Key Points
Myeloablative fractionated busulfan regimen results in low nonrelapse mortality without a higher relapse rate.
This regimen is a viable myeloablative alternative for patients who receive a reduced intensity regimen because of age or comorbidity.
Abstract
Traditional conditioning regimens for patients undergoing allogeneic hematopoietic cell transplantation (allo-HCT) provide suboptimal outcomes, especially for older patients and those with comorbidities. We hypothesized that a fractionated myeloablative busulfan dose delivered over an extended period would reduce nonrelapse mortality (NRM) while retaining antileukemic effects. Here, we performed a phase 2 trial for adults with hematological malignancies receiving matched related or unrelated allo-HCT. Participants received busulfan 80 mg/m2 as outpatients on days −20 and −13 before transplant. Fludarabine 40 mg/m2 was administered on days −6 to −3, followed by busulfan dosed to achieve a target area under the curve of 20 000 mol/min for the whole course. The primary end point was day-100 NRM. Seventy-eight patients were included, with a median age of 61 years (range, 39-70 years), who received transplantation for acute leukemia (24%), myelodysplastic syndrome (27%), or myeloproliferative disease/chronic myeloid leukemia (44%). HCT-specific comorbidity index (HCT-CI) was ≥3 in 34 (44%). With a median follow-up of 36.4 months (range, 2.9-51.5), the 100-day, 1-year, and 3-year NRM rates were 3.8%, 8%, and 9.3%, respectively, without a significant difference in age or HCT-CI score. The 1-year and 3-year relapse incidence was 10% and 18%, respectively. The 3-year overall survival was 80%, without a significant difference in age or HCT-CI score and was similar for patients aged >60 years and those aged <60 years as well as for those with HCT-CI ≥3 and HCT-CI <3. Overall, a myeloablative fractionated busulfan regimen has low NRM without an increase in relapse rate, resulting in promising survival, even in older patients or in patients with comorbidities. This trial was registered at www.clinicaltrials.gov as #NCT02861417.
Introduction
Although allogeneic hematopoietic cell transplantation (allo-HCT) cures patients with high-risk hematological malignancies, less than half of the patients who receive reduced intensity transplantation because of older age and/or comorbidities exhibit a significant survival benefit after transplantation.1-3 Because most patients with high-risk hematological malignancies are older and/or have comorbidities and thus have a higher nonrelapse mortality (NRM),4 there is an unmet need for novel approaches for these patients. Traditional myeloablative conditioning (MAC) regimens reduce the risk of relapse after allo-HCT compared with reduced intensity conditioning (RIC) regimens but are associated with higher rates of NRM, thus negating some benefit of the higher intensity regimen.5-7 To meaningfully improve outcomes, a MAC regimen that reduces relapse without increasing the NRM is needed. A first step toward this goal was to deliver a higher dose of busulfan (Bu) with pharmacokinetic (PK) guidance in combination with fludarabine (Flu), which yielded superior outcomes compared with fixed-dose Bu with Flu.8,9
Next, we hypothesized that lengthening the duration of chemotherapy and administering it over a longer period would reduce its toxicity without altering efficacy. We therefore fractionated Bu by administering it as 6 doses over a 2-week period, combined with Flu.10 We demonstrated the feasibility, safety, and possibly increased efficacy of this MAC regimen in older patients and patients with comorbidities.3,11 Because graft-versus-host disease (GVHD) is the leading contributor to late NRM beyond day 100,12 we added posttransplant cyclophosphamide (PTCy) as a GVHD prophylaxis to this fractionated Bu-MAC regimen. For younger patients, we found that this regimen yielded low rates of severe GVHD and NRM.13
In this study, we hypothesized that further fractionation, administering 6 doses of myeloablative Bu over a 3-week period along with Flu and PTCy may not only further reduce toxicity and NRM but also reduce relapse rate, resulting in better survival in older patients. This regimen may serve as a myeloablative alternative to a RIC regimen for older patients and patients with comorbidities.
Herein, we report the mature results of this phase 2 study.
Methods
Study design and participants
This was an open-label, nonrandomized, phase 2 clinical trial that assessed the safety and efficacy of extended fractionation of Bu and Flu conditioning with PTCy-based GVHD prophylaxis. The first 3 cohorts of this protocol with a shorter Bu schedule and/or haploidentical donors have been published.13 In this fourth cohort designed for patients who were frailer and were candidates for a RIC regimen, we initially included patients aged >60 years or <60 years with comorbidities or a diagnosis of myelofibrosis but later extended enrollment to other diseases and ages because of encouraging results. Thus, eligibility criteria included patients with any hematological malignancy, including acute or chronic leukemia, lymphoma, myeloma or myeloproliferative disease such as myelofibrosis; age ranging from 18 to 70 years; having an 8/8 HLA-matched related or unrelated donor, determined by high-resolution typing; and having adequate organ function with forced expiratory volume (FEV1), forced vital capacity (FVC), and corrected diffusing capacity for carbon monoxide (DLCO) ≥50% of predicted value, ejection fraction ≥50%, creatinine clearance ≥50 mL/min, bilirubin ≤2-times the upper limit of normal, alanine aminotransferase of 200 IU/L, and a Karnofsky performance score of ≥70 or an Eastern Cooperative Oncology Group performance score of 0 to 1, with any number of comorbidities. Exclusion criteria included prior allo-HCT, HIV infection, active hepatitis B and C, any uncontrolled infection, and receipt of inotuzumab or gemtuzumab in the past. This study (The University of Texas MD Anderson Cancer Center protocol 2016-0137) was approved by the institutional review board at MD Anderson Cancer Center. The research was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent before enrollment. This study was registered at ClinicalTrials.gov as #NCT02861417.
