Key Points
The R2-ISS system can stratify patients with MM receiving autologous stem cell transplantation.
Demographic and clinical variables (eg, age and platelet count) significantly affect survival prediction accuracy in the R2-ISS model.
Abstract
The Second Revision of the International Staging System (R2-ISS) was published in 2022 and has been validated in several cohorts of patients with multiple myeloma (MM). In this study, we investigated a total of 860 patients with MM who received an upfront autologous stem cell transplantation between 2001 and 2021. The median age of the patients was 60 years, with a median overall survival (OS) of 123 months and median progression-free survival (PFS) of 70 months. We collected the variables included in the ISS, R-ISS, and R2-ISS systems as well as additional standard variables. Our analyses demonstrated that all 3 ISS series systems (ISS, R-ISS, and R2-ISS) exhibited robust discrimination in terms of both OS and PFS among our study cohort. The ISS system effectively stratified patients into 3 risk groups, whereas the R-ISS system accurately identified patients at extremely high or low risk. The R2-ISS system further refined risk stratification by dividing patients into 4 more balanced risk groups. Furthermore, we specifically focused on identifying variables that distinguished patients with OS < 3 years and OS > 10 years within the low-risk R2-ISS stages (I and II) and high-risk R2-ISS stages (III and IV). Our findings revealed that age, hemoglobin, and 1p deletion significantly influenced the classification of patients in the low-risk R2-ISS stage. Additionally, serum light chain, platelet count, age, and the presence of the t(14;16) translocation were found to affect high-risk classification.
Introduction
Multiple myeloma (MM) is a malignant plasma cell disease characterized by significant variability in prognosis. Numerous efforts have been dedicated to the risk stratification of patients with MM, resulting in the development of various models based on clinical and cytogenetic factors.1 Among these prognostic tools, the International Staging System (ISS) series system has gained prominence and widespread usage. The original ISS system was introduced in 2005 and used 2 common clinical serum variables, albumin (ALB) and β2-microglobulin (B2M).2 As additional clinical and cytogenetic risk factors were identified, the ISS system was subsequently revised by incorporating serum lactate dehydrogenase and chromosomal abnormalities (del17p, t(4;14), and t(14;16)). The revised ISS (R-ISS) system demonstrated improved ability in identifying patients with MM with high-risk profiles.3 In 2022, a second revision of the ISS system was implemented and named the R2-ISS system. The R2-ISS system encompasses ISS stage, lactate dehydrogenase, and chromosomal abnormality information (del17p, t(4;14), and +1q); assigns a weighted score to each parameter; and ultimately establishes a well-balanced 4-stage classification system.4 Several clinical centers at Asia and Australia have recently conducted studies to verify the efficacy of the R2-ISS system for risk stratification.5-11 All these studies have consistently demonstrated effective separation of patients based on the R2-ISS classification.
Autologous stem cell transplantation (ASCT) has been used in the treatment of MM since the early 1990s. For patients deemed suitable candidates for transplantation, it is acknowledged that they stand to derive greater therapeutic benefits from ASCT vs conventional chemotherapy.12-15 Because the R2-ISS system was established from multiple centers, including many clinical trials with or without ASCT treatment, it is important to determine whether this R2-ISS system is still appropriate for stratifying patients with MM treated with ASCT. Therefore, we analyzed a cohort of 860 patients with MM who received an upfront ASCT at a single center. Our analysis involved examining the distribution of ISS and R-ISS stages within the R2-ISS stages. Univariate and multivariate Cox regression analyses were performed to identify risk factors associated with ASCT in patients with MM. Additionally, we conducted a comparative analysis between patients at both the low-risk R2-ISS stages (I and II) and the high-risk R2-ISS stages (III and IV) who exhibited overall survival (OS) <3 years and OS >10 years, with a focus on exploring risk factors that might further refine the classification provided by the R2-ISS system.
Methods
Data source
We analyzed data on 860 patients with MM who received ASCT at the University of Arkansas for Medical Science (UAMS) (median follow-up time, 90.9 months; Q1, 49.0 and Q3, 133.1). These patients were diagnosed with MM during 2000-2018; the median age at transplant was 61 years (range, 30-80 years). All patients were tested for 1q amplification/gain (1q+) and 17p del by fluorescence in situ hybridization (FISH) and had gene expression profiling (GEP), which was used to predict chromosome translocations. Demography and clinical data included sex, race, age, isotype, serum light chain type, urine light chain type, bone marrow plasma cell percentage, albumin, B2M, lactate dehydrogenase, creatine, C-reactive protein, hemoglobin, platelet, monocyte, lymphocyte, serum M protein level, urine M protein level, and calcium. Cytogenetic data included del 1p, del17p, +1q, and chromosol translocations (t(4;14), t(14;16), t1(4;20)) predicted by GEP “spiked” expression.16-19 The detailed methods for FISH, GEP, and predicting chromosomal translocations were published previously.20,21 Data were collected at the time of the first MM diagnosis at UAMS before treatment. The collection of all data was approved by the institutional review board of the University of Arkansas for Medical Sciences, and written informed consent was obtained from all subjects for the procurement of samples.
