Key Points
Consider ACR screening prior to 10 years. ACR levels >100 mg/g should be considered a risk factor for albuminuria.
Renoprotective SCA trials should account for high variability in albuminuria measurements.
Abstract
It is critical to characterize the natural history of albuminuria in patients with sickle cell anemia (SCA); however, these data are currently lacking and affecting evidence-based guidelines. We performed a natural history study of the development of pediatric albuminuria. We identified participants with hemoglobin SS/SB0 thalassemia ≥5 years with albumin to creatinine ratio (ACR) measurements performed at a steady-state clinic visit. Participants were characterized as either persistent, intermittent, or never albuminuria. We determined the prevalence of persistent albuminuria, use of ACR ≥100 mg/g as a predictor, and variation in ACR measurements. We mirrored this study to determine the variation in albuminuria measurements in the SCA murine model. Among 355 participants with HbSS/SB0 thalassemia with 1728 ACR measurements, we identified 17% with persistent and 13% with intermittent albuminuria. Thirteen percent of participants with persistent albuminuria developed an abnormal ACR before 10 years of age. A single ACR measurement ≥100 mg/g was associated with 55.5 times (95% confidence interval, 12.3-527) higher odds of having persistent albuminuria. Among participants with ACR ≥100 mg/g, we identified significant variability in the results of repeated measurements. The median ACR at the initial and next measurements were 175.8 mg/g (interquartile range [IQR], 135-242) and 117.3 mg/g (IQR, 64-292). The human variability in ACR was mirrored by ∼20% variability in albuminuria in murine model. This evidence suggests adopting standards for repeating ACR measurements, consider screening for ACR before 10 years of age, and using an ACR >100 mg/g as a risk factor for progression. Pediatric and murine renoprotective clinical trials need to consider the high variability in repeated ACR measurements.
Introduction
Patients with sickle cell anemia (SCA) are at increased risk for developing chronic kidney disease (CKD) and patients who progress to end-stage kidney disease (ESKD) experience a high mortality.1-3 Albuminuria is a well-established risk factor for progression to ESKD and early mortality.4-6 However, currently the SCA field is lacking longitudinal studies from birth through adulthood to confirm the association of early albuminuria with ESKD and mortality. Of concern, data show that ∼20% of adolescent patients with SCA develop albuminuria and 20% of patients with sickle cell who develop ESKD are aged <30 years.7-11 Furthermore, patients with SCA who progress to ESKD are at an increased risk for early mortality and experience disparities in access to renal transplantation.11,12 With the observation of established pathophysiology in other diseases that albuminuria progresses to ESKD, experts in SCA continue to emphasize the need to prevent the development and progression of albuminuria as a vital component of care.
Several guidelines highlighted monitoring and treating albuminuria as a key component of care; however, each guideline cited a lack of evidence to describe the natural history of the progression to CKD in SCA. The current National Heart, Lung, and Blood Institute Evidence-Based Management of Sickle Cell Disease provides a consensus opinion suggesting that annual screening for albuminuria in patients with SCA should begin by the age of 10 years.13 The American Society of Hematology (ASH) Sickle Cell Guidelines for cardiopulmonary renal complications, based on very low certainty in the evidence regarding effects, suggest initiating therapeutic interventions for patients with albuminuria.14 Finally, the ASH/U.S. Food and Drug Administration (FDA) expert panel on clinical trial end points suggests using a percent decline in albuminuria as an end point for future SCA trials.15 Of concern, these guidelines discussed limited evidence in patients with SCA and relied heavily on extrapolating findings from studies of albuminuria in non-SCA populations. Therefore, it is critical that evidence from patients with SCA confirms these opinions and suggestions for the diagnosis and management of albuminuria.
In addition to the topics addressed in evidence-based guidelines, several important clinical questions remain for albuminuria. First, at what albumin to creatinine ratio (ACR) values should clinicians begin therapeutic interventions? Second, what is the natural course of ACR changes in patients with intermittent albuminuria? Finally, randomized controlled trials for patients with albuminuria and SCA are needed. However, to design a well-powered randomized controlled trial, a major limitation, and considered in the Food and Drug Administration/ASH guidelines, is that we are lacking data on the normal variation in the percent changes in ACR levels. To address these critical questions, we performed a study of the University of Alabama at Birmingham (UAB) Pediatric Sickle Cell Kidney Disease cohort to extract evidence regarding the natural history of albuminuria. In addition, because preclinical studies are integral part of moving novel therapeutics into the clinical stage, we also performed longitudinal study on the natural history of the development and variability of albuminuria in murine SCA model.
