Key Points
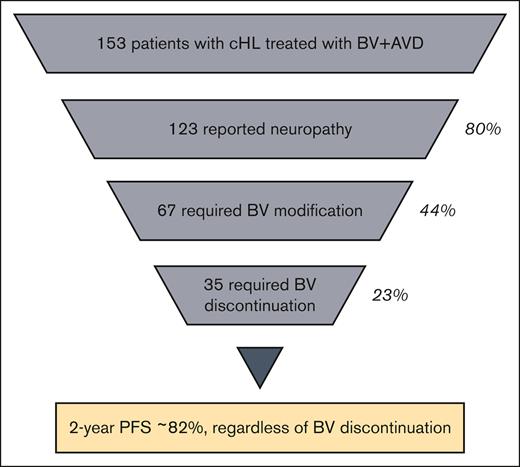
Most patients receiving BV plus AVD reported neuropathy, leading to BV discontinuation in 23%, a rate higher than that described in trials.
BV discontinuation and/or dose reduction from neuropathy did not lead to inferior 2-year PFS.
Abstract
Brentuximab vedotin (BV) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) is increasingly used for frontline treatment of stage III/IV classical Hodgkin lymphoma (cHL). Peripheral neuropathy (PN) was the most common and treatment-limiting side effect seen in clinical trials but has not been studied in a nontrial setting, in which clinicians may have different strategies for managing it. We conducted a multisite retrospective study to characterize PN in patients who received BV + AVD for newly diagnosed cHL. One hundred fifty-three patients from 10 US institutions were eligible. Thirty-four patients (22%) had at least 1 ineligibility criteria for ECHELON-1, including stage, performance status, and comorbidities. PN was reported by 80% of patients during treatment; 39% experienced grade (G) 1, 31% G2, and 10% G3. In total, BV was modified in 44% of patients because of PN leading to BV discontinuation in 23%, dose reduction in 17%, and temporary hold in 4%. With a median follow-up of 24 months, PN resolution was documented in 36% and improvement in 33% at the last follow-up. Two-year progression-free survival (PFS) for the advanced-stage patients was 82.7% (95% confidence interval [CI], 0.76-0.90) and overall survival was 97.4% (95% CI, 0.94-1.00). Patients who discontinued BV because of PN did not have inferior PFS. In the nontrial setting, BV + AVD was associated with a high incidence of PN. In our cohort, which includes patients who would not have been eligible for the pivotal ECHELON-1 trial, BV discontinuation rates were higher than previously reported, but 2-year outcomes remain comparable.
Introduction
Based on results of the ECHELON-1 trial, brentuximab vedotin (BV) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) has become a commonly used regimen for newly diagnosed, advanced-stage classical Hodgkin lymphoma (cHL).1,2 BV is an antibody-drug conjugate (ADC) comprising an anti-CD30 antibody conjugated to the microtubule disrupting agent monomethyl auristatin E (MMAE); it was previously approved for and used in relapsed/refractory cHL.3,4 With longer follow-up of ECHELON-1, BV + AVD demonstrated a superior 6-year overall survival (OS) of 93.9% compared with 89.4% of the previous standard of care: doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).1 This OS benefit will likely make BV + AVD the new standard of care for frontline treatment of advanced cHL, increasing its use across academic and community centers.
Although BV + AVD has lower rates of pulmonary toxicity because of the elimination of bleomycin, it carries unique toxicities, namely cytopenias and peripheral neuropathy (PN).2 Because cytopenias can be mitigated with the administration of primary granulocyte-colony stimulating factor prophylaxis, neuropathy is often the dose-limiting side effect of BV + AVD.