Statistical analysis
For calculations of OS and progression-free survival (PFS), we used the first chemotherapy date at our institution as the start time point. Progression events were defined by the treating physician.
For missing values, the mice package in R4.0.5 was used for multiple imputation. The missing rate of each variable is shown in supplemental Table 1. Supplemental Table 2 shows patient information before and after imputation.
For statistical analysis, CBCgrps,22 survival, and survminer package in R4.0.5 were applied for baseline information, survival curves, and univariable and multivariable Cox analysis. For continuous variables, we transferred the variables into classification variables based on clinical standard (supplemental Table 3).
Results
Patient characteristics at diagnosis
A total of 860 patients with MM who received ASCT and had GEP and FISH data available were analyzed in this study. The median OS time is 90.9 months (Q1, 49.0 and Q3, 133.1), and the median PFS time is 65.4 months (Q1, 29.6 and Q3, 111.7). The median age of these patients was 60.4 years (Q1, 53.0 and Q3, 66.3). Of the total, 39% of patients were female and 61% were male. Immunoglobulin G (IgG; 59%) was the most common isotype, followed by IgA (21%). In total, 17% of these patients were classified with R2-ISS stage I, 27% stage II, 39% stage III, and 17% stage IV. Table 1 shows patient demographic data and laboratory and clinical parameters collected at diagnosis. We used multiple imputations for missing data in the analyses.
Features of patients with MM based on R2-ISS
| Variables . | Total (N = 860) . | I (n = 144) . | II (n = 231) . | III (n = 338) . | IV (n = 147) . | P . |
|---|---|---|---|---|---|---|
| Sex, n (%) | .003 | |||||
| Female | 335 (39) | 54 (38) | 86 (37) | 118 (35) | 77 (52) | |
| Male | 525 (61) | 90 (62) | 145 (63) | 220 (65) | 70 (48) | |
| Race, n (%) | .658 | |||||
| Asian | 3 (0) | 1 (1) | 0 (0) | 1 (0) | 1 (1) | |
| Africa American | 101 (12) | 17 (12) | 23 (10) | 43 (13) | 18 (12) | |
| Native American | 2 (0) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | |
| Pacific Islander | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | |
| White | 753 (88) | 126 (88) | 208 (90) | 292 (86) | 127 (86) | |
| Isotype, n (%) | .102 | |||||
| Biclonal disease | 2 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | |
| Free light chain | 152 (18) | 25 (17) | 34 (15) | 60 (18) | 33 (22) | |
| IgA | 177 (21) | 23 (16) | 55 (24) | 67 (20) | 32 (22) | |
| IgD | 9 (1) | 1 (1) | 1 (0) | 3 (1) | 4 (3) | |
| IgG | 506 (59) | 90 (62) | 134 (58) | 205 (61) | 77 (52) | |
| IgM | 3 (0) | 2 (1) | 0 (0) | 1 (0) | 0 (0) | |
| Nonsecretory | 11 (1) | 3 (2) | 5 (2) | 2 (1) | 1 (1) | |
| Light, n (%) | .002 | |||||
| κ | 550 (64) | 106 (74) | 152 (66) | 214 (63) | 78 (53) | |
| κ + λ | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | |
| λ | 300 (35) | 34 (24) | 75 (32) | 122 (36) | 69 (47) | |
| None | 9 (1) | 4 (3) | 3 (1) | 2 (1) | 0 (0) | |
| Urine light, n (%) | <.001 | |||||
| κ | 435 (51) | 75 (52) | 116 (50) | 175 (52) | 69 (47) | |
| κ + λ | 4 (0) | 2 (1) | 1 (0) | 0 (0) | 1 (1) | |