Methods
Study design and subjects
To investigate the natural history of pediatric sickle cell kidney disease, we performed a retrospective cohort study of participants in our Institutional Review Board–approved UAB Pediatric Sickle Cell Kidney Cohort. It was conducted according to the Declaration of Helsinki. This cohort was established in 2015 and captures all participants with hemoglobin SS (HbSS) and HbSB0 thalassemia aged ≥5 years with at least 1 ACR measurements performed at a steady-state outpatient clinic visit at the Children’s of Alabama. The earliest ACR measurements recorded in our electronic medical records (EMR) are from April 2010, and the data collection was completed in September 2022. To maintain reliability of our primary outcome, we excluded ACR measurements obtained from our participants with annual ACR measurement obtained at satellite clinics. We abstracted ACR measurements, age, and date of visit from the electronic medical record. We recorded current sickle cell therapy. Participants’ ACR measurements were recorded before starting renoprotective therapy (angiotensin converting enzyme inhibitor or angiotensin receptor blocker therapy) and excluded after initiating therapy. We categorized participants with either (1) persistent albuminuria, if 2 of 3 consecutive ACR measurements were ≥30 mg/g; (2) intermittent albuminuria, if only 1 of 3 consecutive ACR measurements were ≥30 mg/g; or (3) never albuminuria, if no ACR measurements were ≥30 mg/g. Participants who had an abnormal ACR on their most recent ACR but had not undergone confirmatory testing were identified as having intermittent albuminuria.
We identified the date that ACR measurement was obtained and age of participants on that measurement with at least 1 ACR level ≥100 mg/g to compare outcomes for albuminuria and to determine the natural history of changes in ACR after an ACR measurement of >100 mg/g. We also identified the age at first ACR measurement and categorized participants as having completed ACR participants before or after 10 years of age.
Statistical analysis
We performed descriptive statistical analyses for continuous data with mean and standard deviation (SD) for normally distributed data and median and interquartile range (IQR) for nonnormally distributed data. We summarized categorical data with counts and percentages. We used t test to compare means and χ2 to compare proportions. We calculated odds ratio and confidence intervals (CIs) to evaluate differences in outcomes (albuminuria by ever and current) among participants who had ACR measurements >100 mg/g. The binary outcome of being categorized in the persistent or in the intermittent group was the sole predictor binary variable of having ACR >100 vs ACR ≤100. No covariates were added owing to a cell with small count. Clopper-Pearson method was used to calculate 95% CI for proportions.
In this study, we did not collect ACR at exactly 1 year after their documented abnormal value. Therefore, we defined the window for 1 year follow-up as visits occurring within 6 to 18 months of the first visit. For cases with multiple visits in this window, we chose the visit closest to 12 months. We limited the analyses to those with data on both visits. To model the trajectory of the ACR over time from their initial visit, we fitted a generalized linear model with gamma distribution using a log link. We used the generalized estimating equations and assumed an autoregressive (lag 1) covariance structure to address the correlation among repeated observations from the same participant. Based on the results of this fitted model, we obtained predicted ACR with 95% CI at a given time point.
Murine SCA study
We used male and female humanized SS mice.16 Based on our previous studies demonstrating the occurrence of hyperfiltration at 12 weeks of age in male and 20 weeks of age in female HbSS, we performed metabolic cage studies before hyperfiltration phase (using 4-weeks-old male and female mice, n = 21) and adult, 16- and 20-week-old males (n = 10) and females (n = 8), respectively (after murine hyperfiltration phase).17 Mice were maintained under constant humidity and temperature conditions, 12:12 light:dark cycle and had ad libitum access to facility chow (Harlan Teklad) and water. Metabolic cage studies were conducted at 1-week intervals for 4 weeks, and 24-hour urinary albumin excretion was measured using GenWay Biotech ELISA kit (cat#GWB-282C17). All animal protocols were approved by the Institutional Animal Care and Use Committees of UAB.
