Key Points
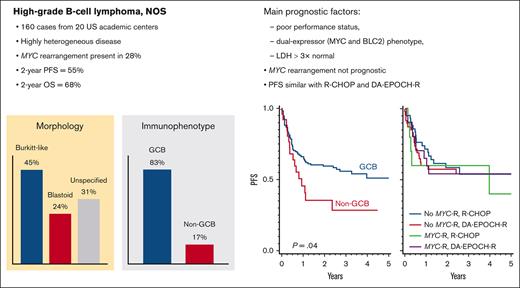
HGBL-NOS is a highly heterogeneous category; PFS (55% at 2 years) did not significantly differ between R-CHOP and intensified regimens.
A dual-expressor (MYC and BCL2) immunophenotype and TP53 alterations, but not MYC alterations, were significantly associated with worse PFS.
Abstract
In this multi-institutional retrospective study, we examined the characteristics and outcomes of 160 patients with high-grade B-cell lymphoma, not otherwise specified (HGBL-NOS)—a rare category defined by high-grade morphologic features and lack of MYC rearrangements with BCL2 and/or BCL6 rearrangements ("double hit"). Our results show that HGBL-NOS tumors are heterogeneous: 83% of patients had a germinal center B-cell immunophenotype, 37% a dual-expressor immunophenotype (MYC and BCL2 expression), 28% MYC rearrangement, 13% BCL2 rearrangement, and 11% BCL6 rearrangement. Most patients presented with stage IV disease, a high serum lactate dehydrogenase, and other high-risk clinical factors. Most frequent first-line regimens included dose-adjusted cyclophosphamide, doxorubicin, vincristine, and etoposide, with rituximab and prednisone (DA-EPOCH-R; 43%); rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP; 33%); or other intensive chemotherapy programs. We found no significant differences in the rates of complete response (CR), progression-free survival (PFS), or overall survival (OS) between these chemotherapy regimens. CR was attained by 69% of patients. PFS at 2 years was 55.2% and OS was 68.1%. In a multivariable model, the main prognostic factors for PFS and OS were poor performance status, lactate dehydrogenase >3 × upper limit of normal, and a dual-expressor immunophenotype. Age >60 years or presence of MYC rearrangement were not prognostic, but patients with TP53 alterations had a dismal PFS. Presence of MYC rearrangement was not predictive of better PFS in patients treated with DA-EPOCH-R vs R-CHOP. Improvements in the diagnostic criteria and therapeutic approaches beyond dose-intense chemotherapy are needed to overcome the unfavorable prognosis of patients with HGBL-NOS.
Introduction
High-grade B-cell lymphoma, not otherwise specified (HGBL-NOS) is a diagnostic category introduced in the revised fourth edition of the World Health Organization (WHO) classification of lymphoid malignancies and retained in both the 2022 WHO and the International Consensus Classification.1-3 This category, along with HGBL with MYC and BCL2 and/or BCL6 rearrangements (often referred to as double-hit lymphoma), replaced the prior designation of “B-cell lymphomas, unclassifiable with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma” (BCLU).4 HGBL-NOS includes lymphomas with Burkitt-like or blastoid morphology but excludes double-hit lymphoma, lymphoblastic lymphoma, blastoid mantle cell lymphoma, or rare related entities like the HGBL with 11q aberration. HGBL-NOS tumors are very rare, accounting for ∼1% to 2% of non-Hodgkin lymphomas, and although they share features with diffuse large B-cell lymphoma (DLBCL), their responsiveness to standard therapies and prognosis have not specifically been examined.5,6 Prior case series used the older BCLU designation and combined HGBL-NOS with double-hit lymphoma cases,7-10 had small sample sizes derived from single institutions,11 or lacked consistent treatment and outcome data.12,13
HGBL-NOS is not a precisely defined diagnostic category. Its definition relies on morphologic features, including the Burkitt-like and blastoid variants; after exclusion of the double-hit lymphoma, it is not defined by cytogenetic or molecular markers.14 With limited diagnostic material, many tumors may be classified as DLBCL with high-grade features, and the WHO restricts the category of HGBL-NOS to the rare lymphomas that absolutely (yet, often, subjectively) cannot be placed in a more specific diagnostic category. Currently, no standard treatment for HGBL-NOS is supported by dedicated research because of the lack of prospective studies in this rare subtype, although many experts recommend intensified chemotherapy beyond rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) based on single-arm studies enrolling patients with various high-risk B-cell lymphomas.3,15-17 The objective of this study was to describe the treatments and outcomes of patients with HGBL-NOS diagnosed in academic centers in the United States based on the 2016 WHO criteria and investigate prognostic factors and potential patient subsets that might derive benefit from intensified therapy.
Methods
Patients
We conducted a multicenter retrospective study of adult patients (aged ≥18 years at diagnosis) with newly diagnosed HGBL-NOS treated between 2016 and 2021 at 20 academic US institutions (supplemental Figure 1A). The institutional review boards of each participating facility approved the research, which was conducted in accordance with the Declaration of Helsinki. Cases of HGBL-NOS were selected locally by investigators (clinicians or pathologists), with a central review of all pathology reports to confirm eligibility criteria and the diagnosis. In 10 participating institutions (submitting 61% of all cases), expert lymphoma hematopathologist investigators undertook a detailed local slide review to confirm fulfillment of WHO criteria for HGBL-NOS. To assess concordance, 6 hematopathology experts (blinded to immunophenotype, molecular tests, or final clinical diagnosis) examined scanned hematoxylin and eosin–stained slides from 15 cases (using ImageScope version 12.4.6, Leica Biosystems, magnification ×20) and assigned Burkitt-like, blastoid, or large cell morphology. We excluded any tumors that had features consistent with those of DLBCL, NOS, Burkitt lymphoma, lymphoblastic lymphoma, or blastoid mantle cell lymphoma. Burkitt lymphoma was considered in the differential diagnosis of Burkitt-like HGBL, NOS, and ruled out based on ≥1 exclusionary criteria: highly pleomorphic morphology, CD10− or BCL2+ immunophenotype, presence of incompatible cytogenetics (eg, translocations of BCL2 or BCL6 but not MYC, or complex karyotype).1,3,14 In addition, cases not tested for MYC rearrangement or those with double-hit cytogenetics were excluded. Cases of HGBL-NOS presenting as a transformation from other low-grade B-cell lymphomas or as a posttransplant lymphoproliferative disorder (PTLD) were eligible. All stagings and immunohistochemical and molecular studies as well as treatments were completed at the discretion of the treating physicians in accordance with the institutional practice.