| λ | 246 (29) | 24 (17) | 53 (23) | 109 (32) | 60 (41) | |
| None | 175 (20) | 43 (30) | 61 (26) | 54 (16) | 17 (12) | |
| PCAsp (%), median (Q1, Q3) | 38 (20, 57.62) | 30 (12.38, 41.25) | 33 (17.5, 52) | 45 (28, 63.75) | 39 (22, 60) | < .001 |
| PCBmBx (%), median (Q1, Q3) | 40 (20, 70) | 29 (10, 40) | 40 (15, 60) | 50 (30, 80) | 50 (30, 80) | < .001 |
| Albumin (g/dL), median (Q1, Q3) | 3.8 (3.4, 4.2) | 4.1 (3.8, 4.4) | 3.9 (3.6, 4.3) | 3.6 (3.2, 4.1) | 3.5 (3.2, 3.95) | < .001 |
| B2M (5.5mg/L), median (Q1, Q3) | 3.7 (2.5, 6.1) | 2.42 (1.9, 2.8) | 3 (2.1, 3.95) | 5.9 (3.7, 8.5) | 5.2 (3.6, 7.8) | < .001 |
| Lactate dehydrogenase (Unit/L), median (Q1, Q3) | 152 (127, 185) | 142 (122.5, 164) | 142 (122.5, 166.5) | 152.5 (126.25, 183.5) | 209 (156, 256.5) | < .001 |
| Creatinine (mg/L), median (Q1, Q3) | 1 (0.8, 1.27) | 0.9 (0.7, 1.1) | 0.9 (0.8, 1.1) | 1.1 (0.9, 1.5) | 1 (0.8, 1.4) | < .001 |
| GFR (ml/min/1.73 m2), median (Q1, Q3) | 69.31 (53.9, 84.73) | 82.97 (67.33, 99.57) | 74.46 (63.61, 87.62) | 63.26 (41.26, 76.12) | 58.95 (44.16, 79.98) | < .001 |
| CRP (mg/L), median (Q1, Q3) | 4.5 (2.21, 6.6) | 4.5 (1.1, 4.5) | 4.5 (4.5, 5.85) | 4.5 (2.67, 7.85) | 4.5 (3.95, 10.1) | .005 |
| Hb (g/dL), mean ± SD | 11.22 ± 2.04 | 12.61 ± 1.65 | 11.84 ± 1.76 | 10.62 ± 2.05 | 10.25 ± 1.74 | < .001 |
| Platelets (10^9/L), median (Q1, Q3) | 219 (171, 269) | 237.5 (198, 277.75) | 231 (188, 280) | 209.5 (163, 257) | 184 (128.5, 251) | < .001 |
| Monocytes (%), median (Q1, Q3) | 8.2 (6.3, 10.62) | 7.9 (6.4, 10.22) | 8.1 (6.45, 10.65) | 8.3 (6.3, 10.6) | 8.6 (6.2, 11.2) | .776 |
| Lymphocytes (%), median (Q1, Q3) | 29.4 (21, 38.1) | 28.7 (22.08, 38.7) | 29.4 (21.5, 37.65) | 30.35 (21.12, 38.9) | 28.7 (20, 34.95) | .468 |
| SM (g/L), median (Q1, Q3) | 2.5 (0.5, 4.2) | 1.7 (0.27, 3.12) | 2.4 (0.7, 3.7) | 3.2 (0.8, 5) | 2.6 (0.36, 4.3) | < .001 |
| UM (g/L), median (Q1, Q3) | 18.5 (0, 730.5) | 0 (0, 226.25) | 0 (0, 395.5) | 246.5 (0, 1434.5) | 206 (0, 1198.5) | < .001 |
| Ca (mmol/L), median (Q1, Q3) | 9.2 (8.8, 9.7) | 9.4 (9, 9.7) | 9.3 (8.9, 9.7) | 9.1 (8.7, 9.8) | 9.1 (8.6, 9.9) | .069 |
| BMI, median (Q1, Q3) | 27.56 (24.7, 31.19) | 27.91 (24.97, 31.41) | 27.54 (24.85, 31.16) | 27.7 (24.83, 31.3) | 26.92 (24.43, 30.68) | .781 |
| MM diagnosis transplant (mth), median (Q1, Q3) | 4.17 (3.23, 5.54) | 4.42 (3.5, 6.02) | 4.07 (3.27, 5.67) | 4.2 (3.27, 5.66) | 3.8 (2.83, 4.98) | .018 |
| OS time by the first chemotherapy cycle (mth), median (Q1,Q3) | 90.9 (49.02, 133.08) | 115.3 (81.71, 153.45) | 100.93 (64.5, 138.48) | 82.85 (42.61, 129.33) | 63.37 (29.93, 106.75) | < .001 |
| OS, n (%) | < .001 | |||||
| 0 | 407 (47) | 92 (64) | 135 (58) | 138 (41) | 42 (29) | |
| 1 | 453 (53) | 52 (36) | 96 (42) | 200 (59) | 105 (71) | |
| PFS time by the first chemotherapy cycle (mth), median (Q1, Q3) | 65.35 (29.55, 111.67) | 87.92 (56.36, 131.4) | 71.57 (37.43, 126.88) | 58.62 (24.22, 95.74) | 42.9 (16.23, 82.6) | < .001 |
| PFS, n (%) | < .001 | |||||
| 0 | 302 (35) | 69 (48) | 100 (43) | 104 (31) | 29 (20) | |
| 1 | 558 (65) | 75 (52) | 131 (57) | 234 (69) | 118 (80) | |