Results
Natural history and variability of albuminuria in human SCA
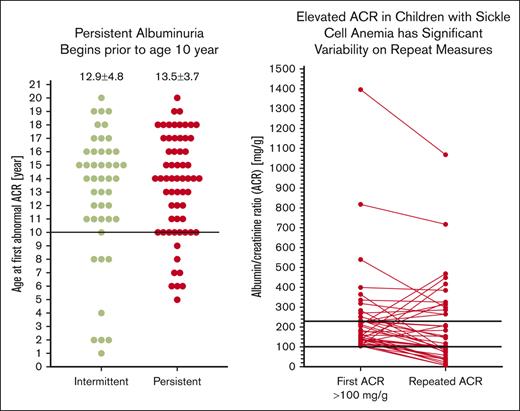
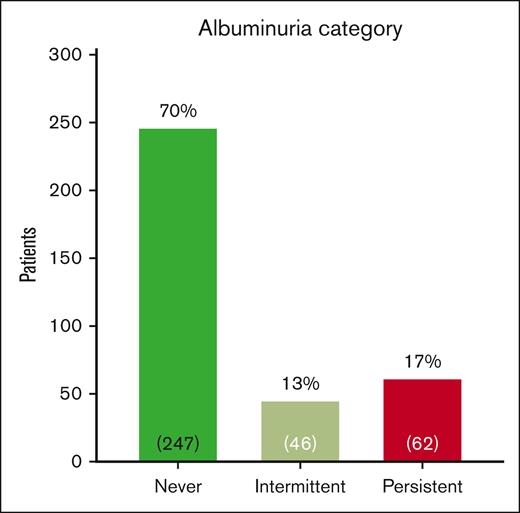
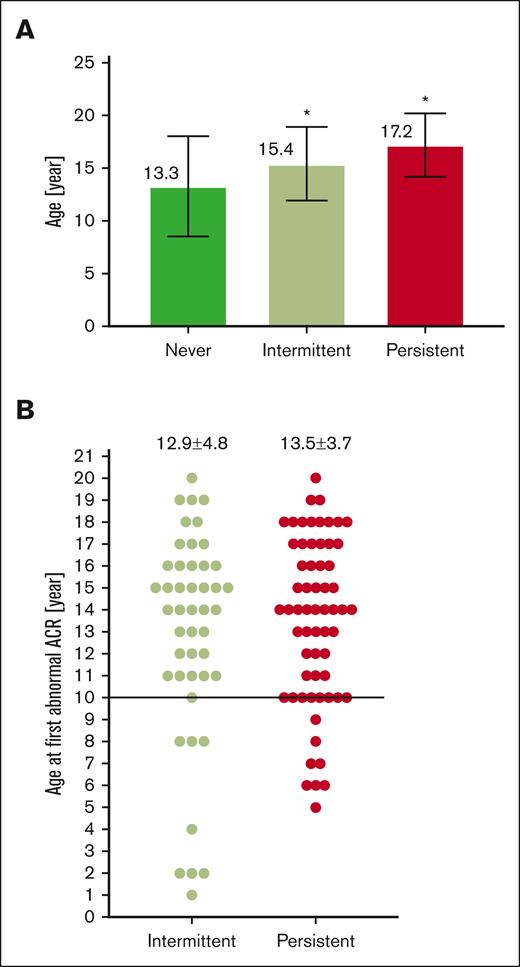
We evaluated 1728 ACR measurements in 355 participants with HbSS/SB0 thalassemia who were aged ≥5 years. The current mean age of participants was 14.2 ± 4.6 years and 172 (48%) were female (Table 1). Fifty-nine percent of participants were prescribed hydroxyurea. The median ACR was 11.7 mg/g (IQR, 7.3-28.4), and the median number of ACR measurements performed was 4 (IQR, 2-7). We identified 62 (17%) of participants in our pediatric cohort as having persistent albuminuria, 46 (13%) with intermittent albuminuria, and 247 (70%) with not yet having albuminuria (never) (Figure 1). As albuminuria progresses over time, we identified that participants with never albuminuria were younger (13.3 ± 4.7 years) than participants with intermittent albuminuria (15.4 ± 3.5 years, P = .007) or persistent albuminuria (17.2 ± 3.0 years, P < .0001; Figure 2A). We did not identify a difference in the age of participants at the time of a first abnormal ACR (intermittent, 12.9 ± 4.8 years vs persistent, 13.5 ± 3.7 years; P = .5; Figure 2B). Among 62 participants with persistent albuminuria, 8 (13%) participants developed their first episode of albuminuria before the age of 10 years. Four of those 8 participants had their first abnormal ACR at ≤6 years of age (Figure 2B).
Summary of similarities and differences or limitations of using humanized HbSS mouse model
| PROS . | CONS . |
|---|---|
| HbSS mice exclusively express human sickle hemoglobin: mouse α-globin genes are replaced with human α-globin HBA1 gene, mouse β-like globin genes replaced with tandemly linked genomic segments of human γ-globin HGB1 and sickle β-globin HBBSgenes. | HbSS mice almost immediately after birth switch HbF to HbS. |
| HbSS mice present hematological similarities with human SCA: erythrocytic sickling, intravascular hemolysis, reticulocytosis, anemia, leukocytosis. | HbSS mice have heterogenic genetic background, which may result in genetic drift. |
| HbSS mice present histopathological similarities with human SCA: multiorgan infarcts, endothelial dysfunction, inflammation, vasculopathy, heart hypertrophy, pulmonary hypertension, hepatic iron overload and fibrosis, nociception. | Owing to species specific characteristics HbSS mice may present with different pharmacokinetics and/or drug metabolism. |
| HbSS mice recapitulates human renal phenotype: early hyperfiltration, progressive albuminuria/proteinuria, progressive GFR decline, glomerulopathy, tubulopathy, hypostenuria, and papillary congestion/necrosis. | |
| HbSS mice are widely available, easy to maintain, cost-effective, suitable for longitudinal studies, provide ability to model disease through pharmacological, genetic, cellular, surgical manipulations, valuable for preclinical studies. | |
| Advantages of using HbSS over other animal SCA models: Humanized porcine SCA animal model represents prolonged HbF to HbS switch (∼12 months); it is unknown if this model recapitulate human disease; limited availability and high cost of maintenance of the model. Baboon model presents a great model to study HbF-inducing treatments (β-like globin gene locus is highly conserved between baboon and man) but does not exhibit SCA pathology (owing to the lack of HbS). Berkeley sickle cell mouse model lacks proper genetic (littermate) controls; has reduced mean corpuscular Hb concentration (mild α-thalassemia phenotype); has exuberant splenic hematopoiesis. SAD mouse express mouse α- and βminor and human SAD hemoglobin transgene (low proportion of human SAD Hb); is not anemic, recapitulates only some SCA-associated kidney defects. |
| PROS . | CONS . |
|---|---|
| HbSS mice exclusively express human sickle hemoglobin: mouse α-globin genes are replaced with human α-globin HBA1 gene, mouse β-like globin genes replaced with tandemly linked genomic segments of human γ-globin HGB1 and sickle β-globin HBBSgenes. | HbSS mice almost immediately after birth switch HbF to HbS. |