Procedures
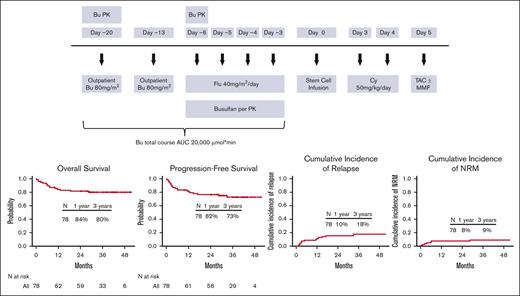
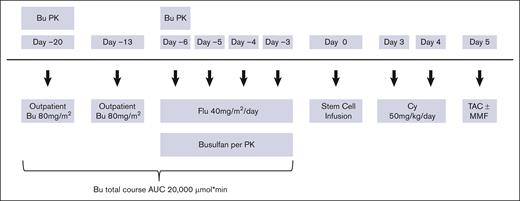
The conditioning regimen consisted of Flu with fractionated delivery of a myeloablative dose of Bu to target an area under the concentration vs time curve of 20 000 ± 12% μmol × min per course. On day −20 and day −13 before allo-HCT, patients received 1 dose of 80 mg/m2 Bu IV in an outpatient infusion clinic. Bu PK analysis was performed after the first dose on day −20, and the results of this analysis were used to calculate Bu dose adjustments for each patient for the day −6 and day −5 doses (supplemental Methods). During inpatient treatment from day −6 to day −3, Flu 40 mg/m2 was administered over 1 hour, once daily IV, and immediately followed by Bu given over 3 hours, once daily IV by controlled rate infusion pump. A second PK analysis was performed after the day −6 dose of Bu to calculate adjustments to the final 2 doses on day −4 and day −3 to meet the target total area under the concentration vs time curve (Figure 1).
Study design. AUC, area under the curve; Cy, cyclophosphamide; MMF, mycophenolate mofetil; TAC, tacrolimus.
Study design. AUC, area under the curve; Cy, cyclophosphamide; MMF, mycophenolate mofetil; TAC, tacrolimus.
GVHD prophylaxis consisted of PTCy 50 mg/kg IV on days +3 and +4, followed by tacrolimus from day +5 (target trough level, 5-15 ng/mL). The last 38 patients who underwent unrelated donor transplant also received mycophenolate mofetil 15 mg/kg per dose (maximum 1000 mg per dose based on actual body weight), IV or by mouth, 3 times a day, starting on day +5 through day +100.
Supportive care was given as per standard practice guidelines for patients receiving allo-HCT and HCT. All patients received mesna 10 mg/kg IV before the first dose of PTCy, which was repeated every 4 hours, for a total of 10 doses. Levetiracetam 500 mg was administered for antiseizure prophylaxis, starting on the evening before Bu and then every 12 hours until 24 hours after the last dose of Bu. Antimicrobial prophylaxis was administered according to institutional protocol. Granulocyte colony-stimulating factor at a dose of 5 μg/kg per day subcutaneously was started on day +7 and continued until neutrophil engraftment, defined as an absolute neutrophil count of >500 × 106 cells per L for 3 consecutive days. Donor bone marrow or granulocyte colony-stimulating factor–primed peripheral blood progenitor cells were procured with standard mobilization protocols and apheresis techniques. All donors provided written informed consent. Unrelated donor bone marrow was obtained through the US National Marrow Donor Program according to applicable guidelines. Follow-ups were usually daily for the first 30 days, then weekly for 3 months, and then as needed, as is standard for patients who have received allo-HCT. We performed routine laboratory tests and imaging as clinically indicated. Maintenance therapy was at the discretion of the treating physician, and 9 patients received at least 1 course: 5 patients had a hypomethylating agent–based regimen and 4 had an FLT-3 inhibitor.
Outcomes
NRM on day 100 was the primary outcome. Secondary objectives were to obtain estimates of efficacy and safety of this regimen. Outcomes analyses included overall survival (OS), progression-free survival (PFS), relapse rate, neutrophil and platelet engraftment, and incidence of acute14 and chronic GVHD (cGVHD). Platelet engraftment was defined as a platelet count of ≥20 × 109 cells per L for 7 consecutive days without platelet transfusion. The Common Terminology Criteria for Adverse Events version 5.0 was used to grade toxicities, which were monitored daily until engraftment or until the day of discharge, whichever was later, and at least weekly thereafter until day 100. Post hoc exploratory analyses were conducted to compare outcomes in patients aged <60 years and those aged >60 years, with an HCT-specific comorbidity index (HCT-CI) score of 0 to 2 or ≥3, low/intermediate or high/very high disease risk index (DRI), and according to the primary hematological disease.
Statistical analysis
The primary objective of the study was to assess the safety of the regimen and estimate NRM rate on day 100. We used Bayesian monitoring rules to monitor the 100-day NRM rate and stop the accrual on a cohort if there was strong evidence at any time that this rate exceeded 20%. The method of Kaplan and Meier was used to estimate the distribution of OS and PFS from the time of transplant. For OS, patients who remained alive were censored at their last follow-up visit. For PFS, patients who remained alive without disease progression were censored at the time of their last follow-up visit. Distributions were compared using the log-rank test. The cumulative NRM incidence was assessed in a competing risks framework with relapse as the competing risk. Distributions were compared using the Gray test.
