who underwent bridging (n = 5 [63%]: brentuximab vedotin, n = 1; rituximab, n = 1; BV-lenalidomide–radiotherapy, n = 1; R-polatuzumab vedotin, n = 1; and pembrolizumab, n = 1). Chemoimmunotherapy was used in 25% of patients who underwent bridging (n = 2: R-BEAM, n = 1; R-GemOx, n = 1). The remaining patient received radiotherapy only.
Toxicity of axi-cel in patients with PMBCL did not significantly differ from that observed in the DLBCL cohort: grade ≥3 cytokine release syndrome and neurotoxicity occurred each in 15% of patients with PMBCL and 12% and 17% of patients with DLBCL, respectively. Prolonged neutropenia was less frequent after axi-cel treatment for PMBCL compared with that for DLBCL, although this difference was not statistically significant (supplemental Table 1). No nonrelapse death occurred in the PMBCL cohort.
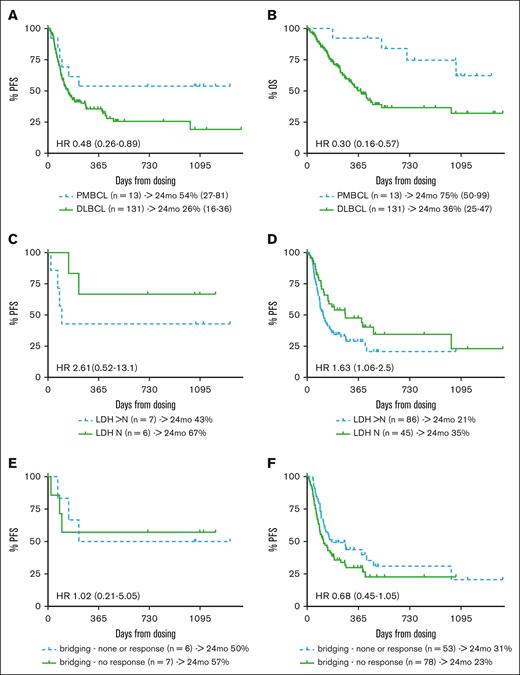
Regarding efficacy, the 85% overall response rate (ORR) and 54% complete response (CR) rate in patients with PMBCL did not differ significantly from the response rates observed in the DLBCL cohort (ORR, 71%; CR, 42%; supplemental Table 1). With a median follow-up of 35 months (range, 14-44 months), 6 relapse/progression events were observed among responders (in 2 of 4 patients who achieved partial remission and in 2 of 7 patients who achieved CR), resulting in 2-year progression-free survival (PFS) and overall survival (OS) probabilities of 54% (95% confidence interval [CI], 27-81) and 75% (95% CI, 50-99), respectively, for patients with PMBCL. This compared favorably with DLBCL outcomes (PFS, 26% [95% CI, 16-36]; hazard ratio, 0.48 [95% CI, 0.26-0.89]; OS, 36% [95% CI 25-47]; hazard ratio, 0.3 [95% CI, 0.16-0.57]; Figure 1A-B). Of note, one of the durable PMBCL responses occurred in a patient who received consolidating radiotherapy for residual metabolic activity (Deauville score, 4) 7 months after axi-cel infusion. Although this did not result in a complete metabolic response, the patient is currently (day 1093 after axi-cel) alive and well, without imaging evidence of lymphoma progression. Consolidating measures were not reported for any other responder. In contrast to the DLBCL cohort, progression events beyond 8 months after axi-cel treatment did not occur in patients with PMBCL.
Survival outcomes of patients treated with axi-cel for PMBCL or DLBCL. (A) PFS and (B) OS of patients treated with axi-cel for PMBCL or DLBCL. PFS according to LDH status (elevated vs normal LDH) for (C) PMBCL and (D) DLBCL; and response to bridging therapy (response vs nonresponse) for (E) PMBCL and (F) DLBCL, as assessed at lymphodepletion among patients with PMBC and DLBCL treated with axi-cel. HR, hazard ratio; LDH, lactate dehydrogenase; mo, month; N, normal.
Survival outcomes of patients treated with axi-cel for PMBCL or DLBCL. (A) PFS and (B) OS of patients treated with axi-cel for PMBCL or DLBCL. PFS according to LDH status (elevated vs normal LDH) for (C) PMBCL and (D) DLBCL; and response to bridging therapy (response vs nonresponse) for (E) PMBCL and (F) DLBCL, as assessed at lymphodepletion among patients with PMBC and DLBCL treated with axi-cel. HR, hazard ratio; LDH, lactate dehydrogenase; mo, month; N, normal.
Of the 6 patients with PMBCL whose disease progressed after axi-cel, follow-up treatment information was available in 4 cases. Of these, 3 patients were treated with checkpoint inhibitors, yielding responses in 2 of them. Another patient achieved a metabolic CR after polatuzumab-based chemoimmunotherapy. Two of 3 responders proceeded to undergo allogeneic stem cell transplantation but subsequently succumbed to nonrelapse mortality. Collectively, 4 patients with PMBCL died after disease progression after axi-cel after a median interval of 16 months (range, 3-32 months), whereas the remaining 2 patients were alive at 14 months after axi-cel failure.