| Age at ASCT date (yr), median (Q1, Q3) | 60.42 (52.96, 66.32) | 57.53 (48.77, 64.8) | 61.1 (54.53, 66.55) | 61.06 (54.58, 66.9) | 59.78 (51.49, 66.19) | .002 |
| ISS, n (%) | < .001 | |||||
| I | 292 (34) | 144 (100) | 111 (48) | 31 (9) | 6 (4) | |
| II | 317 (37) | 0 (0) | 120 (52) | 124 (37) | 73 (50) | |
| III | 251 (29) | 0 (0) | 0 (0) | 183 (54) | 68 (46) | |
| t(4;14), n (%) | < .001 | |||||
| 0 | 753 (88) | 144 (100) | 222 (96) | 302 (89) | 85 (58) | |
| 1 | 107 (12) | 0 (0) | 9 (4) | 36 (11) | 62 (42) | |
| t(14;16), n (%) | .062 | |||||
| 0 | 821 (95) | 142 (99) | 223 (97) | 320 (95) | 136 (93) | |
| 1 | 39 (5) | 2 (1) | 8 (3) | 18 (5) | 11 (7) | |
| t(14;20), n (%) | .759 | |||||
| 0 | 837 (97) | 142 (99) | 225 (97) | 328 (97) | 142 (97) | |
| 1 | 23 (3) | 2 (1) | 6 (3) | 10 (3) | 5 (3) | |
| del1p, n (%) | .085 | |||||
| del1p | 176 (20) | 22 (15) | 45 (19) | 69 (20) | 40 (27) | |
| no del | 684 (80) | 122 (85) | 186 (81) | 269 (80) | 107 (73) | |
| R-ISS, n (%) | < .001 | |||||
| I | 204 (24) | 142 (99) | 62 (27) | 0 (0) | 0 (0) | |
| II | 525 (61) | 2 (1) | 169 (73) | 275 (81) | 79 (54) | |
| III | 131 (15) | 0 (0) | 0 (0) | 63 (19) | 68 (46) | |
| +1q21, n (%) | < .001 | |||||
| Gain 1q21 | 322 (37) | 0 (0) | 65 (28) | 128 (38) | 129 (88) | |
| No gain | 538 (63) | 144 (100) | 166 (72) | 210 (62) | 18 (12) | |
| Del 17p, n (%) | <.001 | |||||
| Del 17p | 83 (10) | 0 (0) | 10 (4) | 38 (11) | 35 (24) | |
| No del | 777 (90) | 144 (100) | 221 (96) | 300 (89) | 112 (76) |
| Variables . | Total (N = 860) . | I (n = 144) . | II (n = 231) . | III (n = 338) . | IV (n = 147) . | P . |
|---|---|---|---|---|---|---|
| Sex, n (%) | .003 | |||||
| Female | 335 (39) | 54 (38) | 86 (37) | 118 (35) | 77 (52) | |
| Male | 525 (61) | 90 (62) | 145 (63) | 220 (65) | 70 (48) | |
| Race, n (%) | .658 | |||||
| Asian | 3 (0) | 1 (1) | 0 (0) | 1 (0) | 1 (1) | |
| Africa American | 101 (12) | 17 (12) | 23 (10) | 43 (13) | 18 (12) | |
| Native American | 2 (0) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | |
| Pacific Islander | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | |
| White | 753 (88) | 126 (88) | 208 (90) | 292 (86) | 127 (86) | |
| Isotype, n (%) | .102 | |||||
| Biclonal disease | 2 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | |
| Free light chain | 152 (18) | 25 (17) | 34 (15) | 60 (18) | 33 (22) | |
| IgA | 177 (21) | 23 (16) | 55 (24) | 67 (20) | 32 (22) | |
| IgD | 9 (1) | 1 (1) | 1 (0) | 3 (1) | 4 (3) | |
| IgG | 506 (59) | 90 (62) | 134 (58) | 205 (61) | 77 (52) | |
| IgM | 3 (0) | 2 (1) | 0 (0) | 1 (0) | 0 (0) | |
| Nonsecretory | 11 (1) | 3 (2) | 5 (2) | 2 (1) | 1 (1) | |
| Light, n (%) | .002 | |||||
| κ | 550 (64) | 106 (74) | 152 (66) | 214 (63) | 78 (53) | |
| κ + λ | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | |
| λ | 300 (35) | 34 (24) | 75 (32) | 122 (36) | 69 (47) | |
| None | 9 (1) | 4 (3) | 3 (1) | 2 (1) | 0 (0) | |
| Urine light, n (%) | <.001 | |||||
| κ | 435 (51) | 75 (52) | 116 (50) | 175 (52) | 69 (47) | |
| κ + λ | 4 (0) | 2 (1) | 1 (0) | 0 (0) | 1 (1) | |
| λ | 246 (29) | 24 (17) | 53 (23) | 109 (32) | 60 (41) | |