The cumulative incidence of acute GVHD (aGVHD) grades 2 to 4 was assessed in a competing risks framework, with competing risks of disease relapse and death without relapse. Patients who did not experience aGVHD, death, or disease relapse by day 100 were censored on day 100. The cumulative incidence of grade 3 to 4 aGVHD was assessed using a similar approach. The cumulative incidence of any cGHVD was assessed in a similar framework with the same competing risks, except that patients who did not experience cGVHD, death, or relapse were censored at the last follow-up date. The cumulative incidence of limited and extensive cGVHD were assessed using a similar approach.
All statistical analyses were performed using R version 4.1.1. All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Between June 2017 and July 2019, 79 patients were registered. One patient progressed soon after the first dose of Bu and therefore was removed from the protocol and administered salvage chemotherapy treatment and a successful transplant with an alternative regimen. For the remaining 78 patients, the median age was 61 years (range, 39-70 years). Forty-three patients (55%) of the cohort were male. Nineteen patients (24%) had acute myeloid leukemia (AML), 9 (47%) were in first complete remission (CR), 5 (26%) were in CR without count recovery, and 5 (26%) had more advanced disease. Twenty-one (27%) had myelodysplastic syndrome (MDS); the Revised International Prognostic Scoring System score was >3.5 in 15 (71%) patients. Thirty-one (40%) patients had myelofibrosis: 12 (39%) had high-risk disease, 13 (42%) intermediate 2–risk disease, and 6 (19%) intermediate 1–risk disease based on the Dynamic International Prognostic Scoring System Plus criteria. Thirty-four patients (44%) had an HCT-CI4 score ≥3. The frequency of common (>2 patients) organ-specific comorbidities was moderate pulmonary in 26 (33%), psychiatric in 25 (32%), mild hepatic in 19 (24%), obesity in 13 (17%), diabetes in 12 (15%), prior solid tumors in 11 (14%), severe pulmonary in 5 (6%), infection in 5 (6%), arrhythmia in 5 (6%), and severe hepatic in 3 (4%) patients. Seventeen patients (22%) had a high/very high DRI.15 The donor was a matched unrelated donor in 49 patients (63%), and 73 (94%) received a peripheral blood progenitor cell graft. Patient and transplant characteristics are summarized in Table 1. The median follow-up duration was 36.4 months (range, 2.9-51.5) for survivors.
Baseline characteristics
| . | Total (N = 78) . |
|---|---|
| Age, median (range) y | 61 (39-70) |
| Age group, y | |
| ≤60 | 34 (44%) |
| >60 | 44 (56%) |
| Sex | |
| Male | 43 (55%) |
| Female | 35 (45%) |
| Race | |
| White | 67 (87%) |
| Other | 10 (13%) |
| Diagnosis | |
| ALL | 3 (4%) |
| AML | 19 (24%) |
| MDS | 21 (27%) |
| Myelofibrosis | 31 (40%) |
| CML | 3 (4%) |
| Multiple myeloma | 1 (1%) |
| Donor | |
| HLA-matched sibling | 29 (37%) |
| HLA-matched 8-of-8 unrelated | 49 (63%) |
| Graft source | |
| Peripheral blood progenitor cells | 73 (94%) |
| Bone marrow | 5 (6%) |
| GVHD prophylaxis | |
| PTCy/tacro | 40 (51%) |
| PTCy/tacro/MMF | 38 (49%) |
| Refined DRI | |
| Low | 3 (4%) |
| Intermediate | 58 (74%) |
| High | 17 (22%) |
| HCT-CI | |
| 0 | 11(14%) |
| 1-2 | 33 (42%) |
| 3-4 | 23 (30%) |
| ≥5 | 11 (14%) |
| Karnofsky performance status | |
| 70 | 7 (9%) |
| 80 | 22 (29%) |
| 90 | 24 (31%) |
| 100 | 24 (31%) |
| . | Total (N = 78) . |
|---|---|
| Age, median (range) y | 61 (39-70) |
| Age group, y | |
| ≤60 | 34 (44%) |
| >60 | 44 (56%) |
| Sex | |
| Male | 43 (55%) |
| Female | 35 (45%) |
| Race | |
| White | 67 (87%) |
| Other | 10 (13%) |
| Diagnosis | |
| ALL | 3 (4%) |
| AML | 19 (24%) |
| MDS | 21 (27%) |
| Myelofibrosis | 31 (40%) |
| CML | 3 (4%) |
| Multiple myeloma | 1 (1%) |
| Donor | |
| HLA-matched sibling | 29 (37%) |
| HLA-matched 8-of-8 unrelated | 49 (63%) |
| Graft source | |
| Peripheral blood progenitor cells | 73 (94%) |
| Bone marrow | 5 (6%) |
| GVHD prophylaxis | |
| PTCy/tacro | 40 (51%) |
| PTCy/tacro/MMF | 38 (49%) |
| Refined DRI | |
| Low | 3 (4%) |
| Intermediate | 58 (74%) |
| High | 17 (22%) |
| HCT-CI | |
| 0 | 11(14%) |
| 1-2 | 33 (42%) |
| 3-4 | 23 (30%) |
| ≥5 | 11 (14%) |
| Karnofsky performance status | |
| 70 | 7 (9%) |
| 80 | 22 (29%) |
| 90 | 24 (31%) |
| 100 | 24 (31%) |
ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; MMF, mycophenolate mofetil; tacro, tacrolimus.