Variables and end points
Investigators collected demographic, clinicopathologic, treatment, and outcome data using a standardized protocol. Serum lactate dehydrogenase (LDH) level was normalized to the local upper limit of normal, and performance status (PS) was reported per the Eastern Cooperative Group scale. Cell of origin was assigned using immunohistochemical analysis and the Hans algorithm.18 Cases with concurrent expression of BCL2 (≥50% cutoff) and MYC (≥40% cutoff) as assessed via immunohistochemistry were designated as dual-expressor lymphoma (DEL). The morphologic features of these neoplasms were classified as Burkitt-like, blastoid, or undetermined. Based on the results of fluorescent in situ hybridization (FISH), we noted the presence of rearrangements of MYC (MYC-R, by definition, only single-hit), BCL2 (BCL2-R), BCL6 (BCL6-R), or extra copies (ECs) of these genes. Presence of TP53 alterations was determined in a subset of patients using DNA sequencing or inferred from strong (≥50%) immunohistochemical staining, which was previously shown to highly correlate with the presence of TP53 mutation.19
Similar to prior observational studies of high-grade lymphomas,20,21 treatment regimens were classified as standard intensity (including R-CHOP with or without high-dose methotrexate for central nervous system [CNS] prophylaxis) or intensified (including dose-adjusted cyclophosphamide, doxorubicin, vincristine, and etoposide, with rituximab and prednisone [DA-EPOCH-R],16 rituximab, cyclophosphamide, doxorubicin, vincristine, methotrexate, ifosfamide, etoposide, and cytarabine [R-CODOX-M/IVAC],22 and rituximab, hyperfractionated cyclophosphamide, doxorubicin, vincristine, methotrexate, and cytarabine [R-hyperCVAD/MA]).23 Because most patients were treated with R-CHOP or DA-EPOCH-R, we further compared the outcomes between these 2 specific regimens.
Overall response and complete response (CR) to therapy were assigned locally by applying the Lugano criteria.24 Progression-free survival (PFS) was defined as the time from diagnosis to recurrence, progression, death, or last follow-up. Overall survival (OS) was calculated from diagnosis to the last follow-up or death.
Statistical analysis
Categorical variables were compared using Fisher exact test, and continuous variables were compared using rank-sum test. Survival analysis was conducted using the Kaplan-Meier method. Considering many overlapping variables, prognostic factors for PFS or OS were selected using classic forward and backward selection. To account for missing data, variable selection was conducted on data sets augmented by multiple imputation using chained equations.25 The imputation models contained the maximum number of baseline variables that supported convergence, including the outcome (cumulative hazard of PFS). Estimates and standard errors were averaged using Rubin rules for 30 imputed data sets. All estimates are provided with 95% confidence intervals (CIs). In this exploratory study, we did not apply corrections for multiple testing and used P < .05 as an indicator of statistical significance. Data analysis was conducted using Stata/SE 17.0 (College Station, TX).
Results
Patients and disease characteristics
The study included 160 patients diagnosed across 20 institutions. Median age was 64 years (range, 18-92 years), with 56% of patients aged ≥60 years at diagnosis (Table 1). The male-to-female ratio was 2.1. Four (2%) patients had HIV infection, with the reported CD4 count ranging from 100 to 524 cells per mm3. Patients often presented with advanced-stage disease: stage III in 6% and stage IV in 63%. Extranodal sites, including the bone marrow (24%), peripheral blood (8%), gastrointestinal tract (22%), liver (12%), kidneys (13%), gonads (4%), and CNS (7%), were involved in 81%. Most patients (69%) had an elevated serum LDH level and high or high-intermediate international prognostic index (IPI; 54%). Nine cases represented transformation from prior indolent lymphoma (follicular lymphoma in 6, and chronic lymphocytic leukemia in 3), and 5 patients developed HGBL-NOS as a PTLD.
Clinical and histopathologic characteristics of patients with HGBL-NOS (N = 160)
| Variable . | . | . |
|---|---|---|
| Age, median (range), y | 64 | 18-92 |
| Sex, n (%) | ||
| Male | 108 | 67.5% |
| Female | 52 | 32.5% |
| Poor PS, n (%)∗ | 33 | 20.6% |
| Stage, n (%)† | ||
| I or II | 48 | 30.6% |
| III or IV | 109 | 69.4% |
| Undetermined | 3 | |
| >1 extranodal site, n (%) | 66 | 41.3% |
| Bone marrow involvement, n (%)∗ | 39 | 24.4% |
| CNS involvement, n (%) | 11 | 6.9% |
| LDH > ULN, n (%)∗ | 110 | 68.8% |
| LDH > 3 × ULN, n (%)∗ | 37 | 23.1% |
| IPI, n (%) | ||
| Low | 34 | 21.3% |
| Intermediate low | 27 | 16.9% |
| Intermediate high | 42 | 26.3% |
| High | 44 | 27.5% |
| Undetermined | 13 | 8.1% |
| Morphology, n (%) | ||
| Burkitt-like | 72 | 45.0% |