PN is a well-known toxicity of BV and appears to be a class effect among ADCs with MMAE payloads, including polatuzumab vedotin, enfortumab vedotin, and tisotumab vedotin.5-8 The leading hypothesis for the mechanism of BV-associated neuropathy implicates nonspecific ADC uptake by CD30– cells, leading to MMAE–mediated microtubule toxicity.9-11 In the landmark AETHERA trial that led to the approval of BV maintenance for relapsed/refractory cHL after autologous stem cell transplant, 56% of patients reported neuropathy (13% grade ≥3), leading to BV discontinuation in 23%.3 Retrospective studies of BV monotherapy have reported rates of neuropathy ranging from 9% to 50%.12-15 However, PN from BV + AVD tends to be more common despite shorter treatment times, because of the compounding neurotoxic effect of vinblastine and the more frequent dosing interval. In ECHELON-1, the overall rate of PN in the BV + AVD group was 67% (11% grade ≥3), leading to drug discontinuation in 6.6% of patients. PN improved or resolved in the majority (85%) of the patients who developed it, but at 5 years, 19% of patients in the BV + AVD arm had ongoing neuropathy.16
To date, there have been no studies outside of prospective clinical trials examining neuropathy from BV + AVD for newly diagnosed cHL. In the nontrial setting, physicians may treat patients with BV + AVD who would not have been eligible for ECHELON-1, including those with preexisting neuropathy or conditions that may predispose them to neuropathy, such as HIV disease. Furthermore, clinicians may have different strategies for managing neuropathy outside of the protocolized setting of a trial. We aimed to understand the real-world incidence of PN in this population, how BV + AVD treatment is modified to mitigate neuropathy, and the impact of these modifications on outcomes for newly diagnosed cHL.
Methods
Study design
We performed a multisite, retrospective cohort study to characterize PN in patients receiving BV + AVD for newly diagnosed cHL at 10 US cancer centers between 1 January 2018 and 1 March 2022. All data were extracted from the electronic medical records of the patients. The study was approved by the institutional review boards of each participating site and conducted according to the Declaration of Helsinki.
Patients
Patients were included if they had biopsy-proven diagnosis of cHL and were planned to receive 6 cycles BV + AVD for newly diagnosed cHL, regardless of stage and comorbidities. Patients were excluded if they were planned to receive <6 cycles of treatment, did not have sufficient data available for analysis, or had not completed treatment before the data cutoff date (1 September 2022).
Outcomes and definitions
The primary end point for the study was the incidence of PN, defined as new or worsening neuropathy attributable to BV + AVD per the treating physician. Secondary outcomes included treatment modification because of neuropathy or other causes (including discontinuation, dose reduction, and dose hold), risk factors associated with neuropathy, medical management of neuropathy, and neuropathy resolution/improvement at last follow-up. Overall response rate, progression-free survival (PFS), and OS were additional secondary outcomes. Response to therapy was assessed by the treating physician according to Lugano criteria.17 PFS and OS were respectively defined as the time from treatment initiation to progression or death and the time from treatment initiation to death, both assessed by the treating physician.
Data collection
Demographic and clinical data (age, gender, weight, Eastern Cooperative Oncology Group [ECOG] performance status, baseline comorbidities, cHL stage, and International Prognostic Score [IPS] for Hodgkin Lymphoma18) were collected from the electronic medical records of the patients. Neuropathy characteristics (incidence, grade, distribution, medications used for treatment, and status at last follow-up) and treatment course (BV and vinblastine discontinuation, dose reductions, and dose holds, as well as total dose received and subsequent therapy if applicable) were also captured. Grading was performed retrospectively using descriptions in clinical documentation and the Common Terminology Criteria for Adverse Effect (CTCAE) version 5.0.
Statistical analysis
Univariate logistic regression was used to assess factors associated with PN. The Bonferroni correction was used to address the problem of multiple comparisons.19 We tested 6 factors, resulting in a significance threshold of P = .008. Selected factors from this analysis were then combined into multivariate logistic regression. In these regressions, initial dose of BV (in mg) was modeled as a continuous variable. Initial dose of BV in mg was calculated using starting dose recorded in mg per kg and patient weight at the start of treatment. We assumed doses were capped at 120 mg, given this recommendation in the prescribing information for BV; this was the general practice at all participating institutions.
PFS and OS were calculated using the Kaplan-Meier method. Univariate Cox proportional hazards models were created to assess the effects of all-cause and neuropathy-specific BV discontinuation as well as BV dose reduction on PFS. To address potential bias because of patients who progressed or died on treatment before the potential development of neuropathy, a sensitivity analysis was performed using only the patients who completed the entire course of therapy (n = 144). All statistical analysis was conducted using R software, version 4.2.2.20,21
Results
Characteristics of patients with cHL receiving BV + AVD
A total of 153 patients across 10 institutions who were planned to receive 6 cycles of BV + AVD for newly diagnosed cHL before September 2022 were identified. Patient characteristics are shown in Table 1. The cohort was 61% male, with a median age of 35 years (range, 18-76), and 22% of the patients were aged >60 years. Most patients receiving BV + AVD had advanced-stage disease (92%); however, a minority of patients (8%) had stage I-II disease at the time of diagnosis. For advanced-stage patients, 41% had a high-risk IPS of 4 to 7, corresponding with worse prognosis.