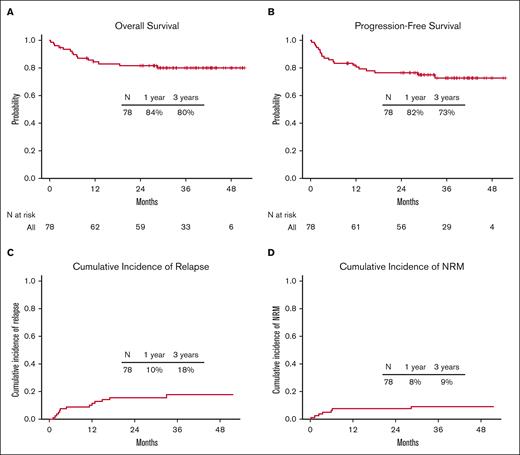
NRM, relapse, and survival
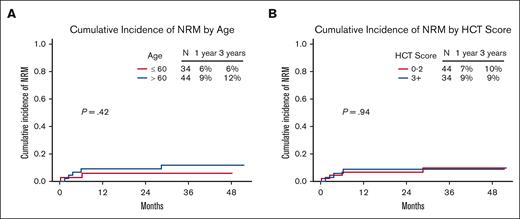
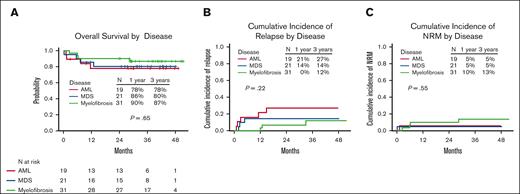
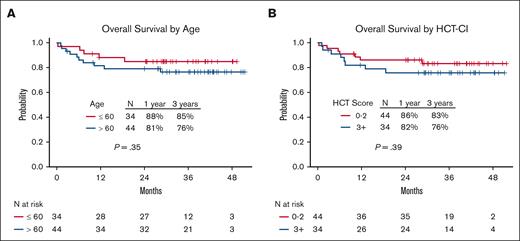
Seven of the 78 patients (9%) died of causes other than relapse. At 100 days, 1 year, and 3 years, the NRM was 3.8% (95% confidence interval [CI], 0-8.1), 8% (95% CI, 2-14), and 9.3% (95% CI, 2.6-15.9), respectively (Figure 2D). There was no significant difference in the 3-year NRM between the older (aged >60 years) and younger (aged ≤60 years) age groups (11.8% [95% CI, 1.9-21.7] and 5.9% [95% CI, 0-13.9], respectively; P = .42]) or between patients with low (0-2) and high (≥3) HCT-CI scores (9.8% [95% CI, 0.4-19.2] and 8.8% [95% CI, 0-18.5], respectively; P = .94) (Figures 3 and 4C).
Outcomes for the entire cohort. (A) OS, (B) PFS, (C) relapse rate, and (D) NRM.
Outcomes for the entire cohort. (A) OS, (B) PFS, (C) relapse rate, and (D) NRM.
NRM according to age and comoborbidity burden. NRM based on (A) age and (B) HCT-CI.
NRM according to age and comoborbidity burden. NRM based on (A) age and (B) HCT-CI.
Outcomes according to the disease. (A) OS, (B) relapse rate, and (C) NRM.
For the entire cohort, 63 of the 78 patients (81%) were alive. The 1-year and 3-year OS rates were 84% (95% CI, 77-93) and 80% (95% CI, 72-90%), respectively (Figure 2A). There was no significant difference in the 3-year OS rates between the older (aged >60 years) and younger (aged ≤60 years) age groups (76% [95% CI, 65-90] and 85% [95% CI, 74-98], respectively; P = .35) or between patients with low (0-2) and high (≥3) HCT-CI scores (83% [95% CI, 72-96] and 76% [95% CI, 62-92], respectively; P = .39) (Figure 5). The 1-year and 3-year OS rates for patients with AML were 78% (95% CI, 61-100) and 78% (95% CI, 61-100), respectively; for patients with MDS, these were 86% (95% CI, 72-100) and 80% (95% CI, 65-100), respectively; and for patients with myelofibrosis, these were 90% (95% CI, 81-100) and 87% (95% CI, 75-100), respectively (Figure 4A). Progression of the primary hematological disease was the most common cause of death in the entire cohort (n = 8 [10%]; Table 4).
OS according to age and comorbidity burden. OS based on (A) age and (B) HCT-CI.
OS according to age and comorbidity burden. OS based on (A) age and (B) HCT-CI.