| Blastoid | 38 | 23.8% |
| Unspecified | 50 | 31.3% |
| Cell of origin (based on IHC), n (%)† | ||
| GCB-like | 131 | 82.9% |
| Non-GCB | 27 | 17.1% |
| Undetermined | 2 | |
| CD10 expression, n (%) | ||
| Positive | 123 | 76.9% |
| Negative | 37 | 23.1% |
| BCL6 expression, n (%)† | ||
| Positive | 120 | 80.0% |
| Negative | 30 | 20.0% |
| Not tested | 10 | |
| BCL2 expression, n (%)† | ||
| Positive | 83 | 53.9% |
| Negative | 71 | 46.1% |
| Not tested | 6 | |
| MYC expression, n (%)† | ||
| Positive | 102 | 71.8% |
| Negative | 40 | 28.2% |
| Not tested | 18 | |
| MUM1 expression, n (%)† | ||
| Positive | 60 | 45.5% |
| Negative | 72 | 54.5% |
| Not tested | 28 | |
| Dual MYC and BCL2 expressor, n (%)† | ||
| Yes | 52 | 37.1% |
| No | 88 | 62.9% |
| Undetermined | 20 | |
| CD5 expression, n (%)† | ||
| Positive | 20 | 14.1% |
| Negative | 122 | 85.9% |
| Not tested | 18 | |
| MYC rearrangement, n (%) | ||
| Yes | 44 | 27.5% |
| No | 116 | 72.5% |
| BCL2 rearrangement, n (%)† | ||
| Yes | 17 | 12.7% |
| No | 117 | 87.3% |
| Not tested | 26 | |
| BCL6 rearrangement, n (%)† | ||
| Yes | 15 | 11.2% |
| No | 119 | 88.8% |
| Not tested | 26 | |
| First-line therapy, n (%) | ||
| DA-EPOCH-R | 68 | 42.5% |
| R-CHOP | 53 | 33.1% |
| R-CODOX-M/IVAC | 11 | 6.9% |
| R-hyperCVAD/MA | 6 | 3.8% |
| Other | 16 | 10.0% |
| Untreated | 4 | 2.5% |
| Unknown | 2 | 1.2% |
| Variable . | . | . |
|---|---|---|
| Age, median (range), y | 64 | 18-92 |
| Sex, n (%) | ||
| Male | 108 | 67.5% |
| Female | 52 | 32.5% |
| Poor PS, n (%)∗ | 33 | 20.6% |
| Stage, n (%)† | ||
| I or II | 48 | 30.6% |
| III or IV | 109 | 69.4% |
| Undetermined | 3 | |
| >1 extranodal site, n (%) | 66 | 41.3% |
| Bone marrow involvement, n (%)∗ | 39 | 24.4% |
| CNS involvement, n (%) | 11 | 6.9% |
| LDH > ULN, n (%)∗ | 110 | 68.8% |
| LDH > 3 × ULN, n (%)∗ | 37 | 23.1% |
| IPI, n (%) | ||
| Low | 34 | 21.3% |
| Intermediate low | 27 | 16.9% |
| Intermediate high | 42 | 26.3% |
| High | 44 | 27.5% |
| Undetermined | 13 | 8.1% |
| Morphology, n (%) | ||
| Burkitt-like | 72 | 45.0% |
| Blastoid | 38 | 23.8% |
| Unspecified | 50 | 31.3% |
| Cell of origin (based on IHC), n (%)† | ||
| GCB-like | 131 | 82.9% |
| Non-GCB | 27 | 17.1% |
| Undetermined | 2 | |
| CD10 expression, n (%) | ||
| Positive | 123 | 76.9% |
| Negative | 37 | 23.1% |
| BCL6 expression, n (%)† | ||
| Positive | 120 | 80.0% |
| Negative | 30 | 20.0% |
| Not tested | 10 | |
| BCL2 expression, n (%)† | ||
| Positive | 83 | 53.9% |
| Negative | 71 | 46.1% |
| Not tested | 6 | |
| MYC expression, n (%)† | ||
| Positive | 102 | 71.8% |
| Negative | 40 | 28.2% |
| Not tested | 18 | |
| MUM1 expression, n (%)† | ||
| Positive | 60 | 45.5% |
| Negative | 72 | 54.5% |
| Not tested | 28 | |
| Dual MYC and BCL2 expressor, n (%)† | ||
| Yes | 52 | 37.1% |
| No | 88 | 62.9% |
| Undetermined | 20 | |
| CD5 expression, n (%)† | ||
| Positive | 20 | 14.1% |
| Negative | 122 | 85.9% |
| Not tested | 18 | |
| MYC rearrangement, n (%) | ||
| Yes | 44 | 27.5% |
| No | 116 | 72.5% |
| BCL2 rearrangement, n (%)† | ||
| Yes | 17 | 12.7% |
| No | 117 | 87.3% |
| Not tested | 26 | |
| BCL6 rearrangement, n (%)† | ||
| Yes | 15 | 11.2% |
| No | 119 | 88.8% |
| Not tested | 26 | |
| First-line therapy, n (%) | ||
| DA-EPOCH-R | 68 | 42.5% |
| R-CHOP | 53 | 33.1% |
| R-CODOX-M/IVAC | 11 | 6.9% |
| R-hyperCVAD/MA | 6 | 3.8% |
| Other | 16 | 10.0% |
| Untreated | 4 | 2.5% |
| Unknown | 2 | 1.2% |
IHC, immunohistochemistry; ULN, upper limit of normal.
Percentage calculation includes missing data on PS in 7 (4.4%) bone marrow involvement in 8 (5.0%) and LDH in 12 (7.5%) patients.
Percentage calculation excludes missing data.
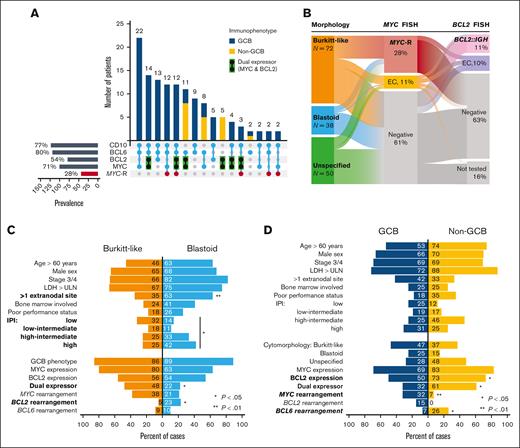
The morphologic features were described as Burkitt-like in 45%, blastoid in 24%, or unspecified in 31% of the patients (supplemental Table 1). Overall, 83% of cases had a germinal center B-cell–like (GCB) immunophenotype, whereas 17% were non-GCB, and 2 cases were undetermined (supplemental Table 2). Excluding cases with missing data, immunophenotypic analysis showed that these neoplasms tested positive for CD20 in 96%, BCL6 in 80%, CD10 in 77%, MYC in 72%, BCL2 in 54%, MUM1/IRF4 in 46%, CD5 in 14% (all negative for cyclin D1 immunohistochemistry or FISH for t[11;14]), and CD30 in 7% (6 of 81 cases tested) of the cases. The median Ki-67 rate was 95% (range, 50%-100%). A DEL immunophenotype was present in 37% of the cases, and a Burkitt-like immunophenotype (CD10+/BCL6+/BCL2−) was present in 32% (Figure 1A). Two patients with blastoid tumors tested positive for terminal deoxynucleotidyl transferase (TdT) but otherwise had mature B-cell lymphomas; both had IGH::BCL2 rearrangement, and 1 had transformed from follicular lymphoma. In situ hybridization for Epstein-Barr virus–encoded small RNA tested positive in 8% of cases (8 of 103 tested).