| HbSS mice present hematological similarities with human SCA: erythrocytic sickling, intravascular hemolysis, reticulocytosis, anemia, leukocytosis. | HbSS mice have heterogenic genetic background, which may result in genetic drift. |
| HbSS mice present histopathological similarities with human SCA: multiorgan infarcts, endothelial dysfunction, inflammation, vasculopathy, heart hypertrophy, pulmonary hypertension, hepatic iron overload and fibrosis, nociception. | Owing to species specific characteristics HbSS mice may present with different pharmacokinetics and/or drug metabolism. |
| HbSS mice recapitulates human renal phenotype: early hyperfiltration, progressive albuminuria/proteinuria, progressive GFR decline, glomerulopathy, tubulopathy, hypostenuria, and papillary congestion/necrosis. | |
| HbSS mice are widely available, easy to maintain, cost-effective, suitable for longitudinal studies, provide ability to model disease through pharmacological, genetic, cellular, surgical manipulations, valuable for preclinical studies. | |
| Advantages of using HbSS over other animal SCA models: Humanized porcine SCA animal model represents prolonged HbF to HbS switch (∼12 months); it is unknown if this model recapitulate human disease; limited availability and high cost of maintenance of the model. Baboon model presents a great model to study HbF-inducing treatments (β-like globin gene locus is highly conserved between baboon and man) but does not exhibit SCA pathology (owing to the lack of HbS). Berkeley sickle cell mouse model lacks proper genetic (littermate) controls; has reduced mean corpuscular Hb concentration (mild α-thalassemia phenotype); has exuberant splenic hematopoiesis. SAD mouse express mouse α- and βminor and human SAD hemoglobin transgene (low proportion of human SAD Hb); is not anemic, recapitulates only some SCA-associated kidney defects. |
GFR, glomerular filtration rate; HbF, fetal hemoglobin; HbS, sickle hemoglobin; SAD, Hemoglobin beta S-Antilles-D Punjab.
Categorization of albuminuria in UAB Pediatric Sickle Cell Kidney Cohort. Patients with HbSS and HbSB0 thalassemia ≥ 5 years of age with ACR measurements performed at a steady-state outpatient clinic visit were included. Never albuminuria is defined as no ACR measurements ≥30 mg/g, intermittent albuminuria as 1 of 3, and persistent albuminuria as 2 of 3 consecutive ACR measurements ≥30 mg/g.
Categorization of albuminuria in UAB Pediatric Sickle Cell Kidney Cohort. Patients with HbSS and HbSB0 thalassemia ≥ 5 years of age with ACR measurements performed at a steady-state outpatient clinic visit were included. Never albuminuria is defined as no ACR measurements ≥30 mg/g, intermittent albuminuria as 1 of 3, and persistent albuminuria as 2 of 3 consecutive ACR measurements ≥30 mg/g.
Occurrence of albuminuria in pediatric patients with SCA by age. (A) Average age of SCA pediatrics by albuminuria category, ∗P < .05 vs never albuminuria; (B) Average age at first ACR >30 mg/g by albuminuria category.
Occurrence of albuminuria in pediatric patients with SCA by age. (A) Average age of SCA pediatrics by albuminuria category, ∗P < .05 vs never albuminuria; (B) Average age at first ACR >30 mg/g by albuminuria category.
Because the National Institutes of Health (NIH) recommends obtaining and initial ACR measurement by age 10 years, we analyzed the timing of initial ACR measurement. The median age of the initial ACR measurement for participants was age of 9 years (IQR, 6-13). One hundred thirty nine out of 355 participants (39%) had their initial ACR obtained after 10 years of age. We did not identify a significant difference in the age of participants at their initial ACR by albuminuria status (persistent, 10.4 ± 4.1 years; intermittent, 9.1 ± 4.5 years; never, 9.3 ± 4.2 years; P = .18). Second, we analyzed the percent of participants with albuminuria identified on their initial ACR measurement. Among 26 participants with persistent albuminuria with an initial ACR measurement obtained before age of 10 years, 6 (23%) were identified with albuminuria on their first measurement. Finally, among 28 participants with persistent albuminuria and an ACR initially measured after age of 10 years, 9 (32%) had albuminuria on their first measurement.