| None | 175 (20) | 43 (30) | 61 (26) | 54 (16) | 17 (12) | |
| PCAsp (%), median (Q1, Q3) | 38 (20, 57.62) | 30 (12.38, 41.25) | 33 (17.5, 52) | 45 (28, 63.75) | 39 (22, 60) | < .001 |
| PCBmBx (%), median (Q1, Q3) | 40 (20, 70) | 29 (10, 40) | 40 (15, 60) | 50 (30, 80) | 50 (30, 80) | < .001 |
| Albumin (g/dL), median (Q1, Q3) | 3.8 (3.4, 4.2) | 4.1 (3.8, 4.4) | 3.9 (3.6, 4.3) | 3.6 (3.2, 4.1) | 3.5 (3.2, 3.95) | < .001 |
| B2M (5.5mg/L), median (Q1, Q3) | 3.7 (2.5, 6.1) | 2.42 (1.9, 2.8) | 3 (2.1, 3.95) | 5.9 (3.7, 8.5) | 5.2 (3.6, 7.8) | < .001 |
| Lactate dehydrogenase (Unit/L), median (Q1, Q3) | 152 (127, 185) | 142 (122.5, 164) | 142 (122.5, 166.5) | 152.5 (126.25, 183.5) | 209 (156, 256.5) | < .001 |
| Creatinine (mg/L), median (Q1, Q3) | 1 (0.8, 1.27) | 0.9 (0.7, 1.1) | 0.9 (0.8, 1.1) | 1.1 (0.9, 1.5) | 1 (0.8, 1.4) | < .001 |
| GFR (ml/min/1.73 m2), median (Q1, Q3) | 69.31 (53.9, 84.73) | 82.97 (67.33, 99.57) | 74.46 (63.61, 87.62) | 63.26 (41.26, 76.12) | 58.95 (44.16, 79.98) | < .001 |
| CRP (mg/L), median (Q1, Q3) | 4.5 (2.21, 6.6) | 4.5 (1.1, 4.5) | 4.5 (4.5, 5.85) | 4.5 (2.67, 7.85) | 4.5 (3.95, 10.1) | .005 |
| Hb (g/dL), mean ± SD | 11.22 ± 2.04 | 12.61 ± 1.65 | 11.84 ± 1.76 | 10.62 ± 2.05 | 10.25 ± 1.74 | < .001 |
| Platelets (10^9/L), median (Q1, Q3) | 219 (171, 269) | 237.5 (198, 277.75) | 231 (188, 280) | 209.5 (163, 257) | 184 (128.5, 251) | < .001 |
| Monocytes (%), median (Q1, Q3) | 8.2 (6.3, 10.62) | 7.9 (6.4, 10.22) | 8.1 (6.45, 10.65) | 8.3 (6.3, 10.6) | 8.6 (6.2, 11.2) | .776 |
| Lymphocytes (%), median (Q1, Q3) | 29.4 (21, 38.1) | 28.7 (22.08, 38.7) | 29.4 (21.5, 37.65) | 30.35 (21.12, 38.9) | 28.7 (20, 34.95) | .468 |
| SM (g/L), median (Q1, Q3) | 2.5 (0.5, 4.2) | 1.7 (0.27, 3.12) | 2.4 (0.7, 3.7) | 3.2 (0.8, 5) | 2.6 (0.36, 4.3) | < .001 |
| UM (g/L), median (Q1, Q3) | 18.5 (0, 730.5) | 0 (0, 226.25) | 0 (0, 395.5) | 246.5 (0, 1434.5) | 206 (0, 1198.5) | < .001 |
| Ca (mmol/L), median (Q1, Q3) | 9.2 (8.8, 9.7) | 9.4 (9, 9.7) | 9.3 (8.9, 9.7) | 9.1 (8.7, 9.8) | 9.1 (8.6, 9.9) | .069 |
| BMI, median (Q1, Q3) | 27.56 (24.7, 31.19) | 27.91 (24.97, 31.41) | 27.54 (24.85, 31.16) | 27.7 (24.83, 31.3) | 26.92 (24.43, 30.68) | .781 |
| MM diagnosis transplant (mth), median (Q1, Q3) | 4.17 (3.23, 5.54) | 4.42 (3.5, 6.02) | 4.07 (3.27, 5.67) | 4.2 (3.27, 5.66) | 3.8 (2.83, 4.98) | .018 |
| OS time by the first chemotherapy cycle (mth), median (Q1,Q3) | 90.9 (49.02, 133.08) | 115.3 (81.71, 153.45) | 100.93 (64.5, 138.48) | 82.85 (42.61, 129.33) | 63.37 (29.93, 106.75) | < .001 |
| OS, n (%) | < .001 | |||||
| 0 | 407 (47) | 92 (64) | 135 (58) | 138 (41) | 42 (29) | |
| 1 | 453 (53) | 52 (36) | 96 (42) | 200 (59) | 105 (71) | |
| PFS time by the first chemotherapy cycle (mth), median (Q1, Q3) | 65.35 (29.55, 111.67) | 87.92 (56.36, 131.4) | 71.57 (37.43, 126.88) | 58.62 (24.22, 95.74) | 42.9 (16.23, 82.6) | < .001 |
| PFS, n (%) | < .001 | |||||
| 0 | 302 (35) | 69 (48) | 100 (43) | 104 (31) | 29 (20) | |
| 1 | 558 (65) | 75 (52) | 131 (57) | 234 (69) | 118 (80) | |
| Age at ASCT date (yr), median (Q1, Q3) | 60.42 (52.96, 66.32) | 57.53 (48.77, 64.8) | 61.1 (54.53, 66.55) | 61.06 (54.58, 66.9) | 59.78 (51.49, 66.19) | .002 |