Clinicopathologic characteristics of HGBL-NOS. (A) Prevalence of the most common immunophenotypes with or without MYC rearrangement (only phenotypes with n ≥ 2 are shown); (B) prevalence of MYC and/or BCL2 alterations, via FISH, in morphologic subtypes; distribution of characteristics based on panel B tumor morphology and (C) cell of origin determined via immunohistochemistry (P values from Fisher exact test).
Clinicopathologic characteristics of HGBL-NOS. (A) Prevalence of the most common immunophenotypes with or without MYC rearrangement (only phenotypes with n ≥ 2 are shown); (B) prevalence of MYC and/or BCL2 alterations, via FISH, in morphologic subtypes; distribution of characteristics based on panel B tumor morphology and (C) cell of origin determined via immunohistochemistry (P values from Fisher exact test).
FISH revealed the presence of MYC-R in 28%, MYC-EC in an additional 11%, BCL2-R in 13% (percentage based on tested cases), BCL2-EC in 12%, BCL6-R in 11%, and BCL6-EC in 10% of the cases (Figure 1B). Nine cases were subjected to FISH for 11q aberration and all showed negative results. Of 26 tested tumors, 10 (39%) had a TP53 alteration: 6 of 10 were tested via gene sequencing, and 6 of 20 using p53 expression via immunohistochemistry in >50%-positive cells, which has been associated with TP53 alterations.
Comparing HGBL-NOS based on morphology (Figure 1C; supplemental Table 1), blastoid tumors were more likely than Burkitt-like tumors to involve multiple extranodal sites (63% vs 35%, respectively; P = .005), had overall higher IPIs (Mantel-Haenszel P = .012), were more likely to harbor the IGH::BCL2 rearrangement (23% vs 5%, respectively; P = .028), but were less likely to be designated as DEL (22% vs 48%, respectively; P = .016); MYC-R was somewhat more frequent in Burkitt-like HGBL (38% vs 21% in blastoid; P = .089), but there was no significant difference in the prevalence of Burkitt-like (CD10+/BCL6+/BCL2−) immunophenotype (37% vs 35%, respectively; P = .99). Furthermore, 5 of 6 transformed follicular lymphoma cases were blastoid tumors, whereas all PTLD tumors had Burkitt-like morphology.
A GCB immunophenotype was predominant in all morphologic subtypes: Burkitt-like (86%), blastoid (89%), and undetermined (74%). Non-GCB tumors were more likely to have a DEL immunophenotype (61% vs 32% in GCB tumors; P = .016) and carry BCL6-R (26% vs 7%, respectively; P = .022) and only rarely had MYC-R (7% vs 32%, respectively; P = .009; Figure 1D; supplemental Table 2).
We did not observe any significant clinical or histopathologic differences between patients who did (61%; n = 97) or did not (39%; n = 63) undergo expert hematopathology review for this study (supplemental Table 3). When 6 expert hematopathology investigators examined the morphology of 15 selected cases, concordance was achieved in only 2 (13%) cases, and >50% of pathologists agreed on the morphology assignment in 9 (60%) cases (supplemental Figure 2). Thus, the interrater reliability for HGBL morphology assignment was very poor (Cohen κ = 0.27).
Treatment
Among the 154 patients who received systemic therapy, 97% received rituximab with the initial chemotherapy regimen, which was DA-EPOCH-R in 43% of patients, R-CHOP in 33%, R-CODOX-M/IVAC in 7%, and R-hyperCVAD/MA in 4%. The median number of chemotherapy courses was 6 (range, 1-8). Among 139 patients with recorded data, 57 (41%) received intrathecal and 26 (19%) systemic CNS prophylaxis (12% received both). Furthermore, 20 of 144 (14%) patients with recorded data had consolidative radiation therapy, and 6 of 144 (4%) patients underwent autologous stem cell transplantation in the first remission. Compared with patients receiving first-line R-CHOP (n = 53), those receiving DA-EPOCH-R (n = 68) were, on average, younger, and, more often had, advanced-stage disease or Burkitt-like histology but without a significant difference in the frequency of MYC-R (19% vs 31%, respectively; P = .15; supplemental Table 4).
The overall response rate (ORR) for first-line therapy was 80.3% (95% CI, 72.8-86.5), and the CR rate was 69% (95% CI, 60.7-76.4). There was no significant difference between patients receiving R-CHOP or those receiving more intensive chemotherapy regimens in terms of the ORR (82% vs 81%, respectively; P = .99) or CR (66% vs 74%; P = .32). HGBL-NOS recurred in 59 (38%) patients, of whom 40 (68%) died. The most common second-line regimens included ifosfamide, carboplatin, and etoposide (27%); dexamethasone, high-dose cytarabine, and platinum (11%); or gemcitabine and oxaliplatin (11%). An anti-CD20 antibody was included in 85% of salvage regimens. The ORR for salvage therapy was 33.3% (95% CI, 20.8-47.9), and CR was attained in 9.8% (95% CI, 3.2-21.4); these proportions were even lower for the 36 patients who experienced relapse or progression within 6 months from the initial diagnosis (ORR, 13.9%; CR, 5.6%). Thirteen patients received CD19-directed chimeric antigen receptor (CAR) T-cell therapy, with responses noted in 7 (54%) and CR in 4 (31%). However, 43% (3 of 7) of patients responding to CAR T-cell therapy experienced further progression.