Baseline characteristics of patients with cHL treated with BV + AVD
| Characteristics . | Overall (n = 153), n (%) . |
|---|---|
| Median age, y (range) | 35 (18-76) |
| >60 y | 33 (22%) |
| Sex | |
| Male | 93 (61%) |
| cHL stage | |
| I-II | 13 (8%) |
| III-IV | 140 (92%) |
| Histology | |
| Nodular sclerosis | 101 (66%) |
| Mixed cellularity | 18 (12%) |
| Lymphocyte-rich | 7 (5%) |
| Unknown/not available | 27 (18%) |
| ECOG status | |
| 0 | 80 (52%) |
| 1 | 58 (38%) |
| 2 | 10 (7%) |
| 3-4 | 4 (3%) |
| IPS (advanced-stage patients only) | |
| 0-3 | 82 (59%) |
| 4-7 | 57 (41%) |
| Comorbidities | |
| PN | 9 (6%) |
| Diabetes mellitus | 8 (5%) |
| Pulmonary disease | 15 (10%) |
| HIV infection | 7 (5%) |
| Cardiovascular disease | 16 (10%) |
| None | 86 (56%) |
| Meets ≥1 exclusion criteria for ECHELON-1 | 34 (22%) |
| Median number of cycles BV + AVD (range) | 6 (1-6) |
| Median starting dose, mg/kg (range) | 1.2 (0.9-1.2) |
| Median number of treatments BV received (range) | 11 (1-12) |
| Characteristics . | Overall (n = 153), n (%) . |
|---|---|
| Median age, y (range) | 35 (18-76) |
| >60 y | 33 (22%) |
| Sex | |
| Male | 93 (61%) |
| cHL stage | |
| I-II | 13 (8%) |
| III-IV | 140 (92%) |
| Histology | |
| Nodular sclerosis | 101 (66%) |
| Mixed cellularity | 18 (12%) |
| Lymphocyte-rich | 7 (5%) |
| Unknown/not available | 27 (18%) |
| ECOG status | |
| 0 | 80 (52%) |
| 1 | 58 (38%) |
| 2 | 10 (7%) |
| 3-4 | 4 (3%) |
| IPS (advanced-stage patients only) | |
| 0-3 | 82 (59%) |
| 4-7 | 57 (41%) |
| Comorbidities | |
| PN | 9 (6%) |
| Diabetes mellitus | 8 (5%) |
| Pulmonary disease | 15 (10%) |
| HIV infection | 7 (5%) |
| Cardiovascular disease | 16 (10%) |
| None | 86 (56%) |
| Meets ≥1 exclusion criteria for ECHELON-1 | 34 (22%) |
| Median number of cycles BV + AVD (range) | 6 (1-6) |
| Median starting dose, mg/kg (range) | 1.2 (0.9-1.2) |
| Median number of treatments BV received (range) | 11 (1-12) |
Forty-four percent of patients had some comorbidity at diagnosis, including broadly defined cardiovascular disease in 10% and pulmonary disease in 10%. Notably, 9 patients (6%) had preexisting PN at baseline, most commonly because of diabetes mellitus (3 patients). Thirty-four patients (22%) in our cohort would have been ineligible for the pivotal ECHELON-1 based on ≥1 exclusion criteria, including stage (8% early stage), performance status (3% ECOG 3+), preexisting neuropathy (6%), and other comorbidities, including history of other malignancies and HIV infection (10%).
The median number of cycles of BV + AVD received was 6 (range, 1-6). Out of the 136 patients for whom BV dosage data were available, 133 (98%) received a starting dose of BV of 1.2 mg/kg. Assuming a planned cumulative dose of 14.4 mg/kg (12 treatments × 1.2 mg/kg), 66% of the patients received at least 75% of the planned total dose of BV.
Incidence, characteristics, and risk factors for BV + AVD neuropathy
Overall, 123 patients (80%) developed PN during BV + AVD treatment (Table 2). The median time to onset of PN was 2 months (range, 0.23-13); grade 1 neuropathy was reported in 60 patients (39%), grade 2 in 47 (31%), and grade 3 in 15 (10%). In nearly all cases, PN was either sensory predominant (74%) or mixed sensory and motor (25%), with the caveat that the quality of neuropathy could not be ascertained by chart review in 7% of the patients.