For analyzing ACR ≥100 mg/g as a risk factor for persistent albuminuria, we tested the hypothesis that ACR ≥100 mg/g was associated with persistent albuminuria among the 108 participants in our cohort with at least 1 abnormal ACR measurement. Forty-five participants in our cohort were identified with at least 1 ACR measurement ≥100 mg/g; 43 (96%; 95% CI, 85.0-99.5) of these participants were categorized as having persistent albuminuria. We identified 63 participants with 1 episode of ACR ≥30 mg/g but no episodes of ACR ≥100 mg/g. Only 19 (30%; 95% CI, 16.6-39.7) of these participants with ACR between 30 and 100 mg/g were diagnosed with persistent albuminuria. The odds of having persistent albuminuria among participants with ACR ≥100 mg/g were 55 times (95% CI, 11-227) higher than among participants with albuminuria but no episodes of ACR ≥100 mg/g.
As per the natural history of intermittent albuminuria, 46 participants had only 1 of 3 consecutive ACR measurements ≥30 mg/g. Among these 46 participants, 11 (24%) were diagnosed with albuminuria on their most recent measurement and awaited a repeat ACR measurement to determine if they had persistent albuminuria. Thirty participants (65%) had no additional episodes of ACR measurements ≥30 mg/g. Only 5 (11%) participants with intermittent albuminuria had at least 1 subsequent ACR ≥30 mg/g but were never identified with 2 of 3 consecutive measurements ≥30 mg/g, during their time in this cohort.
For analyzing natural history of albuminuria variability after an ACR ≥100 mg/g, we identified 41 participants in our cohort with a first episode of ACR ≥100 mg/g and a subsequent ACR measurement. Twenty-four participants were administered hydroxyurea during this time, 10 participants underwent transfusion therapy, and 7 did not undergo any sickle cell–modifying therapy. The median ACR for the first episode of ACR ≥100 mg/g was 175.8 (IQR, 135-242). The median ACR on the next measurement was 117.3 (IQR, 64-292). (Figure 3) Overall, 9 (22%) participants had an increase in their ACR (median increase, 72% [IQR, 13%-157%]) on their next ACR measurement and 32 participants (78%) had a decrease in their ACR (median decrease, 45% [IQR, 13%-74%]) on their next ACR measurements (Figure 3). We identified no difference in the variability of the next ACR measurement by sickle cell therapy (P = .4). Twenty-four (59%) of the participants continued to have ACR >100 mg/g, 13 (32%) had an ACR between 30 and 100 mg/g, and 4 participants (10%) had a normal ACR on their next ACR measurement. We identified a significant median difference in the initial ACR with the next ACR measurements of −58.9 mg/g (IQR, −5.2 to −113, P = .003).
Subsequent ACR among participant with first ACR >100 mg/g. The median of the first episode of ACR >100 mg/g was 175.8 (IQR, 135-242), while next ACR was 117.3 (IQR, 64-292). Among the participants 9 (22%) has an increase (median increase, 72% [IQR, 13%-157%] and 32 [78%]) had a decrease (median decrease, 45% [IQR, 13%-74%]) in their susequent ACR.
Subsequent ACR among participant with first ACR >100 mg/g. The median of the first episode of ACR >100 mg/g was 175.8 (IQR, 135-242), while next ACR was 117.3 (IQR, 64-292). Among the participants 9 (22%) has an increase (median increase, 72% [IQR, 13%-157%] and 32 [78%]) had a decrease (median decrease, 45% [IQR, 13%-74%]) in their susequent ACR.
It was also of interest to estimate the 1-year change in albuminuria and its SD for future clinical trials. We identified 30 patients with a repeat ACR within 6 months of the initial ACR ≥100 mg/g. The results of these analyses showed that 1-year observed mean change in ACR was decreased for 95.4 mg/g (baseline to 1 year, 215.1-119.7 mg/g) with a SD of (132.7 mg/g) (Figure 4A). This estimate represented a 44% reduction in ACR at 1 year. The observed correlation between baseline and 1 year ACR was 0.34.
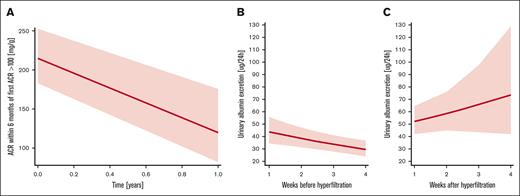
Natural history of albuminuria in pediatrics and murine model of SCA. (A) Variability in ACR after first episode of albuminuria ≥100 mg/g in pediatrics SCA, 1-year observed mean change in ACR was a decrease of 95.4 mg/g (baseline to year 1, 215.1-119.7 mg/g) with a SD of (132.7 mg/g); (B) variability in urinary albumin excretion in murine SCA model before hyperfiltration phase presents with downward trend over a time of 4 weeks (n = 21); (C) variability in urinary albumin excretion in murine SCA model after hyperfiltration phase presents with upward trend over a time of 4 weeks (n = 18).
Natural history of albuminuria in pediatrics and murine model of SCA. (A) Variability in ACR after first episode of albuminuria ≥100 mg/g in pediatrics SCA, 1-year observed mean change in ACR was a decrease of 95.4 mg/g (baseline to year 1, 215.1-119.7 mg/g) with a SD of (132.7 mg/g); (B) variability in urinary albumin excretion in murine SCA model before hyperfiltration phase presents with downward trend over a time of 4 weeks (n = 21); (C) variability in urinary albumin excretion in murine SCA model after hyperfiltration phase presents with upward trend over a time of 4 weeks (n = 18).