Survival and prognostic factors
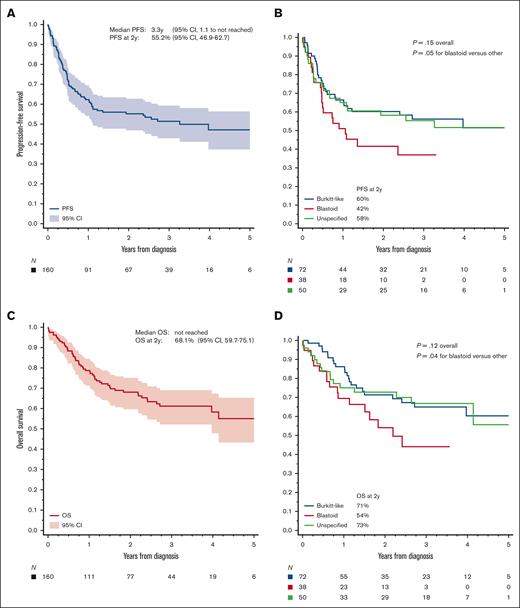
The median follow-up from diagnosis was 2.9 years (95% CI, 2.6-3.2). The median PFS was 3.3 years (95% CI, 1.1 to not reached), with a 2-year estimate of 55.2% (95% CI, 46.9-62.7) and a 3-year estimate of 51.4% (95% CI, 42.9-59.3; Figure 2A). Most progression events occurred early, at a median of 5.3 months from diagnosis (range, 0-39 months). We observed no statistically significant difference between morphologic subtypes, although patients with blastoid tumors had somewhat worse outcome (Figure 2B). Of 59 recurrences, 16 (27%) involved the CNS; a detailed analysis of the incidence for CNS recurrence and CNS-directed therapy is provided in a separate analysis.26 The median OS was not reached, with a 2-year OS estimate of 68.1% (95% CI, 59.7-75.1; Figure 2C), which was worse for blastoid HGBL (Figure 2D). The OS after progression was poor, with a median of 7.3 months (95% CI, 5.1-11.1) and a 2-year OS of 18.2% (95% CI, 8.0-31.6).
Survival outcomes in HGBL-NOS. (A) PFS for all patients and (B) based on the tumor morphology; (C-D) analogous curves for OS; P values were obtained using log-rank test.
Survival outcomes in HGBL-NOS. (A) PFS for all patients and (B) based on the tumor morphology; (C-D) analogous curves for OS; P values were obtained using log-rank test.
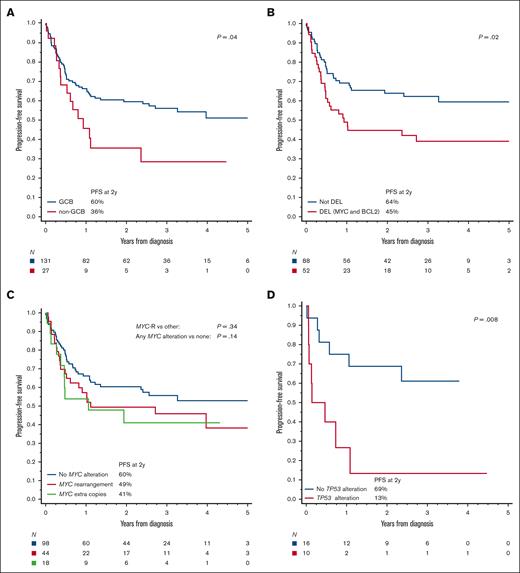
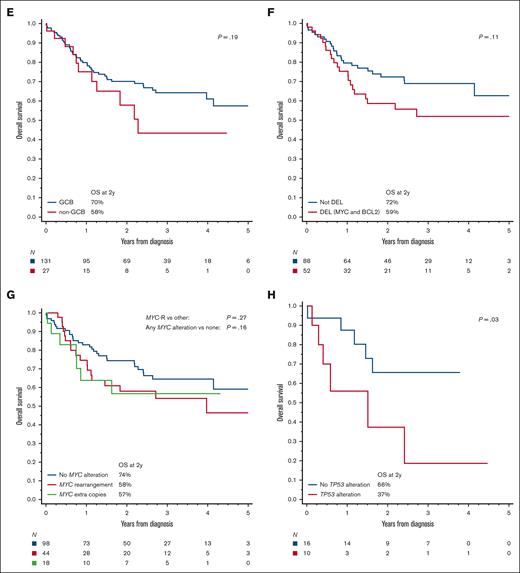
Upon univariate analysis, the PFS was significantly worse for patients with poor PS, advanced-stage disease, bone marrow involvement, high LDH level, non-GCB immunophenotype, DEL immunophenotype, or BCL2 expression than for other patients (Figure 3A-B; supplemental Figures 3A-D and 5B-E; supplemental Table 5). Presence of MYC-R or MYC-EC was not associated with a statistically significantly worse PFS (Figure 3C), whereas TP53 alteration or overexpression carried a particularly poor prognosis (Figure 3D). PFS was also worse in the presence of BCL2 or BCL6 rearrangements or ECs (supplemental Figure 5E-F). Higher IPI was associated with a poorer PFS, but age-adjusted IPI or Burkitt lymphoma IPI was more discriminating (supplemental Figure 3E-F; supplemental Table 6). We observed no significant difference in outcomes between cases that did and did not undergo expert pathology review (supplemental Figure 5A,G). For the OS, the univariate prognostic factors were similar (supplemental Table 5), except that the differences by cell of origin, BCL2 expression, or DEL status did not reach statistical significance, and BCL6 rearrangements/EC were not prognostic (Figure 3E-H; supplemental Figures 4 and 5H-L). Seventy percent of deaths were considered lymphoma-related by the investigators, and 7% were due to treatment toxicity. Upon multivariable analysis, the 3 independent factors most robustly associated with PFS and OS were poor PS, LDH > 3 × upper limit of normal, and DEL phenotype (Table 2). The presence of TP53 mutation was not included in the multivariable model because of the extent of missing data, but after adjusting for the other factors, it had a prognostic significance for PFS among patients who were tested (supplemental Table 7).
Biologic risk factors in HGBL-NOS. PFS based on (A) the cell of origin via immunohistochemistry, (B) DEL phenotype (MYC and BCL2 expression via immunohistochemistry), (C) presence of MYC alterations via FISH, and (D) presence of TP53 alterations (by sequencing or p53 IHC); (E-H) analogous curves for OS; P values were obtained using log-rank test (log-rank test for trend as indicated).
Biologic risk factors in HGBL-NOS. PFS based on (A) the cell of origin via immunohistochemistry, (B) DEL phenotype (MYC and BCL2 expression via immunohistochemistry), (C) presence of MYC alterations via FISH, and (D) presence of TP53 alterations (by sequencing or p53 IHC); (E-H) analogous curves for OS; P values were obtained using log-rank test (log-rank test for trend as indicated).