Incidence and characteristics of neuropathy from BV + AVD
| Characteristic . | Overall (n=153), n (%) . |
|---|---|
| Any neuropathy | 123 (80%) |
| Maximal grade | |
| 1 | 60 (39%) |
| 2 | 47 (31%) |
| 3 | 15 (10%) |
| Distribution | |
| Sensory only | 84 (55%) |
| Motor only | 1 (1%) |
| Mixed sensory and motor | 29 (19%) |
| Unknown | 9 (6%) |
| Median time to onset, mo (range) | 2 (0.23-13) |
| Characteristic . | Overall (n=153), n (%) . |
|---|---|
| Any neuropathy | 123 (80%) |
| Maximal grade | |
| 1 | 60 (39%) |
| 2 | 47 (31%) |
| 3 | 15 (10%) |
| Distribution | |
| Sensory only | 84 (55%) |
| Motor only | 1 (1%) |
| Mixed sensory and motor | 29 (19%) |
| Unknown | 9 (6%) |
| Median time to onset, mo (range) | 2 (0.23-13) |
Neuropathy was graded retrospectively using modified CTCAE v5.0 criteria (grade 1: asymptomatic or mild symptoms, including paresthesias or observed decreased deep tendon reflexes; grade 2: moderate symptoms, limiting function or instrumental activity of daily living (ADL); grade 3: severe symptoms, limiting self-care ADL).
In univariate logistic regression examining the risk factors for the development of PN of any grade, no factors examined (age, sex, stage, IPS, baseline neuropathy, and diabetes mellitus) had a significant association. However, higher total BV dose (in mg) during initial infusion was significantly associated with the risk of grade ≥2 neuropathy in univariate analysis: the odds ratio (OR) per 10 mg increase in BV dose was 1.31 (95% confidence interval [CI], 1.10-1.58; P = .002). This association held in the multivariate regression when adjusted for age, baseline neuropathy, and diabetes mellitus (OR, 1.34; 95% CI, 1.12-1.62; P = .002). Patients receiving an initial dose of BV ≥80 mg were significantly more likely to develop grade 2+ PN (OR, 3.57; 95% CI, 1.61-8.65; P = .001). In the multivariate regression, age was not significantly associated with the risk of grade 2+ neuropathy (OR, 1.02; 95% CI, 1.00-1.04; P = .13).
Management of neuropathy associated with BV + AVD
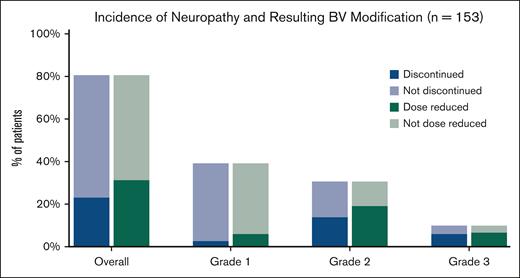
The primary strategy for management of neuropathy was discontinuation or dose reduction of BV. In total, BV was modified in 67 patients (44%) owing to neuropathy. This resulted in BV discontinuation in 35 patients (23%), dose reduction alone in 26 (17%), and hold of ≥1 doses in 6 (4%) (Table 3). Discontinuation and dose reduction rate by neuropathy grade can be seen in Figure 1. Of the 15 patients who had grade 3 neuropathy, treatment was discontinued in 9, dose was reduced in 3, and held and then dose reduced in 3. In patients for whom BV was discontinued, the number of treatments omitted was 4 (range, 1-10) or 33% of the total planned dose. In patients for whom BV dose was reduced, the majority were reduced to 0.9 mg/kg (in 32/52 patients) and fewer to 0.6 mg/kg (7 patients) or 0.8 mg/kg (6 patients). As a result, in these patients, a median of 15% of the planned total dose of BV was not administered (range, 4%-27%). BV was discontinued for a reason other than neuropathy in 24 patients (16%), including pneumonitis (8 patients, 5%), infection (5 patients, 3%), and cytopenias (5 patients, 3%).