Natural history and variability of albuminuria in murine SCA
To further evaluate natural variation in albuminuria, we complemented clinical data with murine SCA data. Our group had previously published data on the natural history of the development of albuminuria in SCA murine model.17 In this study, we demonstrated that the natural history of albuminuria in HbSS mice was different before and after hyperfiltration. Based on linear regression modeling, we observed a significant difference in the trajectory of albuminuria over time in young (before hyperfiltration) and adult mice (after their hyperfiltration phase [P = .02]). Young HbSS mice presented with downward trend in albuminuria over time (Figure 4B), with average values of albuminuria 47.6 ± 27.2 (week 1), 32.6 ± 20.3 (week 2), 32.0 ± 24.3 (week 3), and 31.3 ± 16.1 μg per 24 hours (week 4). Subsequent to hyperfiltration phase, older HbSS mice had upward trend (Figure 4C) in albuminuria over time (week 1, 49.7 ± 23.6; week 2, 74.5 ± 66.9; week 3, 55.9 ± 82.2; week 4, 73.0 ± 86.4 μg per 24 hours, respectively). Finally, we analyzed the variability in albuminuria levels at these different stages of glomerular filtration. Preceding hyperfiltration, the average albuminuria over a time of 4 weeks in young HbSS mice was 35.9 ± 7.8 μg per 24 hours (±21.7%), whereas in adult mice, after hyperfiltration, it was 63.3 ± 12.4 μg per 24 hours (±19.6%). These findings mirrored human data in which pediatrics and adults presented with high degree of variability in urinary albumin excretion.
Discussion
Our study provides critical information to understand the natural history and variability in albuminuria in pediatric SCA and provides critical evidence for care guidelines and clinicians monitoring patients for CKD. The Kidney Disease Improving Global Outcomes (KDIGO) defines CKD for albuminuria as either having 1 ACR measurement ≥300 mg/g or 2 of 3 consecutive ACR measurements ≥30 mg/g.18 Our data highlight the importance of obtaining 2 additional ACR measurements to confirm persistent albuminuria because 40% of our cohort had intermittent rather than persistent albuminuria. In line with our results, other studies have also showed that not all the changes in mild elevations in ACR are evidential of kidney disease progression.19,20 Thus, future research studies of SCA kidney disease need to adopt this more rigorous definition to reduce the categorization bias in studies using a single ACR measurement from 30 to 300 mg/g as albuminuria. The KDIGO guidelines also recommend performing ACR measurements on an early morning urine sample but allowing a random urine sample to be obtained. We provide patients with a urine specimen collection container to return at their next clinic visit and schedule these patients for an early morning follow-up appointment. This strategy allows for an early morning void to be collected in clinic if their first morning void is not obtained. Finally, urinalysis to detect proteinuria is considered a third line technique. Patients with severely increased albuminuria (≥300 mg/g) have excellent sensitivity and specificity for a positive urine dipstick; however, the sensitivity of a urine dipstick is poor (58%) for patients with measured ACR between 30 and 300 mg/g.21 Therefore, if resources are present in future research, we recommend that patients with a positive urine dipstick have ACR measurements performed on the confirmatory visit.
Second, our study confirms the observation from prior studies suggesting ACR ≥100 mg/g as a prognostic value for persistent albuminuria. Between 75% and 90% of pediatric and adult patients with sickle cell disease (SCD) with ACR ≥100 mg/g will have repeated albuminuria on subsequent measurements; in contrast, between only 30% and 50% of patients with a single episode of albuminuria ≥30 but <100 mg/g will have an abnormal ACR levels on the next 2 measurements.10,22 In addition, longitudinal study in adults with SCA provides evidence that ACR ≥100 mg/g is associated with rapidly declining estimated Glomerular Filtration Rate (eGFR) and CKD progression.10 These data suggest that clinicians should intensify plans for monitoring and treating patients with ACR ≥100 mg/g. Furthermore, considering the high predictive value of ACR magnitude, a cut-point of an ACR ≥100 mg/g can be considered as either an inclusion criteria or prognostic marker in future clinical trials of SCA kidney disease.