Outcomes according to age, DRI, and HCT-CI stratification
| . | OS . | P value . | PFS . | P value . | NRM . | P value . | Relapse . | P value . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 y | 3 y | 1 y | 3 y | 1 y | 3 y | 1 y | 3 y | |||||
| All patients | 84.4% (76.7%-92.9%) | 80.1% (71.5%-89.7%) | 82% (73.9%-91%) | 72.7% (63%-84%) | 7.7% (1.7%-13.6%) | 9.3% (2.6%-15.9%) | 10.3% (3.5%-17.1%) | 18.0% (8.8%-27.2%) | ||||
| Age, y | .35 | .35 | .42 | .65 | ||||||||
| ≤60 | 88.1% (77.9%-99.8%) | 85% (73.7%-98%) | 88.1% (77.9%-99.8%) | 75% (59.3%-94.9%) | 5.9% (0%-13.9%) | 5.9% (0%-13.9%) | 6.0% (0%-14.2%) | 19.1% (2.0%-36.2%) | ||||
| >60 | 81.5% (70.7%-93.9%) | 76.3% (64.5%-90.4%) | 77.3% (65.8%-99.7%) | 69.9% (57.4%-85.1%) | 9.1% (0.5%-17.7%) | 11.8% (1.9%-21.7%) | 13.6% (3.4%-23.9%) | 18.3% (6.7%-30.0%) | ||||
| HCT-CI | .39 | .25 | .94 | .17 | ||||||||
| <3 | 86.1% (76.4%-97.1%) | 83.2% (72.4%-95.5%) | 86.2% (76.6%-97.1%) | 78.4% (66.8%-92.1%) | 6.8% (0%-14.4%) | 9.8% (0.4%-19.2%) | 6.9% (0%-14.6%) | 11.7% (1.9%-21.5%) | ||||
| ≥3 | 82% (69.9%-96.1%) | 75.7% (62.3%-91.9%) | 76.5% (63.5%-92.1%) | 65.7% (50.7%-85%) | 8.8% (0%-18.5%) | 8.8% (0%-18.5%) | 14.7% (2.6%-26.8%) | 25.5% (9.4%-41.6%) | ||||
| DRI | <.0001 | .0001 | .13 | .0069 | ||||||||
| Low/intermediate | 93.4% (87.4%-99.9%) | 88.2% (80.3%-96.8%) | 91.8% (85.2%-99%) | 80.2% (70.1%-91.8%) | 4.9% (0%-10.4%) | 6.9% (0.3%-13.4%) | 3.3% (0%-7.8%) | 12.9% (3.5%-22.3%) | ||||
| High | 49.9% (30.3%-82.1%) | 49.9% (30.3%-82.1%) | 46.3% (27.6%-77.8%) | 46.3% (27.6%-77.8%) | 17.6% (0%-36.5%) | 17.6% (0%-36.5%) | 36% (11.7%-60.3%) | 36% (11.7%-60.3%) | ||||
| Disease | ||||||||||||
| AML | 77.9% (60.9%-99.7%) | 77.9% (60.9%-99.7%) | 73.3% (55.7%-96.4%) | 67.7% (49.4%-92.8%) | 5.3% (0%-15.6%) | 5.3% (0%-15.6%) | 21.4% (2.2%-40.7%) | 27.1% (6.0%-48.1%) | ||||
| MDS | 85.7% (72%-100%) | 80.4% (64.8%-99.7%) | 81% (65.8%-99.6%) | 81% (65.8%-99.6%) | 4.8% (0%-14.1%) | 4.8% (0%-14.1%) | 14.3% (0%-29.7%) | 14.3% (0%-29.7%) | ||||
| Myelofibrosis | 90.3% (80.5%-100%) | 86.7% (75.4%-99.8%) | 90.3% (80.5%-100%) | 74.9% (59.9%-93.6%) | 9.7% (0%-20.3%) | 13.3% (0.9%-25.8%) | 0% (0%-0%) | 11.8% (0%-25.2%) | ||||
| . | OS . | P value . | PFS . | P value . | NRM . | P value . | Relapse . | P value . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 y | 3 y | 1 y | 3 y | 1 y | 3 y | 1 y | 3 y | |||||
| All patients | 84.4% (76.7%-92.9%) | 80.1% (71.5%-89.7%) | 82% (73.9%-91%) | 72.7% (63%-84%) | 7.7% (1.7%-13.6%) | 9.3% (2.6%-15.9%) | 10.3% (3.5%-17.1%) | 18.0% (8.8%-27.2%) | ||||
| Age, y | .35 | .35 | .42 | .65 | ||||||||
| ≤60 | 88.1% (77.9%-99.8%) | 85% (73.7%-98%) | 88.1% (77.9%-99.8%) | 75% (59.3%-94.9%) | 5.9% (0%-13.9%) | 5.9% (0%-13.9%) | 6.0% (0%-14.2%) | 19.1% (2.0%-36.2%) | ||||
| >60 | 81.5% (70.7%-93.9%) | 76.3% (64.5%-90.4%) | 77.3% (65.8%-99.7%) | 69.9% (57.4%-85.1%) | 9.1% (0.5%-17.7%) | 11.8% (1.9%-21.7%) | 13.6% (3.4%-23.9%) | 18.3% (6.7%-30.0%) | ||||
| HCT-CI | .39 | .25 | .94 | .17 | ||||||||
| <3 | 86.1% (76.4%-97.1%) | 83.2% (72.4%-95.5%) | 86.2% (76.6%-97.1%) | 78.4% (66.8%-92.1%) | 6.8% (0%-14.4%) | 9.8% (0.4%-19.2%) | 6.9% (0%-14.6%) | 11.7% (1.9%-21.5%) | ||||
| ≥3 | 82% (69.9%-96.1%) | 75.7% (62.3%-91.9%) | 76.5% (63.5%-92.1%) | 65.7% (50.7%-85%) | 8.8% (0%-18.5%) | 8.8% (0%-18.5%) | 14.7% (2.6%-26.8%) | 25.5% (9.4%-41.6%) | ||||
| DRI | <.0001 | .0001 | .13 | .0069 | ||||||||
| Low/intermediate | 93.4% (87.4%-99.9%) | 88.2% (80.3%-96.8%) | 91.8% (85.2%-99%) | 80.2% (70.1%-91.8%) | 4.9% (0%-10.4%) | 6.9% (0.3%-13.4%) | 3.3% (0%-7.8%) | 12.9% (3.5%-22.3%) | ||||
| High | 49.9% (30.3%-82.1%) | 49.9% (30.3%-82.1%) | 46.3% (27.6%-77.8%) | 46.3% (27.6%-77.8%) | 17.6% (0%-36.5%) | 17.6% (0%-36.5%) | 36% (11.7%-60.3%) | 36% (11.7%-60.3%) | ||||
| Disease | ||||||||||||
| AML | 77.9% (60.9%-99.7%) | 77.9% (60.9%-99.7%) | 73.3% (55.7%-96.4%) | 67.7% (49.4%-92.8%) | 5.3% (0%-15.6%) | 5.3% (0%-15.6%) | 21.4% (2.2%-40.7%) | 27.1% (6.0%-48.1%) | ||||
| MDS | 85.7% (72%-100%) | 80.4% (64.8%-99.7%) | 81% (65.8%-99.6%) | 81% (65.8%-99.6%) | 4.8% (0%-14.1%) | 4.8% (0%-14.1%) | 14.3% (0%-29.7%) | 14.3% (0%-29.7%) | ||||
| Myelofibrosis | 90.3% (80.5%-100%) | 86.7% (75.4%-99.8%) | 90.3% (80.5%-100%) | 74.9% (59.9%-93.6%) | 9.7% (0%-20.3%) | 13.3% (0.9%-25.8%) | 0% (0%-0%) | 11.8% (0%-25.2%) | ||||
Median follow-up period for surviving patients: 36.4 months (range, 2.9-51.5).