Neuropathy management strategies in patients receiving BV + AVD
| Treatment modification . | Overall (n = 153), n (%) . |
|---|---|
| BV modification | 67 (44%) |
| Dose reduction | 26 (17%) |
| Discontinuation | 19 (12%) |
| Dose reduction followed by discontinuation | 16 (11%) |
| Dose hold followed by dose reduction | 6 (4%) |
| Vinblastine modification | 26 (17%) |
| Hold or discontinuation | 16 (10%) |
| Dose reduction | 10 (7%) |
| BV continued | 9 (6%) |
| Treatment modification . | Overall (n = 153), n (%) . |
|---|---|
| BV modification | 67 (44%) |
| Dose reduction | 26 (17%) |
| Discontinuation | 19 (12%) |
| Dose reduction followed by discontinuation | 16 (11%) |
| Dose hold followed by dose reduction | 6 (4%) |
| Vinblastine modification | 26 (17%) |
| Hold or discontinuation | 16 (10%) |
| Dose reduction | 10 (7%) |
| BV continued | 9 (6%) |
Because BV modification was our primary interest, more granular data were collected about discontinuation and dose reduction and the temporal relationship between these events than for vinblastine.
Incidence of PN by grade and resulting discontinuation/dose reduction in patients receiving BV + AVD for newly diagnosed Hodgkin lymphoma (n = 153). Dark blue sections represent patients in each group for whom the BV component of treatment was discontinued as a result of neuropathy (median number of treatments omitted = 4) and dark green represent those for whom BV dose was reduced.
Incidence of PN by grade and resulting discontinuation/dose reduction in patients receiving BV + AVD for newly diagnosed Hodgkin lymphoma (n = 153). Dark blue sections represent patients in each group for whom the BV component of treatment was discontinued as a result of neuropathy (median number of treatments omitted = 4) and dark green represent those for whom BV dose was reduced.
In a small subset of patients (26/153; 17%), neuropathy was managed by modifying vinblastine. BV was continued at a reduced dose in 6 patients. In 3 patients, BV was continued at full dose and vinblastine modification was the only strategy for mitigating neuropathy. In 16 of 26 patients for whom vinblastine was modified (10% of total), it was temporarily held or discontinued, whereas, in the other 10 (7%), the dose was reduced.
Half of patients with neuropathy (62/123) were prescribed medications for symptom management (Table 4). The most commonly prescribed medication was gabapentin in 53 patients (43%), followed by duloxetine (12 patients, 10%) and vitamin B supplementation (12 patients, 10%). A minority of patients were documented to have tried alternatives to medication for neuropathy alleviation, including acupuncture (3%) and exercise therapy (3%).
Medical management of neuropathy from BV + AVD
| Medication or therapy . | Patients with neuropathy (n = 123), n (%) . |
|---|---|
| Gabapentin | 53 (43%) |
| Duloxetine or other SNRI | 12 (10%) |
| Vitamin B complex | 12 (10%) |
| Pregabalin | 8 (7%) |
| Exercise therapy | 5 (4%) |
| Alpha lipoic acid | 4 (3%) |
| Acupuncture | 5 (4%) |
| Amitryptiline or other TCA | 3 (2%) |
| Other | 7 (6%) |
| None | 48 (39%) |
| Medication or therapy . | Patients with neuropathy (n = 123), n (%) . |
|---|---|
| Gabapentin | 53 (43%) |
| Duloxetine or other SNRI | 12 (10%) |
| Vitamin B complex | 12 (10%) |
| Pregabalin | 8 (7%) |
| Exercise therapy | 5 (4%) |
| Alpha lipoic acid | 4 (3%) |
| Acupuncture | 5 (4%) |
| Amitryptiline or other TCA | 3 (2%) |
| Other | 7 (6%) |
| None | 48 (39%) |
Numbers may not sum to 100% as in some cases, patients were prescribed more than 1 medication. SNRI, serotonin and norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Neuropathy resolution
The median follow-up time in the cohort was 24 months (range, 0.33-87). Among patients with neuropathy, data were available regarding the PN status of the patient at the last follow-up for 116 (94%). Of these patients, neuropathy had resolved in 41 (35%) or improved by at least 1 grade in 44 (38%). Complete resolution was more common in patients who had less severe toxicity: 45% of those with grade 1 neuropathy had resolution vs 26% with grade 2 and 13% with grade 3 neuropathy.
In the remaining 31 patients (27%), neuropathy was unchanged at the last follow-up. Thus, in total, 75 patients (65%) had persistent neuropathy. Twenty patients (13% of all patients) had ongoing grade 2+ neuropathy at the last follow-up. Three of these patients had preexisting neuropathy before receiving BV + AVD. Median follow-up time for those with ongoing grade 2+ neuropathy was 23 months, in line with the overall cohort. Patients with >12 months of follow-up were numerically more likely to have neuropathy improvement or resolution than those with shorter follow-up (72% vs 55%, P = .13).