Multivariable models for PFS and OS in HGBL-NOS
| Variable . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Poor PS | 1.91 | (1.12-3.26) | .018 | 2.42 | (1.35-4.36) | .0031 |
| LDH > 3 × ULN | 2.40 | (1.44-4.02) | .0009 | 2.57 | (1.42-4.64) | .0018 |
| DEL phenotype | 1.97 | (1.23-3.16) | .0050 | 1.81 | (1.03-3.17) | .038 |
| Variable . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Poor PS | 1.91 | (1.12-3.26) | .018 | 2.42 | (1.35-4.36) | .0031 |
| LDH > 3 × ULN | 2.40 | (1.44-4.02) | .0009 | 2.57 | (1.42-4.64) | .0018 |
| DEL phenotype | 1.97 | (1.23-3.16) | .0050 | 1.81 | (1.03-3.17) | .038 |
HR, hazard ratio.
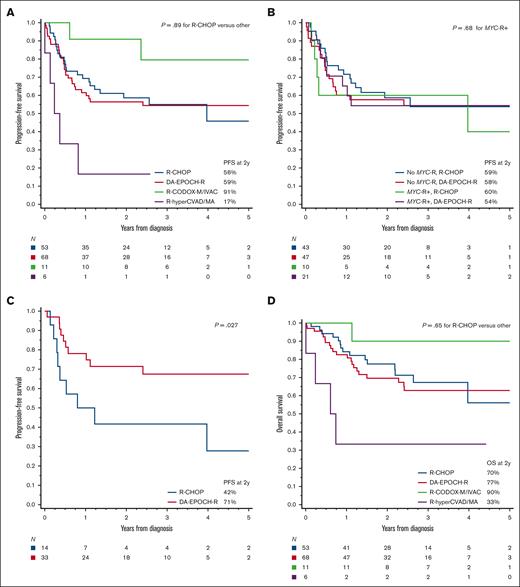
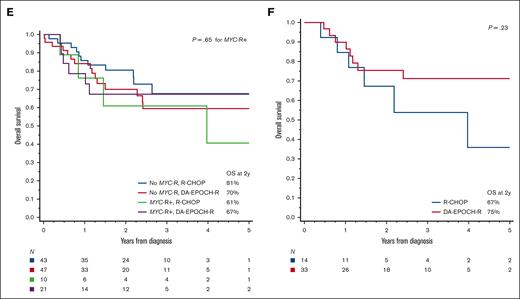
When survival outcomes were examined based on the first-line therapy, we observed no significant difference between patients treated with R-CHOP and those treated with more intensive regimens (Figure 4A,D). The divergent outcomes of patients receiving R-CODOX-M/IVAC or R-hyperCVAD/MA were difficult to interpret, considering the very small sample size and high likelihood of selection bias. There was, however, no significant difference specifically between R-CHOP and DA-EPOCH-R therapy, even with further stratification by MYC-R (P for interaction = 0.55; Figure 4B,E), age-adjusted IPI (P for interaction = 0.30; supplemental Figure 6), or in a multivariable model adjusting for multiple prognostic factors (n = 121; adjusted hazard ratio for PFS = 0.76; 95% CI, 0.41-1.41; P = .38; supplemental Table 8). No factor could discern differential outcomes between R-CHOP or DA-EPOCH-R, with 1 exception; in an exploratory evaluation that excluded transformed lymphomas and PTLD (n = 112), we observed a significant interaction between chemotherapy regimen and the Burkitt-like morphology (P for interaction = .022). In Burkitt-like tumors, DA-EPOCH-R showed a PFS advantage (P = .027; Figure 4C) that was maintained after stratification based on age-adjusted IPI (P = .017). The OS difference was not statistically significant (P = .23; Figure 4F).
Survival outcomes in HGBL-NOS based on the first-line therapy. (A) PFS among patients treated with R-CHOP, DA-EPOCH-R, R-CODOX-M/IVAC, or R-hyperCVAD/MA; P values compare R-CHOP with other (intensive) regimens; (B) PFS among patients treated with R-CHOP or DA-EPOCH-R, stratified based on the presence or absence of MYC rearrangement (MYC-R), P values compare MYC-R+ groups treated with R-CHOP or DA-EPOCH-R; (C) PFS among patients with Burkitt-like HGBL-NOS (excluding transformed and PTLD cases) treated with R-CHOP or DA-EPOCH-R; and (D-F) analogous curves for OS.
Survival outcomes in HGBL-NOS based on the first-line therapy. (A) PFS among patients treated with R-CHOP, DA-EPOCH-R, R-CODOX-M/IVAC, or R-hyperCVAD/MA; P values compare R-CHOP with other (intensive) regimens; (B) PFS among patients treated with R-CHOP or DA-EPOCH-R, stratified based on the presence or absence of MYC rearrangement (MYC-R), P values compare MYC-R+ groups treated with R-CHOP or DA-EPOCH-R; (C) PFS among patients with Burkitt-like HGBL-NOS (excluding transformed and PTLD cases) treated with R-CHOP or DA-EPOCH-R; and (D-F) analogous curves for OS.
Discussion
In this study, we described the characteristics, treatments, and outcomes of patients with HGBL-NOS as diagnosed in academic practices using the WHO classification criteria. Our comprehensive analysis illustrates the heterogeneity of the rare HGBL-NOS category from all perspectives. We observed that survival outcomes of patients with HGBL-NOS were worse than those historically reported for patients with DLBCL. The DEL immunophenotype was associated with a worse PFS but MYC-R was not. The outcomes did not appear to significantly improve with intensified regimens frequently applied in clinical practice, except those of the de novo Burkitt-like subgroup. Our findings highlight the need for future research needed for a better definition and dedicated management of these aggressive lymphomas.