Adverse events of grade 3 or more
| . | Total (N = 78) . |
|---|---|
| Febrile neutropenia | 35 (45%) |
| On days 0-4 after transplantation | 18 (23%) |
| Gastrointestinal | |
| Mucositis | 12 (15%) |
| Nausea | 5 (6%) |
| Diarrhea | 12 (15%) |
| Ileus | 1 (1%) |
| Gastrointestinal bleeding | 1 (1%) |
| Pulmonary | |
| Idiopathic pneumonia syndrome | 15 (19%) |
| Cardiovascular | |
| CHF | 2 (3%) |
| Hypertension | 3 (4%) |
| Thromboembolic event | 1 (1%) |
| Liver | |
| Increased total bilirubin | 9 (12%) |
| Increased ALT | 3 (4%) |
| Renal | 4 (5%) |
| Neurological | |
| Headache | 1 (1%) |
| Confusion | 2 (3%) |
| Hematological | |
| Red cell aplasia because of major ABO incompatibility | 2 (3%) |
| Fluid overload | 5 (6%) |
| Hemorrhagic cystitis | 2 (3%) |
| . | Total (N = 78) . |
|---|---|
| Febrile neutropenia | 35 (45%) |
| On days 0-4 after transplantation | 18 (23%) |
| Gastrointestinal | |
| Mucositis | 12 (15%) |
| Nausea | 5 (6%) |
| Diarrhea | 12 (15%) |
| Ileus | 1 (1%) |
| Gastrointestinal bleeding | 1 (1%) |
| Pulmonary | |
| Idiopathic pneumonia syndrome | 15 (19%) |
| Cardiovascular | |
| CHF | 2 (3%) |
| Hypertension | 3 (4%) |
| Thromboembolic event | 1 (1%) |
| Liver | |
| Increased total bilirubin | 9 (12%) |
| Increased ALT | 3 (4%) |
| Renal | 4 (5%) |
| Neurological | |
| Headache | 1 (1%) |
| Confusion | 2 (3%) |
| Hematological | |
| Red cell aplasia because of major ABO incompatibility | 2 (3%) |
| Fluid overload | 5 (6%) |
| Hemorrhagic cystitis | 2 (3%) |
ABO, A, B, and O blood types; ALT, alanine aminotransferase; CHF, congestive heart failure.
Causes of death
| . | N (%) Total = 15 of 78 (19%) . |
|---|---|
| Progression of primary hematological disease | 8 (10%) |
| Idiopathic pneumonia syndrome | 3 (4%) |
| Acute coronary syndrome/CHF | 1 (1%) |
| aGVHD | 1 (1%) |
| cGVHD | 1 (1%) |
| Secondary graft failure | 1 (1%) |
| . | N (%) Total = 15 of 78 (19%) . |
|---|---|
| Progression of primary hematological disease | 8 (10%) |
| Idiopathic pneumonia syndrome | 3 (4%) |
| Acute coronary syndrome/CHF | 1 (1%) |
| aGVHD | 1 (1%) |
| cGVHD | 1 (1%) |
| Secondary graft failure | 1 (1%) |
CHF, congestive heart failure.
The 1-year and 3-year PFS rates of the entire cohort were 82% (95% CI, 74-91) and 73% (95% CI, 63-84), respectively (Figure 2B). There was no significant difference in 3-year PFS between the older (aged >60 years) and younger (aged ≤60 years) age groups (70% [95% CI, 57-85] and 75% [95% CI, 59-95], respectively; P = .35) or between patients with low (0-2) and high (≥3) HCT-CI scores (78% [95% CI, 67-92] and 66% [95% CI, 51-85], respectively; P = .25; supplemental Figure 1). The 1-year and 3-year PFS rates of patients with AML were 73% (95% CI, 56-96) and 68% (95% CI, 49-93), respectively; for patients with MDS, these were 81% (95% CI, 66-100) and 81% (95% CI, 66-100), respectively; and for patients with myelofibrosis, these were 90% (95% CI, 81-100) and 75% (95% CI, 60-94), respectively.