Outcomes from BV + AVD treatment
A secondary aim of the study was to characterize treatment outcomes in patients receiving BV + AVD for newly diagnosed cHL. Regarding best response to treatment, 136 patients (89%) attained a complete response (CR), 9 patients (6%) had a partial response, 1 had stable disease, and 6 had progressive disease. Response data were unavailable for 1 patient. Five patients died on treatment, with sepsis or septic shock given as the cause of death in 4 patients.
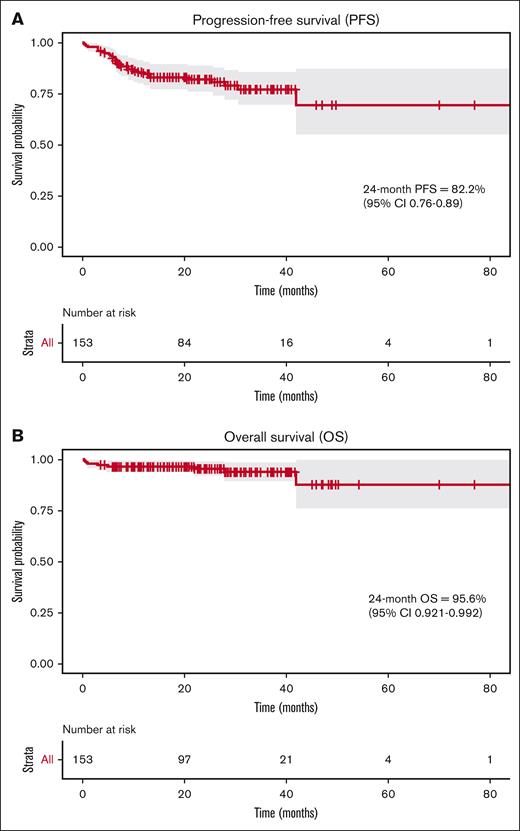
Two-year PFS for the entire cohort was 82.2% (95% CI, 0.76-0.89) and 2-year OS was 95.6% (95% CI, 0.92-0.99) (Figure 2). Outcomes were similar for the advanced-stage subgroup (140/153 patients), with 2-year PFS of 82.7% (95% CI, 0.76-0.90), and OS of 97.4% (95% CI 0.94-1). For patients who progressed on BV + AVD and were candidates for further treatment (n = 22), all received a non-BV–containing salvage regimen initially, but 2 patients went on to receive a subsequent BV-containing regimen after later relapse.
Two-year survival outcomes for patients treated with BV + AVD for newly diagnosed cHL (n = 153). (A) PFS is shown. (B) OS is shown. Survival times were calculated using the Kaplan-Meier method and progression was defined by treating physician notes.
Two-year survival outcomes for patients treated with BV + AVD for newly diagnosed cHL (n = 153). (A) PFS is shown. (B) OS is shown. Survival times were calculated using the Kaplan-Meier method and progression was defined by treating physician notes.
We also tested whether development of neuropathy or discontinuation of either BV or vinblastine would lead to inferior 2-year PFS. Because patients who died or progressed on treatment may not have been treated long enough to develop neuropathy or require treatment modification, we limited these analyses to the subgroup of patients who completed the entire treatment course (n = 144). In this group, presence of neuropathy and discontinuation of BV because of neuropathy were not associated with inferior survival (supplemental Table 1). The 2-year PFS for patients who completed treatment but discontinued BV early was 84.7% (95% CI, 0.73-0.98) compared with 88.2% for those who did not discontinue BV (95% CI, 0.82-0.95). Similarly, dose reduction of BV and modification of vinblastine were not associated with worse 2-year PFS.
Discussion
In this retrospective cohort study, we sought to characterize the incidence and management of PN from BV + AVD in patients with newly diagnosed cHL. We found a high incidence of neuropathy, with 80% of the patients in our cohort developing any neuropathy attributable to treatment. This rate is numerically higher than the overall rate of neuropathy reported in the pivotal ECHELON-1 trial (67%). This difference may be explained by the presence of older patients with more baseline comorbidities like PN and HIV, both of which were excluded from that study.
A key finding of this study is that BV was discontinued in 23% of the patients because of neuropathy, leading to a median 4 omitted treatments of BV. This discontinuation rate is higher than previously described, including the 6.6% discontinuation rate owing to neuropathy in the ECHELON-1 study. This finding suggests that physicians treating outside a trial have a lower threshold for drug discontinuation, which could have implications for efficacy of the overall regimen in the real world. Despite this high rate of discontinuation, this study finds no decrement in efficacy. We observed excellent 2-year outcomes in line with ECHELON-1 and did not detect a significant impact of discontinuation on 2-year PFS.