As previously reported for CARTs in patients with LBCL,8-11 elevated lactate dehydrogenase in PMBCL patients was associated with inferior PFS of the PMBCL cohort (Figure 1C), although this difference was not statistically significant in the small sample investigated here. Interestingly, bridging therapy and response to bridging had no effect on the outcome of axi-cel treatment in PMBCL: patients not responding to bridging therapy displayed a 2-year PFS similar to patients who had not received bridging or had responded to bridging (57% vs 50%; Figure 1E). In contrast, nonresponders to bridging in the axi-cel DLBCL cohort tended to have a poorer outcome compared with bridging responders and patients who had not received bridging. However, this effect was weaker when the whole patient sample was observed as well as in comparison with other studies8,9,12 (Figure 1F).
The only fully published study specifically addressing CARTs in PMBCL is that by Crombie et al,5 who reported on outcomes of 33 patients with r/r PMBCL treated with axi-cel. They observed similar ORR (78%), CR rate (69%), and 2-year PFS (64%) and OS (78%) compared with that observed in this study. Our data are also in line with a recent Italian real-world analysis reporting significantly superior 12-month PFS after axi-cel treatment for r/r PMBCL compared with LBCL (65% vs 47%), albeit the observation time was short.13
Limitations of our study comprise the small sample size and the retrospective nature inherent to registry reports, whereas the long observation time and the good characterization of the PMBCL subset represent strengths. In summary, safety outcomes after axi-cel treatment for PMBCL beyond second treatment line seem comparable with those observed for DLBCL. Unlike in DLBCL, late progression events hardly occur in PMBCL, and bridging failure is not associated with poorer outcome. Along with delayed mortality after axi-cel treatment failure,14 this results in substantially better survival of patients with PMBCL treated with axi-cel compared with patients with DLBCL. The reasons for the superior susceptibility of PMBCL to CARTs remain speculative, but it is likely that the specific immune environment pertinent to PMBCL plays a key role.15
In conclusion, axi-cel is a highly effective salvage option for patients with multiple r/r PMBCL, and exploration of axi-cel in earlier treatment lines as well as in a prospective trial context should be considered.
Contribution: M.-L.S. designed the research, collected and analyzed the data, and wrote the manuscript; W.A.B., F.A.A., M.v.B., V.V., E.M.W.-D., M. Subklewe, C.D.B., B.G., R.M., D.M., R.S., M. Stelljes, M.S.T., G.W., and N.K. collected patient data and reviewed the manuscript; and P.D. designed the research, collected and analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: B.G. reports consultancy for Roche, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Gilead, AbbVie, Janssen, and Novartis and research funding from Roche. C.D.B. reports honoraria and travel funds from BMS, Amgen, Novartis, Jazz, Gilead, and Janssen. D.M. reports consultancies for AbbVie, Roche, BMS, Hexal, Novartis, MSD, Celgene, Janssen-Cilag, Pfizer, AstraZeneca, and Jazz Pharma. E.M.W.-D. reports consultancies for Novartis and Kite/Gilead. F.A.A. reports honoraria from Novartis, Gilead, BMS, Janssen, Takeda, and Therakos/Mallinckrodt and research funding from Therakos/ Mallinckrodt. G.W. reports honoraria from Gilead, Novartis, Takeda, Clinigen, and Amgen. M.-L.S. reports honoraria and travel funds from Kite/Gilead, Takeda, Jannsen, and Sobi. M.v.B. reports honoraria and travel funds from Gilead, Novartis, Janssen, Takeda, and Daiichi Sankyo. M. Stelljes reports honoraria from Kite/Gilead, Jazz, MSD, Novartis, Pfizer, and BMS/Celgene. M.S.T. reports consultancies for AstraZeneca, BMS, Kite/Gilead, Janssen, Roche, and Novartis; and research support from Kite/Gilead, Regeneron, and Roche. M. Subklewe reports consultancies for Amgen, BMS, Gilead, Janssen, Novartis, Pfizer, Seagen; research support from Amgen, BMS, Gilead, Miltenyi, MorphoSys, Novartis, Roche, Seagen; and speaker’s bureau fees from Amgen, BMS, Gilead, Novartis, Pfizer, and Takeda. N.K. reports honoraria from Kite/Gilead, Jazz, MSD, Neovii Biotech, Novartis, Riemser, Pfizer, and BMS and research support from Neovii, Riemser, Novartis, and BMS. P.D. reports consultancy for AbbVie, AstraZeneca, Bluebird Bio, Gilead, Janssen, Novartis, Riemser, and Roche; speaker’s bureau fees from AbbVie, AstraZeneca, Gilead, Novartis, Riemser, and Roche; and research support from Riemser. R.M. reports honoraria from Kite/Gilead and Novartis. R.S. reports honoraria from Kite/Gilead and Novartis. V.V. reports honoraria and travel funds for Gilead, BMS/Celgene, and Janssen; and honoraria from and consultancies for Novartis, Gilead, Janssen, and BMS/ Celgene. W.A.B. reports honoraria and travel funds from Gilead, Novartis, Miltenyi, and Janssen; and research support from Miltenyi.
Correspondence: Maria-Luisa Schubert, Department of Hematology and Oncology, University Hospital Heidelberg, Im Neuenheimer Feld 410, N/A 69120 Heidelberg, Germany; e-mail: maria-luisa.schubert@med.uni-heidelberg.de.
References
Author notes
Data are available on request from the corresponding author, Maria-Luisa Schubert (maria-luisa.schubert@med.uni-heidelberg.de).
The full-text version of this article contains a data supplement.