The cumulative incidence of relapse for the entire cohort at 1 year and 3 years was 10% (95% CI, 4-17) and 18% (95% CI, 9-27), respectively (Figure 2C). Patients with high/very high DRI had an increased risk of disease relapse compared with those with low/intermediate risk (at 1 year: 36% [95% CI, 12-60] vs 3% [95% CI, 0-8], respectively; and at 3 years: 36% [95% CI, 12-60] vs 13% [95% CI, 4-22], respectively; P = .0069; supplemental Figure 2C). The cumulative incidence of relapse at 1 year and 3 years was 21% (95% CI, 2-41) and 27% (95% CI, 6-48), respectively, for patients with AML; 14% (95% CI, 0-30) and 14% (95% CI, 0-30), respectively, for patients with MDS; and 0% (95% CI, 0-0) and 12% (95% CI, 0-25), respectively, for patients with myelofibrosis (Figure 4B; Table 2).
Engraftment and chimerism
The median time to neutrophil engraftment, platelet engraftment, and unsupported platelet count >50 × 109 cells per L after transplantation was 17 days (range, 12-33 days), 25 days (range, 7-964 days), and 39 days (range, 11-764 days), respectively. No primary graft failure occurred, but 1 patient developed secondary graft failure on day +37 after transplant and subsequently died. T-cell and myeloid chimerism reached a median of 100% on day 30 and remained 100% at 1-year follow-up (supplemental Table 2).
GVHD
At 100 days after transplant, the cumulative incidence of aGVHD grades 2 to 4 was 34.6% (95% CI, 24-45), and the cumulative incidence of aGVHD grades 3 to 4 was 5% (95% CI, 0-10). The cumulative incidence of limited cGVHD at 1 year was 1% (95% CI, 0-4), and at 3 years, was 3% (95% CI, 0-6). The cumulative incidence of extensive cGVHD at 1 year was 6% (95% CI, 1-12), and at 3 years, was 17% (95% CI, 9-26). The overall cumulative incidence of cGVHD at 1 year and at 3 years was 8% (95% CI, 2-14) and 20% (95% CI, 11-29), respectively. Of patients who were evaluable, 42% remained on immunosuppression at 1-year after transplant.
Toxicity
Of the 78 patients, 69 (88%) developed a grade ≥3 toxicity (Table 3). The most frequent grade ≥3 adverse events included febrile neutropenia (45%), bacterial (35%) and viral (12%) infections, mucositis (15%), diarrhea (15%), increased bilirubin (12%), and pulmonary toxicity (19%). Approximately half of the episodes of febrile neutropenia occurred between day 0 and 4, likely reflecting cytokine release after cell infusion. Details of infections are listed in supplemental Table 1.
Overall, there were 15 deaths (19%), of which 8 (10%) were due to recurrence of the underlying malignancy, 3 (4%) from idiopathic pneumonia syndrome, and 1 (1%) each from aGVHD, cGVHD, acute coronary syndrome, and secondary graft failure (Table 4).
We observed a minor decrease in patients’ blood counts from day −20 to day −6 after the first 2 doses of Bu were administered during the outpatient phase of treatment. Median white blood cell counts on day −20 and day −6 were 6.3 × 109/L (range, 1.3 × 109/L-33.6 × 109/L) and 2.15 × 109/L (range, 0.4 × 109/L-9.3 × 109/L), respectively. This reduction did not result in any infections during the outpatient period. Median platelet counts on day −20 and day −6 were 169 × 109/L (range, 9 × 109/L-623 × 109/L) and 61 × 109/L (range, 8 × 109/L-417 × 109/L), respectively.
Discussion
In this phase 2 trial, a MAC regimen with fractionated Bu administered over an extended 3-week period, with PTCy-based GVHD prophylaxis, resulted in a favorable 3-year OS rate of 80% while retaining low rates of 3-year NRM and relapse of 9% and 18%, respectively. More than half of the study cohort was aged >60 years and 44% had HCT-CI scores of ≥3. Although this cohort had a high proportion of older patients and with high comorbidities, they had similar outcomes compared with the younger and fitter participants.
Hematological malignancies are most often diagnosed in older individuals. With an aging population, an increasing number of older patients with significant comorbidities are potential candidates for allo-HCT, which may provide the optimal management of their disease. However, advanced age is one of the major barriers for undergoing allo-HCT.16 Therefore, an effort should be made to facilitate safe and effective conditioning regimens that minimize regimen-related toxicity and mortality while maintaining disease control with low relapse rates.
Traditionally, MAC regimens comprise 4 to 6 days of chemotherapy and result in decreased risk of relapse, albeit with higher NRM compared with RIC regimens. RIC has been associated with reduced NRM but also with increased risk of relapse.5 A randomized phase 3 trial by the Blood and Marrow Transplant Clinical Trials Network group compared the outcomes of 356 adult patients with AML or MDS who received either MAC or RIC regimens.7 There was a trend toward improved 18-month OS rates for the patients who received MAC compared with those who received RIC regimens (77.5% vs 67.7%; P = .07). In a long-term follow-up report of that trial, the 4-year transplant-related mortality was higher for patients who received MAC than for those who received RIC (25.1% vs 9.9%; P < .001), yet the use of RIC was associated with a fourfold higher risk of relapse in multivariate analysis (P < .001), leading to a significant OS advantage for the MAC arm (hazard ratio, 1.54; P = .03).6 Therefore, we sought to develop a conditioning regimen that would provide the improved disease control of a MAC regimen while minimizing toxicity. Fractionation of chemotherapy was developed to increase antitumor effects in AML, allowing for early recruitment of leukemic cells into the cell cycle after initial chemotherapy.17-20 In a previous trial, we showed the feasibility of PK-guided fractionated dosing of Bu over 2 weeks, which enabled the safe administration of myeloablative doses of Bu.10,13 Although direct between-trial comparisons cannot be made, we can appreciate that in this study, an extension of Bu fractionation over 3 weeks with PTCy provided favorable PFS and OS rates while maintaining low NRM and toxicity. The rate of severe mucositis was 15%, which was lower than the 27% seen in our previous study of fractionated Bu dosing over a 2-week period3 and much lower than the >60% seen with myeloablative regimens7; It was similar to the 12% to 18% rate of severe mucositis observed with reduced intensity regimens.3,7 Furthermore, lower gastrointestinal toxicity and PTCy GVHD prophylaxis were associated with a low death rate of 1% each from aGVHD and cGVHD, respectively (Table 4).