Unsurprisingly, in the nontrial setting, there was significant variability in the management of neuropathy across institutions and patients. Some physicians maintained the dose frequency and intensity, whereas others made dose reductions even in the setting of low-grade toxicity, and a minority opted to modify vinblastine rather than BV. These practices often differed from the management algorithm in BV prescribing information, which recommends dose reduction for grade 2 neuropathy and therapy hold for grade 3 toxicity.22 This study suggests a more conservative approach can be safely taken without an impact on outcomes.
In our cohort, half of patients with neuropathy were prescribed some medication for symptom management, most often gabapentin. Only 12 patients were prescribed duloxetine, the only guideline-recommended medication for chemotherapy-induced PN. These findings suggest a gap between evidence and practice in treatment of chemotherapy-induced PN. Moreover, very few patients used acupuncture or exercise therapy, interventions with less conclusive evidence supporting their use but minimal associated harms.23 Because we did not collect temporal data about the therapies prescribed, we were unable to analyze whether certain medications used in our study were associated with resolution or treatment of neuropathy. Our findings support the notion that neuropathy from BV + AVD resolves or improves over time in most patients but can lead to persistent symptoms in a minority, evidenced by the 13% with ongoing grade ≥2 neuropathy at the last follow-up.
Interestingly, we found that a minority of patients treated with BV + AVD had early-stage (stage I/II) disease, despite the regimen being approved only for advanced-stage patients. Although the rationale for opting for BV + AVD over ABVD in these cases was not collected, many of these patients were older or had baseline pulmonary disease, which may have led clinicians to opt for BV + AVD to eliminate the risk of bleomycin-induced pneumonitis. For early-stage patients treated with the regimen, we did not observe a difference in neuropathy characteristics or outcomes. BV has been studied in several phase 2 trials in the early-stage population for newly diagnosed cHL, with favorable initial results.24-26
Outside of neuropathy, one surprising toxicity observed in our study was suspected BV-associated pneumonitis. Despite a low incidence of pulmonary toxicity in the BV + AVD arm of ECHELON-1 (2%), in our cohort, 5% developed pulmonary toxicity requiring BV discontinuation.2 This complication is not well-described, though phase 1 studies of BV noted a high rate of lung injury when BV was added to ABVD (44%). In 1 retrospective study of 123 adult and pediatric patients receiving BV for cHL, 5% developed pneumonitis,27 and several other case reports have detailed the finding.28-31 Our findings emphasize the need to consider BV-associated pneumonitis in patients who develop pulmonary symptoms during treatment.
Our study has several limitations, most notably its retrospective observational design. Relying on treating physician notes may have limited our ability to identify and grade neuropathy accurately, leading to neuropathy underreporting and/or inaccurate grading. Likewise, assessing improvement and resolution of neuropathy retrospectively was challenging given the inconsistent documentation about the status of symptoms during the follow-up period. We mitigated these concerns by only recording data when the status of neuropathy could be ascertained with certainty and otherwise recording “unknown/not available.” Similarly, use of medications for neuropathy may have been underreported, because we relied on physician notes rather than patient medication lists for this data. Lastly, our follow-up was limited to 2 years, which may have limited the ability to determine whether BV discontinuation or dose reduction impacts late relapse as well as track long-term resolution of neuropathy.
Future studies should focus on identifying the underlying mechanism and risk factors for neuropathy associated with BV, which may allow for improved patient selection for this regimen.9,10,32 Studies of MMAE ADCs like BV and polatuzumab vedotin have only identified cumulative dose, patient weight, and serum albumin concentration as risk factors.11,15 Age has been shown to be an important risk factor for neuropathy from traditional chemotherapy (eg, taxanes); however, thus far, this relationship has not been borne out with ADCs, including in this study.33,34 More data on this topic would be helpful given that older patients may not have the same survival benefit from BV + AVD as their younger counterparts.35 Future studies of BV combination regimens may consider omitting vinblastine to minimize toxicity, as has been trialed in patients with limited-stage disease,36 or lowering the dose or number of cycles of BV. Although follow-up is still short, the SWOG S1826 trial recently demonstrated improved PFS and lower rates of neuropathy with nivolumab + AVD than with BV + AVD, which may lead to a new treatment paradigm for newly diagnosed cHL in the future.37 Finally, although our study suggests that treatment modifications because of neuropathy do not have a deleterious effect on outcomes, more work is also needed to identify optimal management strategies for PN to maximize the quality of life of patients during and after treatment.