| ISS, n (%) | < .001 | |||||
| I | 292 (34) | 144 (100) | 111 (48) | 31 (9) | 6 (4) | |
| II | 317 (37) | 0 (0) | 120 (52) | 124 (37) | 73 (50) | |
| III | 251 (29) | 0 (0) | 0 (0) | 183 (54) | 68 (46) | |
| t(4;14), n (%) | < .001 | |||||
| 0 | 753 (88) | 144 (100) | 222 (96) | 302 (89) | 85 (58) | |
| 1 | 107 (12) | 0 (0) | 9 (4) | 36 (11) | 62 (42) | |
| t(14;16), n (%) | .062 | |||||
| 0 | 821 (95) | 142 (99) | 223 (97) | 320 (95) | 136 (93) | |
| 1 | 39 (5) | 2 (1) | 8 (3) | 18 (5) | 11 (7) | |
| t(14;20), n (%) | .759 | |||||
| 0 | 837 (97) | 142 (99) | 225 (97) | 328 (97) | 142 (97) | |
| 1 | 23 (3) | 2 (1) | 6 (3) | 10 (3) | 5 (3) | |
| del1p, n (%) | .085 | |||||
| del1p | 176 (20) | 22 (15) | 45 (19) | 69 (20) | 40 (27) | |
| no del | 684 (80) | 122 (85) | 186 (81) | 269 (80) | 107 (73) | |
| R-ISS, n (%) | < .001 | |||||
| I | 204 (24) | 142 (99) | 62 (27) | 0 (0) | 0 (0) | |
| II | 525 (61) | 2 (1) | 169 (73) | 275 (81) | 79 (54) | |
| III | 131 (15) | 0 (0) | 0 (0) | 63 (19) | 68 (46) | |
| +1q21, n (%) | < .001 | |||||
| Gain 1q21 | 322 (37) | 0 (0) | 65 (28) | 128 (38) | 129 (88) | |
| No gain | 538 (63) | 144 (100) | 166 (72) | 210 (62) | 18 (12) | |
| Del 17p, n (%) | <.001 | |||||
| Del 17p | 83 (10) | 0 (0) | 10 (4) | 38 (11) | 35 (24) | |
| No del | 777 (90) | 144 (100) | 221 (96) | 300 (89) | 112 (76) |
Ca, calcium; CRP, C-reactive protein; Hb, hemoglobin; GFR, glomerular filtration rate; PCAsp, plasma cell percentage by bone marrow aspiration; PCBmBx, plasma cell percentage by bone marrow biopsy; Q, quartile; SD, standard deviation; SM, serum monoclonal protein; t(4;14), t(4;14) chromosome translocation; t(14;16), t(14;16) chromosome translocation; t(14;20), t(14;20) chromosome translocation; UM, urine monoclonal protein.
Multivariable Cox regression analysis for PFS and OS
| Variables . | HR for PFS . | 95% CI for PFS . | P for PFS . | HR for OS . | 95% CI for OS . | P for OS . |
|---|---|---|---|---|---|---|
| B2M, high | 1.304 | 1.027, 1.656 | .003 | 1.461 | 1.122, 1.901 | .005 |
| Hb, low | 1.298 | 1.047, 1.609 | .017 | 1.289 | 1.020, 1.629 | .034 |
| Age at ASCT date, old | 1.505 | 1.258, 1.800 | <.001 | 1.806 | 1.481, 2.202 | <.001 |
| 1p del | 1.274 | 1.033, 1.571 | .023 | 1.357 | 1.078, 1.710 | .009 |
| 1q21 gain | 1.489 | 1.245, 1.781 | <.001 | 1.523 | 1.249, 1.856 | <.001 |
| 17p del | 1.790 | 1.379, 2.324 | <.001 | 2.154 | 1.625, 2.855 | <.001 |
| Variables . | HR for PFS . | 95% CI for PFS . | P for PFS . | HR for OS . | 95% CI for OS . | P for OS . |
|---|---|---|---|---|---|---|
| B2M, high | 1.304 | 1.027, 1.656 | .003 | 1.461 | 1.122, 1.901 | .005 |
| Hb, low | 1.298 | 1.047, 1.609 | .017 | 1.289 | 1.020, 1.629 | .034 |
| Age at ASCT date, old | 1.505 | 1.258, 1.800 | <.001 | 1.806 | 1.481, 2.202 | <.001 |
| 1p del | 1.274 | 1.033, 1.571 | .023 | 1.357 | 1.078, 1.710 | .009 |
| 1q21 gain | 1.489 | 1.245, 1.781 | <.001 | 1.523 | 1.249, 1.856 | <.001 |
| 17p del | 1.790 | 1.379, 2.324 | <.001 | 2.154 | 1.625, 2.855 | <.001 |
CI, confidence interval; Hb, hemoglobin; HR, hazard ratio.