Our results compare favorably with those of previous reports on RIC regimens. In a trial by the Cancer and Leukemia Group B (CALGB)/Blood and Marrow Transplant, a Bu/Flu-based RIC regimen was used in 114 older patients with AML.21 Two-year NRM, disease-free survival, and OS rates were 15%, 42%, and 48%, respectively. The relapse rate at 2 years was 44%. A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of patients with AML/MDS who underwent RIC allo-HCT revealed a 2-year OS rate of 42%; and 43%, 31%, and 34% for the 40 to 54 years, 55 to 59 years, 60 to 64 years, and ≥65 years age groups, respectively.2 In a CIBMTR analysis of patients with myelofibrosis who underwent RIC allo-HCT, the 5-year OS rate was 47%, whereas the 5-year NRM and relapse rates were 24% and 48%, respectively.1 With mature outcomes of the 3-year OS rate of 80%, our results are also as good as, if not better than, those of previous experiences with MAC, which also included younger patients with AML in CR.7 It is noteworthy that our results hold up over a long follow-up period.6
Furthermore, we have observed continuously improving outcomes in our consecutive trials with evolving MAC regimens despite the progressive inclusion of older patients and patients with higher comorbidities. In a randomized trial comparing low and high dose of fractionated Bu administered on a 2-week schedule with tacrolimus and methotrexate GVHD prophylaxis, the 1-year OS rate was 59% in both treatment arms; 1-year relapse rates were 32% vs 30%, respectively; and NRM rates were 21% vs 22%, respectively.10 More recently, a cohort of younger patients with a median age of 47 years undergoing matched donor transplantation who received fractionated Bu on a shorter 2-week schedule with PTCy GVHD prophylaxis had a 2-year OS rate of 76%, with 2-year relapse and NRM rates of 28% and 10%, respectively.13 In this study, by lengthening the fractionated Bu regimen to 3 weeks and starting on day −20 instead of day −13, we were able to extend the MAC regimen to older patients, with a median age of 61 years, and to patients with higher comorbidities, with a 3-year OS, relapse rate, and NRM of 80%, 18%, and 9%, respectively.
One possible limitation of our study pertains to the degree of generalization of our results to other transplant centers because administration of fractionated Bu with PK studies is time consuming and requires dedicated staff. We believe that the favorable patient outcomes justify the effort, assuming these results can be reproduced at other centers. Results need to be studied in a randomized controlled trial, perhaps in a cooperative group multicenter setting that compares our regimen with Flu and Bu or with a Flu and melphalan RIC regimen in older patients. Another limitation is the higher relapse rate that was observed in patients with higher DRI (supplemental Figure 2C). We are currently investigating whether the incorporation of other antileukemic agents to the conditioning regimen could potentially further reduce relapse, especially in these high-risk patients. The longer 3-week fractionated Bu regimen may be particularly suitable for longer administration and achievement of synergy with active targeted oral agents. Trials with venetoclax, sorafenib, cladribine, and thiotepa are underway. We are also evaluating the necessity and benefit of fractionating Bu by comparing the longer 3-week regimen reported here with a shorter 2-week regimen reported previously.10,13
In conclusion, a fractionated Bu plus Flu MAC regimen results in low rates of NRM and promising OS, especially for patients who are older or less fit with hematological malignancies and undergoing allo-HCT.
Acknowledgments
This study was funded by Cancer Center support grant P30CA016672 (US National Cancer Institute, National Institutes of Health). The grant provider had no role in the study design, data collection, data analysis, interpretation of the results, or writing of the report.
Authorship
Contribution: U.R.P. conceptualized and oversaw the study, enrolled patients, interpreted data, ensured regulatory compliance of the trial, wrote the manuscript, and had the final responsibility to submit it for publication; O.P., R.S.M., A.O., J.C., A.M.A., G.A.-A., Q.B., A.M.G., C.M.H., J.S.I., P.K., I.K., D.M., Y.N., B.O., N.S., S.A.S., J.L.R., K.R., M.H.Q., and E.J.S. enrolled patients, provided clinical support, and monitored clinical responses during the study; R.B. contributed to the data analysis, generated figures, and wrote the statistical analysis section of the manuscript; R.E.C. and B.S.A. enrolled patients and conceptualized the study; R.S.M., R.B., and U.R.P. had full access to the raw data; and all authors approved of the manuscript text.
Conflict-of-interest disclosure: U.R.P. has received research support from Bayer, AbbVie, Incyte, and Novartis. Q.B. has received research support from GlaxoSmithKline, Stemline, Acrotech, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Uday R. Popat, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: upopat@mdanderson.org.
References
Author notes
The underlying data of this study are available upon reasonable request from the corresponding author, Uday R. Popat (upopat@mdanderson.org).
The full-text version of this article contains a data supplement.