A potential biologic rationale to explain the correlation between intermittent albuminuria and lower ACR measurements (30-100 mg/g) could be related to the impact of hemolysis on albuminuria. It is established that glomerular albuminuria occurs, but this excess albumin can be reabsorbed in the proximal tubules via the megalin or cubilin receptors.23,24 Relevant to SCA, plasma free heme or hemoglobin (Hb) is one of several ligands that competitively binds to megalin or cubilin and inhibits the reuptake of albumin in the proximal tubules. Data suggest that introduction of free Hb into the proximal tubules decreases tubular albumin reuptake, and this tubular albuminuria can be reversed by infusion of haptoglobin.25 Therefore, it is plausible that fluctuating levels of hemolysis in patients with SCA may contribute to intermittent, lower levels of tubular albuminuria which is not associated with glomerular pathology. This concept that hemolysis affects lower levels of albuminuria in sickle cell can be considered when interpreting human data of therapeutic interventions for albuminuria. In a study by Bartolucci et al, initiation of hydroxyurea, which should decrease the amount of free heme/Hb, significantly decrease ACR levels in patients with lower levels of albuminuria but do not significantly change ACR levels in patients with established proteinuria.26 In contrast, a study of losartan, with known benefit in patients with diabetes with glomerular proteinuria, the greatest reduction in ACR is identified in patients with proteinuria at baseline but not in patients with SCA with lower levels of albuminuria.27
Third, this study provides critical evidence to support earlier screening for albuminuria and further highlights the need to improve implementation of screening guidelines in patients with SCA. The current NIH guidelines suggest screening for albuminuria by age 10 years; however, only 60% of our cohort participants achieved this metric. Among those participants with persistent albuminuria, we identified that ∼30% of participants had abnormal values on their first measurement; this suggests that albuminuria likely is developing before our initial measurement. Limitations to adoption of standards-of-care are a well-described issue in patients with SCA. Annual transcranial Doppler imaging to identify stroke risk is a vital component of care guidelines for all children with SCA aged from 2 to 16 years.13,28,29 Despite having a well-established benefit,30 a recent evaluation of transcranial Doppler screening in over 5000 children with SCA identified successful annual screening rates of only 50%.31 This highlights the critical need of identifying barriers to screening and facilitators for improving the implementation of clinical care practice guidelines.
It will also be important to determine which patients may need earlier screening with ACR. Prior work from this cohort establishes an association between hyperfiltration, starting from 7 to 10 years of age, and the development of persistent albuminuria.32 A second risk factor for the early development of albuminuria is the presence of high-risk APOL1 mutations, which occur in ∼10% of patients with SCA.33-37 Studies suggest that albuminuria occurs in patients with high-risk variants of APOL1 either through insertion of pores in the podocytes or through mitochondrial dysfunction.38 In children with SCA, 25% of patients with an APOL1 high-risk mutation develop albuminuria before the age of 10 years as compared with <5% of patients without high-risk APOL1.39 These studies of hyperfiltration or APOL1 status that focused on the time to develop albuminuria provide evidence to suggest that screening for albuminuria should occur earlier than 10 years of age.39 Because screening for APOL1 mutations is not standard of care, we suggest that annual universal screening for albuminuria should begin by 5 to 6 years of age, especially focusing on patients with hyperfiltration. In addition to clinical care, clinical studies of therapeutics to prevent the development of albuminuria should consider starting interventions as early as 6 years of age.
Understanding the benefit and risk of novel therapeutics on preventing albuminuria in patients with SCA often first involves establishing a benefit in the SCA murine model. Our study further highlights the need to understand the natural history of albuminuria in the SCA murine model. The male humanized–sickle cell murine model develops hyperfiltration at ∼12 weeks of age. During the hyperfiltration phase, we identify a rapid increase in albuminuria. However, after the period of hyperfiltration, at 16 weeks of age, the male HbSS have a rapid decline and normalization of albuminuria. Then, from 20 to 24 weeks, the male HbSS progress to demonstrate a persistent increase in albuminuria. Therefore, our data suggest 2 different approaches for evaluating renoprotective therapies in the male humanized SCA murine model. First, studies focused on preventing the hyperfiltration effect on albuminuria must understand that there is an already established decline in albuminuria immediately after hyperfiltration. Second, long-term studies aimed at reducing persistent albuminuria should begin to evaluate changes in albuminuria by 20 weeks when the second phase of progressive, persistent albuminuria occurs.
A fifth important question in this study addresses the lack of data to understand the natural history for patients with intermittent albuminuria. We acknowledge that children or adults with repeated episodes of intermittent albuminuria may progress to persistent albuminuria during their life. However, in our pediatric cohort, only 11% of pediatric patients with intermittent albuminuria had at least 1 additional episode of albuminuria during this cohort study. More time is required to evaluate the long-term association between 1 episode of albuminuria and progressive CKD. Without this long-term data, we suggest that initiating renoprotective agents for a single ACR level between 30 and 100 mg/g is not indicated.