Patient outcomes according to the stages identified by ISS, R-ISS, and R2-ISS
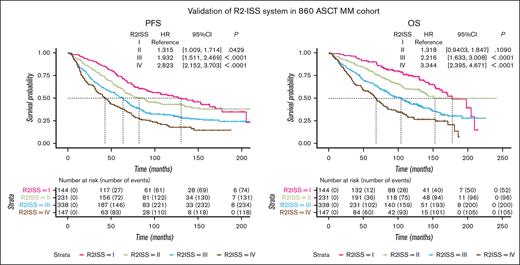
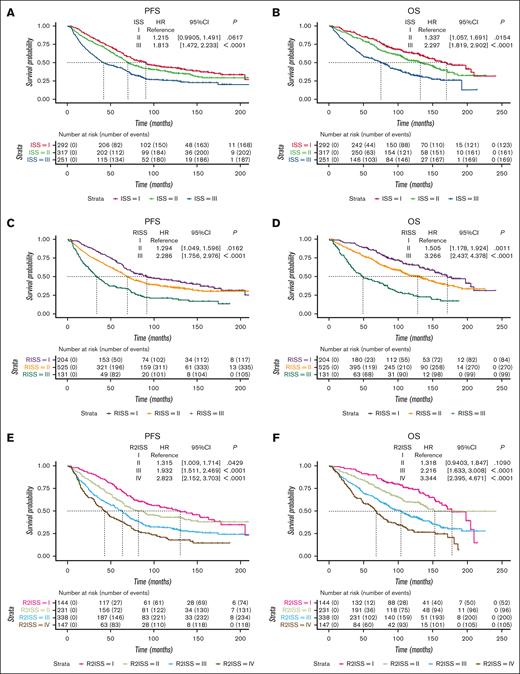
Survival curves were generated to assess PFS and OS across different stages. In the ISS system, patients at stages I, II, and III exhibited median PFS of 90.7 months, 69.9 months, and 42.0 months, respectively. The corresponding median OS values were 170.1, 132.5, and 75.3 months (Figures 1A,B). In the R-ISS system, patients at stages I, II, and III had median PFS values of 91.8, 69.3, and 33.9 months, respectively. The median OS values were 171.3, 128.3, and 49.0 months, respectively (Figures 1C,D). For the R2-ISS system, patients t stages I, II, III, and IV displayed median PFS values of 130.3, 82.2, 63.6, and 42.9, respectively. The median OS values were ∼177.5, 135.6, 103.8, and 68.2 months, respectively (Figures 1E,F).
Survival curves of ISS series systems in a cohort of 860 patients with MM treated with ASCT. (A-B) PFS and OS curves of ISS system. (C-D) PFS and OS curves of R-ISS system. (E-F) PFS and OS curves of R2-ISS system. CI, confidence interval; HR, hazard ratio.
Survival curves of ISS series systems in a cohort of 860 patients with MM treated with ASCT. (A-B) PFS and OS curves of ISS system. (C-D) PFS and OS curves of R-ISS system. (E-F) PFS and OS curves of R2-ISS system. CI, confidence interval; HR, hazard ratio.
Relationship of ISS, R-ISS, and R2-ISS distribution
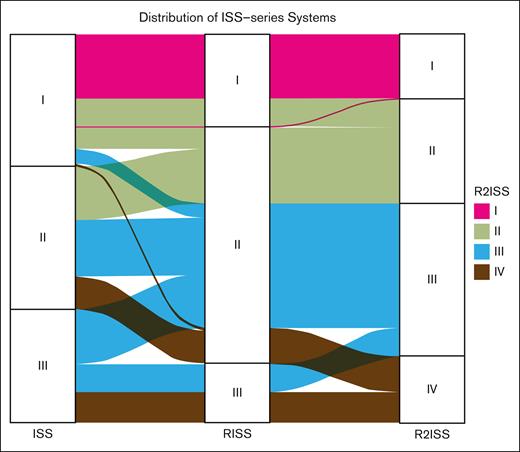
Considering the developmental progression from the ISS system to the R-ISS and R2-ISS systems, our aim is to investigate the redistribution of patients across these stages. We observed that some patients initially classified to ISS stages I and III were redistributed to R-ISS stage II, highlighting the improved capability of R-ISS to identify patients at extremely high or low risk (Figure 2). Moving from R-ISS to R2-ISS, we observed further redistribution of patients from R-ISS stage II to R2-ISS stages II, III, and IV. Additionally, redistribution was also observed among patients at R-ISS stages I and III (Figure 2). Overall, the R2-ISS system demonstrated an evenly distributed classification, whereas the R-ISS system exhibited better performance in recognizing patients at extremely high risk.
Distribution alluvial diagram of ISS series systems. Four color panels represent 4 R2-ISS stages of patients with MM.
Distribution alluvial diagram of ISS series systems. Four color panels represent 4 R2-ISS stages of patients with MM.