In conclusion, our results reaffirm the significance of PN as a treatment-limiting and potentially long-lasting toxicity of BV + AVD treatment. Reassuringly, treatment modification or early truncation did not impact clinical outcomes in this study. Clinicians should educate patients about the likelihood for this side effect and monitor for it closely.
Acknowledgment
This work was supported by the Leonard and Estelle Siegel Fund in Hematology and Oncology.
Authorship
Contribution: J.T.B., H.J.B., and J.S. designed the research; J.T.B., J.A., K.A., J.J.P., M.A.S., S.L., J.N., C.B.W., M.D., F.F., and M.E.H. collected the data; S.M.B., N.E., S.G., M.A.S., M.R.M., E.C.A., B.H., S.F.H., N.N.S., E.A.C., S.D.N., S.K.B., D.J.L., and J.S provided clinical input and reviewed the data from their institutions; J.T.B. and C.C. performed statistical analysis; J.T.B. and J.S. wrote the manuscript; and all authors reviewed and critically revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: S.M.B. was a consultant for TG Therapeutics, AbbVie, and Arcellx. N.E. was a consultant for Merck, ADC Therapeutics, Eli Lilly, and Novartis; served on speakers bureaus for Incyte and BeiGene; and received research funding from BeiGene. S.G. was a consultant for ADC Therapeutics, Kite, AstraZeneca, AbbVie, and Genentech. M.A.S. was a consultant for Kite. M.R.M. reports stock ownership in Schrodinger. C.B.W. was a consultant for Pfizer. B.H. was a consultant for Bristol Myers Squibb (BMS), Novartis, GenMab, TG Therapeutics, and Eli Lilly; received honoraria from MJH Life Sciences, BeiGene, Artiva, and DAVA Oncology; received research funding from Genentech, Crispr Therapeutics, MorphoSys, Caribou Biosciences, and Repare Therapeutics; and travel grants from MJH Life Sciences. S.F.H. was a consultant for SeaGen, Janssen, Bayer, Genentech, AbbVie, Flatiron, Novartis, BeiGene, AstraZeneca, ADC Therapeutics, Epizyme, Merck, TG Therapetucis, Tyme, and Arvinas; and received honoraria from SeaGen, Pharmacyclics, and AstraZeneca; research funding from Celgene, DTRM Biopharma, TG Therapeutics, Debiopharm Group, and Agios; and travel grants from Celgene, Bayer, Pharmacyclics and Genentech. N.N.S. was a consultant for Kite, Loxo/Eli Lilly, TG Therapeutics, Incyte, Novartis, Juno, and Janssen; reported stock ownership in Tundra Targeted Therapeutics; and received research funding from Miltenyi Biotec, Loxo/Eli Lilly, and Adaptive Biotechnologies and travel grants from Miltenyi Biotec. M.E.H. was a consultant for AstraZeneca, Genzyme, Janssen, AbbVie, and Karyopharm; and received research funding from Acerta Pharma and Hematology/Oncology Pharmacy Association. E.A.C. was a consultant for Novartis, BeiGene, Kite, Tessa, and Juno. S.D.N. was a consultant for Pharmacyclics, Roche, Rafael, and FortySeven/Gilead. S.K.B. was a consultant for Affimed, Daiichi Sankyo, and Kyowa Kirin and received honoraria from Kyowa Kirin, SeaGen, and Acrotech. D.J.L. was a consultant for ADC Therapeutics, Calithera, Epizyme, and MorphoSys and received research funding from Curis and Triphase. J.S. was a consultant for SeaGen, BMS, AstraZeneca, Pharmacyclics, Adaptive Biotechnologies, and Atara and received research funding from SeaGen, Celgene, Pharmacyclics, Merck, BMS, Incyte, AstraZeneca, and Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Jakub Svoboda, University of Pennsylvania Perelman School of Medicine, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: jakub.svoboda@pennmedicine.upenn.edu.
References
Author notes
Presented in abstract form at the 2023 meeting of the American Society of Clinical Oncology and 2023 International Conference on Malignant Lymphoma.
Deidentified original data are available upon reasonable request from the corresponding author, Jakub Svoboda (jakub.svoboda@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.