Studying HGBL-NOS is difficult because of exceptional intra- and interinstitutional variability of diagnostic criteria. Although our study is limited by lack of central pathology review, we felt it was unfeasible to undertake this approach considering the very low concordance among experts about the morphologic HGBL criteria.1-3 Indeed, in our assessment, perfect concordance among academic hematopathologists was achieved in only 13% of examined cases, and >50% concordance was observed for 60% of cases. Therefore, we took a hybrid approach, accepting cases diagnosed as HGBL, NOS by academic hematopathologists, with a central review of pathology reports and a detailed local direct slide review in half of the participating institutions. This methodology allowed us to reflect the real-life practice most relevant to clinicians and patients facing the diagnosis of HGBL-NOS today. We acknowledge that it is limited by the highly variable thresholds to apply the Burkitt-like or blastoid morphology criteria, particularly in smaller biopsy specimens.14 An alternative approach with a formal central pathology review leading to rejection of nearly 50% of cases was undertaken for the genomic study of HGBL by the Lymphoma/Leukemia Molecular Profiling Project (LLMPP).13 Of note, the LLMPP found no evident differences in the cell of origin, MYC-R, high-grade gene expression profiling (GEP) signature, or any cytogenetic or genomic feature between cases confirmed as HGBL-NOS or those rejected by the central pathology review. Similarly, we observed no meaningful differences in any clinicopathologic characteristics or outcomes among HGBL-NOS cases submitted with or without local expert slide review. These findings question the overall applicability of morphologic criteria to distinguish a single HGBL, NOS entity and the reproducibility of a central pathology review. Efforts to define HGBL using more objective criteria are needed to move forward in the field. In this context, recent studies have described a molecular HGBL or “dark-zone” GEP signature, which is highly specific to GCB tumors and can subsume both HGBLs, NOS and double-hit HGBL categories.27-29 Being a potentially separate entity, 17% of HGBL-NOS in our series had non-GCB immunophenotype. These tumors typically lacked MYC-R, showed frequent DEL status and significantly worse survival from other HGBL, NOS. In the LLMPP study, 25% of centrally confirmed HGBL-NOS also showed an activated B-cell GEP.13 These consistent observations suggest that non-GCB HGBLs-NOS are important, distinct from GCB HGBL, and require further molecular characterization. This subset might hypothetically contain some of the most aggressive lymphomas from the genomic MCD (MYD88/CD79B-mutated) class, which has been associated with CDKN2A/B deletions, frequent extranodal dissemination, and high risk of relapse after R-CHOP.30-32
We noted a lack of prognostic significance of (single-hit) MYC-R in HGBL-NOS, which is consistent with observations in DLBCL from the large study by the Lunenburg Lymphoma Biomarker Consortium.33 In contrast, survival was exceptionally poor among patients with HGBL-NOS with TP53 alterations or overexpression. Few patients in our cohort underwent testing for TP53 alterations or p53 immunohistochemical analysis, and the testing pattern might have been biased, so the observation is only hypothesis-generating and will need further study for validation. Our observed prevalence of TP53 alterations (39%) was similar to that of the LLMPP analysis (34%)13 and markedly higher than DLBCLs (10%-20%).30,32,34,35 Lymphomas in which MYC-R or the dark-zone GEP signature occur in conjunction with TP53 mutations are associated with a particularly aggressive course, so this pairing might be a future candidate marker for a subset of HGBL-NOS tumors.36,37 Considering our small data set, we could not further dissect the potential interaction of MYC-R and TP53 alterations. Of note, although the intensified DA-EPOCH-R regimen has high efficacy for lymphomas with MYC-R,16,38 it did not overcome the poor prognostic impact of TP53 alterations in HGBL in another study.39
One of our main objectives was to investigate whether intensified immunochemotherapy regimens could improve outcomes for patients with HGBL-NOS. Such an association was observed in retrospective series of double-hit lymphoma,20,40 leading to a change in clinical practice and its subsequent extrapolation to HGBL-NOS by experts.3,41 Similarly to Burkitt or double-hit lymphomas, HGBL-NOS recurs early, mostly during the first year of follow-up. The 2-year PFS of 55% is lower than that for DLBCL NOS, for which recent multicenter clinical trials and observational studies have reported a 70% 2-year PFS after R-CHOP.27,33,42 It is comparable with the outcomes of double-hit HGBL from population-based studies (eg, 52% 2-year freedom from progression in a recent large series from British Columbia; 56% 3-year relapse-free survival after R-CHOP in the United States).28,40 We did not observe PFS or OS differences between R-CHOP and other dose-intense regimens, or DA-EPOCH-R specifically, even accounting for MYC-R and other prognostic factors. Nevertheless, the highly heterogeneous nature of HGBL-NOS, which includes non-GCB tumors, transformed lymphomas, and PTLD might hamper detection of some associations. Of note, in the subset of de novo Burkitt-like HGBL-NOS, DA-EPOCH-R showed a PFS advantage, although this finding needs to be interpreted with caution because of multiple exploratory analyses performed without statistical correction.
Considering variable biology, inconsistent diagnostic patterns, and interinstitutional variation in management, trying to formulate a uniform treatment approach for HGBL, NOS would likely be a misguided approach. Enrolling patients in clinical trials, ideally using specific molecular criteria, remains of paramount importance. Examples of such studies include phase 2 trials for tumors with MYC-R (DA-EPOCH-R,16 R-CHOP with lenalidomide,43 or the “CARMEN” regimen17), biomarker-driven DA-EPOCH-R Nordic trial for high-risk lymphomas (defined by MYC-R, TP53 alterations, DEL immunophenotype, or CD5 expression),44 or the ZUMA-12 trial of CAR T-cell therapy for patients with high-risk DLBCL/HGBL.45 Outside of trials, our results can cautiously support several clinical propositions. DA-EPOCH-R appears advantageous for de novo Burkitt-like HGBL-NOS. It is supported by the efficacy of this regimen in Burkitt lymphoma, double-hit HGBL, and single-institution series of BCLU or HGBL.11,16,29,38 For other HGBL-NOS, alternative options should be considered because of relatively poor outcomes, but R-CHOP is acceptable, because we found no statistically significant evidence of benefit for more intensive regimens even in the presence of (single-hit) MYC-R. We noted a high PFS in a very small (n = 11) subgroup receiving R-CODOX-M/IVAC, precluding meaningful statistical testing but consistent with phase 2 trial experience in high-risk DLBCL.15 This potentially toxic regimen should be applied only by clinicians with expertise, and it may be the preferred choice for younger patients with, or at high risk of, CNS involvement. For the minority of non-GCB HGBLs, a subset analysis of the POLARIX trial suggests that polatuzumab vedotin in combination with R-CHP may improve outcomes for DEL or activated B-cell lymphomas, although its specific efficacy in non-GCB HGBL-NOS remains to be evaluated.42 Furthermore, based on the subset analyses of the PHOENIX trial, ibrutinib may have a role in the treatment of patients with non-GCB/DEL tumors of specific genomic classes.46 Finally, HGBL-NOS with TP53 alterations needs further study, but it constitutes a group with a particularly urgent need for innovative approaches.