Additional risk factors
In addition to the variables used for constructing the R2-ISS stages, we used additional clinical and molecular variables. All continuous variables were categorized, and univariable Cox analysis was conducted (supplemental Table 4). Firstly, variables with P values < .05 were included in the multivariate Cox analysis, revealing that hemoglobin level, age at transplant date, and presence of 1p deletion could serve as independent variables in addition to those used for constructing R2-ISS stages. We further investigated factors that influenced survival in the R2-ISS system. Patients with MM with an OS < 3 years and those with an OS > 10 years within the low-risk R2-ISS stages (stages I and II) and the high-risk R2-ISS stages (stages III and IV) were compared. Variables with P <0.05 in the multivariable cox are presented in Table 2. We found that age affected stratification of the low-risk R2-ISS stages, whereas serum light chain, platelet count, age, and t(14;16) affected stratification of the high-risk stages (supplemental Tables 5 and 6).
Discussion
Over the past 2 decades, extensive research has been conducted to understand the prognosis of MM. Initially, investigations focused on clinical laboratory indicators,23 identifying risk factors such as isotype, calcium levels, platelet count, ALB, B2M, and lactate dehydrogenase. Subsequently, the introduction of metaphase cytogenetics and FISH provided insight into chromosomal abnormalities, whereas GEP, deep gene sequencing,24 and proteomics25 further contributed to our understanding of risk. Whole genome sequencing26 facilitated the identification of high-risk mutations, and more recently, single-cell RNA sequencing27 has aided in recognizing microenvironment prognostic markers. These ongoing discoveries have led to the development of various prognostic models, including the original Durie-Salmon system,23 ISS series systems, GEP models,20,28-31 and the International Myeloma Working Group consensus.1
Among these models, the ISS series systems have gained popularity because of their simplicity. However, despite several studies that have validated the R2-ISS system in different clinical settings, there is currently a lack of validation cohorts specifically focusing on transplantation and MM. To address this gap, we conducted a validation study using a large UAMS MM cohort that underwent transplant.
Notably, the study's cohort comprised 12% of African American patients, whereas ∼20% of patients with MM in the United States are of African American descent. This is consistent with already reported publications that African American patients with MM are less likely to undergo ASCT.32-34 We also noted that the median age within this cohort was 61 years, compared with the US median of 69 years. This is because patients with MM who receive ASCT are usually younger than 65 years old.
Our results demonstrate the robust separation capabilities of all ISS series systems. Furthermore, we investigated the relationship between the ISS series systems and illustrated their distribution patterns (Figure 2). The original 3-stage ISS system was revised to improve its ability to identify patients at extremely high or low risk, resulting in the development of the R-ISS system. Subsequently, the R2-ISS system was introduced as a 4-stage system to further stratify patients with median-risk MM within the R-ISS classification.
We also explored independent risk factors beyond the variables used in the ISS series systems. Our findings revealed that hemoglobin and age at transplant date are independent risk factors for both PFS and OS in ASCT MM. Moreover, we investigated factors that further improve predictions using the R2-ISS. In the low-risk stage (R2-ISS stages I and II), patients who lived longer than 10 years had lower levels of B2M and were younger at the time of ASCT. Conversely, at the high-risk stages (R2-ISS stages III and IV), patients who lived longer than 10 years exhibited higher levels of albumin and platelets and lower levels of lactate dehydrogenase. Additionally, these patients had a lower frequency of 17p deletion and were younger at the time of transplant that those at low-risk stages. Although albumin, B2M, lactate dehydrogenase, and 17p deletion are already included in the R2-ISS system, differences were observed between patients with an OS >10 years and those with >3 years at a given stage. This discrepancy can be attributed to the fact that in the construction of the R2-ISS system, important variables were transformed into binary categories and assigned weighted scores, leading to a potential distortion.
In conclusion, we validated the ISS series in an ASCT-treated MM cohort enrolled in the UAMS Myeloma Center. All 3 ISS series systems can stratify patients with MM treated with ASCT. Despite not being included in the current staging systems, hemoglobin, age, and chromosome 1p deletion are associated with relative high hazard ratios for PFS and OS in our cohort.
Acknowledgments
The authors are indebted to the clinicians of the Myeloma Institute for Research and Therapy for referring patients to this study and to all the patients who have helped them in their pursuit of a cure.
This work was financially supported by National Institutes of Health National Cancer Institute grant U54CA272691 (J.D.S. Jr).
Authorship
Contribution: W.G., A.Z., and J.D.S. Jr designed the study; D.E.M., C.B., and J.D.S. Jr performed the data acquisition; W.G. and A.Z. performed the statistical analysis and visualized the data with help from M.Z., G.T., and W.G.; A.Z., and J.D.S. Jr wrote the manuscript; M.N.M., O.M, and H.P. critically discussed, reviewed, and edited the manuscript; and all authors approved the final manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Shaughnessy Jr, Department of Internal Medicine, University of Arkansas for Medical Sciences, 4301 W Markham St, Slot 776, Little Rock, AR 72205; e-mail: jdshaughnessy@uams.edu.
References
Author notes
∗W.G. and A.Z. contributed equally to this study.
The clinical data used in this article are available on request from the corresponding author, John D. Shaughnessy Jr (jdshaughnessy@uams.edu).
The full-text version of this article contains a data supplement.