Outcomes of patients with relapsed or refractory HGBL-NOS appear to be dismal, with short postprogression OS and low rates of response to salvage chemotherapy. Because 31% of patients with HGBL-NOS are primary refractory, and most progressions occur within 1 year from diagnosis, patients with relapsed or refractory HGBL-NOS will now likely receive second-line CD19-directed CAR T cells (axicabtagene ciloleucel or lisocabtagene maraleucel) based on recent randomized trials.47,48 In our cohort, responses to CAR T-cell therapy (applied as third or subsequent line(s) of therapy) were lower and less durable than reported that for DLBCLs, but the number of patients was small. We note that the registration trials of CAR T-cell therapy for B-cell lymphomas did not observe a lower response rate for the HGBL tumors (mostly double-hit lymphoma).49 Evaluating the effectiveness of this modality in HGBL-NOS will require more clinical experience.
The HGBL-NOS category had been retained by the 2022 WHO and International Consensus classifications, without changes to its definition, and is likely to remain an infrequent diagnosis of exclusion.1,2 Our results provide a comprehensive clinicopathologic description of HGBL-NOS tumors diagnosed using current criteria, which result in too much heterogeneity to guide subtype-specific clinical research. Patients with HGBL-NOS should be enrolled in trials for DLBCL NOS, particularly those guided by molecular or cytogenetic biomarkers. With only half of the patients surviving 3 years without progression, novel treatment approaches need to be identified for both the GCB HGBL (which can now be distinguished by GEP) and the particularly unfavorable non-GCB tumors. Testing of HGBL-NOS for TP53 alterations may help identify patients at the highest risk of poor outcomes with standard cytotoxic chemotherapy.
Authorship
Contribution: A.S.Z. and A.J.O. designed the study; M.G., A.K.B., S.L., L.J.M., H.K., M.A.A.-L., M.L.X., and K.N.N. conducted pathology review; A.S.Z. and A.J.O. analyzed the data and drafted the paper; and all authors collected the data, interpreted the results, and edited and approved the final manuscript.
Conflict-of-interest disclosure: N.E. reports financial support from Pharmacyclics, Seattle Genetics, BeiGene, TG Therapeutics, Novartis, and Incyte. D.J.L. reports financial support from MorphoSys, Calithera, Karyopharm, Epizyme, Curis Inc, ADC Therapeutics, and Triphase. M.E.H. reports financial support from AbbVie, Acerta Pharma, AstraZeneca, Genzyme, and Karyopharm. G.S.N. NanoString, Roche, TG Therapeutics, Zai Lab, Bristol Myers Squibb (BMS)/Celgene, Selvita, Ryvu Therapeutics, MorphoSys US Inc, Kymera Therapeutics, Kite Pharma Inc, Karyopharm, Incyte, Genentech Inc, F. Hoffmann-La Roche Ltd, Daiichi Sankyo Inc, Curis Inc, Celgene Corporation/BMS, Blueprint Medicines Corporation, and Bantam Pharmaceutical. J.S.-S. served on the speaker’s bureu for Seagen and consulted for (advisory boards) TG Therapeutics, AbbVie, ADC therapeutics, Janssen, MorphoSys/Incyte, and MassiveBio. S.K.K. reports financial support from Radyus Research, Karyopharm, and Incyte Pharmaceuticals. M.L.X. reports financial support from Pure Marrow and Blueprint Medicines. P.T. reports financial support from TG Therapeutics, Genentech, ADC Therapeutics, Epizyme, Targeted Oncology, Physician Education Review, and Lilly USA. S.D.S. reports financial support from Portola Pharmaceuticals, Numab Therapeutics AG, Nanjing Pharmaceuticals Co Ltd, MorphoSys, Merck Sharp/Dohme Corp, Kymera Therapeutics, KITE pharma, Karyopharm, Incyte Corporation, Genentech, Epizyme, Enterome, Denovo Biopharma, BeiGene, Bayer, AstraZeneca, ADC Therapeutics, AbbVie, and Viracta Therapeutics. D.A.B. reports financial support from Kite/Gilead, Seagen, Novartis, and Nurix Therapeutics. U.F. reports financial support from Kite, a Gilead Company, MorphoSys, Caribou pharma, and Checkmate Pharma. M.K. reports financial support from AbbVie, AstraZeneca, Celgene/Bristol Myers Squibb, Adaptive Biotechnologies, ADC Therapeutics, BeiGene, Impact Bio, TG Therapeutics, Novartis, Seagen, Celgene, and Genentech. B.H. reports financial support from Viracta Therapeutics and BMS. R.K. reports financial support from Kite, BMS/Celgene, Eusa, Takeda, Pharmacyclics, Genentech/Roche, Karyopharm, BeiGene, Calithera, MorphoSys/Incyte, and AstraZeneca. J.M.V. reports financial support from AstraZeneca, Janssen, Lilly, AbbVie, Johnson & Johnson, Daiichi Sankyo, Pharmacyclics, MorphoSys, and Kite (a Gilead Company). A.J.O. reports financial support from Genmab, Precision Bio, Adaptive Biotechnologies, Celldex, Acrotech Biopharma, Schrodinger, TG Therapeutics, and Genentech. The remaining authors declare no competing financial interests.
Correspondence: Adam J. Olszewski, Department of Medicine, Warren Alpert Medical School of Brown University, Rhode Island Hospital, 593 Eddy St, Providence, RI 02903; e-mail: adam_olszewski@brown.edu.
References
Author notes
Presented in abstract form at the 63rd American Society of Hematology Annual Meeting and Exposition (11 December 2021 to 14 December 2021).
Data are available on request from the corresponding author, Adam J. Olszewski (adam_olszewski@brown.edu). To protect patient privacy, individual participant data cannot be shared.
The full-text version of this article contains a data supplement.