Finally, this study provides critical data on human and murine variability in albuminuria measurements. A recent randomized controlled intervention trial for sickle cell kidney disease proposed using an inclusion criteria for ACR of ≥100 mg/g and a primary outcome of a 30% decline in ACR at 12 months based on the Food and Drug Administration/ASH expert panel on clinical trial end points.15,40 This suggestion of 30% decline in ACR as a primary outcome was extrapolated from non-SCD albuminuria studies. This study provides critical data regarding the significant variability in ACR measurements after an initial ACR measurement of ≥100 mg/g. The next ACR measurement showed that ∼75% of participants had a decline in ACR. We also estimated the change at 1 year of −95 mg/g and an estimated 1-year change of 44% without the use of a renoprotective agent. Similar to pediatric data, the corresponding magnitude of albuminuria variability was observed before and subsequent to renal involvement in murine model of SCA. In particular, a 21.7% variability in albuminuria, with downward trend over time in young HbSS mice, to a great extent, matches inherent fluctuations in ACR in SCA pediatrics data and confirms that this model provides an excellent tool for identifying processes involved in long-term renal outcomes in patients with transient as well as persistent albuminuria. In addition, a 19.6% variability in albuminuria, with upward trend over time, past hyperfiltration phase in adult HbSS mice, corroborates our finding and correlates to renal characteristics of adults with SCA. Furthermore, 24-hour urine collection is not feasible in clinical practice to accurately measure albuminuria in pediatrics, thus ACR ratio is commonly used. However, preclinical studies have the advantage of addressing this concern and should use the most flawless approaches to avoid well-known interferences with albuminuria estimation related to murine creatinine tubular secretion. Tubular secretion accounts for ∼50% and 35% of excreted creatinine in male and female mice, respectively.41 Moreover, with increased plasma creatinine, the contribution of tubular creatinine secretion is even greater. Therefore, by eliminating this large secretory component, murine studies will provide rigorous, reproducible, and accurate preclinical data on urinary measurements of renal outcomes. Our data on variability in ACR measurements clearly demonstrate the need of future studies aimed to prevent or reduce albuminuria to include a placebo arm. Single arm studies using a percent reduction in subsequent ACR measurements are at high risk of introducing bias based on the natural variability identified in this study.
This study provides vital information needed to understand the natural history and variability of albuminuria in pediatric SCA. A few limitations are important to note. First, KDIGO recommends that patients with ACR ≥30 mg/g but <300 mg/g undergo 2 further early morning urine samples within 2 months. This study evaluated patients’ next 2 subsequent ACR measurements, but those measurements were often not obtained within 2 months. CKD is defined as patients having >3 months of abnormal kidney function; therefore, we anticipate that our abnormal ACR measurements, obtained after the initial 2-month confirmation period, maintain the requirements of identifying patients with CKD. Second, although we did not find an association between SCD therapy and variability in ACR on a second measurement, this study did not evaluate risk factors for and therapies to prevent the development of albuminuria. We only report the current SCD therapy in this longitudinal study rather than therapy at each measurement. Prospective studies are currently evaluating the impact of disease modifying therapies on the development and progression of albuminuria. Third, we did not always perform 1-year ACR measurements after an initial episode of albuminuria ≥100 mg/g. Therefore, we could only estimate the 1-year change and variability in ACR. It is important to prospectively study 1-year change and variability in ACR before conducting randomized trials of renoprotective agents.
In conclusion, our study provides critical data to support clinical care practice, guideline development, and research study design. Our findings have important implications for clinicians’ decision for albuminuria screening. The current NIH guidelines recommend screening by age of 10 years; our data suggest that screening for albuminuria by 6 years of age in patients with SCA will capture those patients with early albuminuria. In addition, repeated ACRs should be performed in all patients with ACR ≥30 mg/g before initiating renoprotective therapies, especially if the initial measurement is <100 mg/g. Moreover, our data highlight the need for additional research into the role of SCD modifying therapies that reduce hemolysis as well as renoprotective agents in patients with albuminuria. Using our data, clinical trials must account for the higher-than-expected variability in repeated ACR levels when designing pediatric intervention trials to reduce albuminuria including the need for a placebo arm.
Acknowledgments
The authors acknowledge the patients and families who participated in this research as well as the SCD clinical team at Children’s of Alabama/The University of Alabama at Birmingham (UAB).
This study was supported by grants from UAB AMC21 Multi-PI6724 award, National Heart, Lung, and Blood Institute, R00HL144817 (M.K.), and National Institutes of Health R25DK112731 and R01HL153386-01 (J.L.).
Authorship
Contribution: M.K. and J.L. conceived and designed research; K.B., M.H., and M.K. performed experiments; I.A., J.L., C.I., K.B., M.H., and M.K. analyzed data; J.L. and M.K. drafted the manuscript; M.K., I.A., K.B., M.H., C.I., and J.L. edited and revised the manuscript; and all authors approved the final submission.
Conflict-of-interest disclosure: J.L. is a consultant for sickle cell kidney disease research for Novartis, Forma Therapeutics, and Bioproducts laboratory; served as the chair of the End-Organ Panel for ASH/FDA clinical trial endpoints workshop; served as a panel member of Cardiopulmonary and Kidney Disease, ASH Sickle Cell Clinical Practice Guidelines on Sickle Cell Disease and was on the steering committee for the Novartis funded STEADFAST study (www.clinicaltrials.gov #NCT04053764). The remaining authors declare no competing financial interests.
Correspondence: Jeffrey Lebensburger, Division of Pediatric Hematology Oncology, The University of Alabama at Birmingham, MSPH 1600 7th Ave South, Lowder 512, Birmingham, AL 35233; e-mail: jlebensburger@uabmc.edu.
References
Author notes
Data are available on request from the corresponding author, Jeffrey Lebensburger (jlebensburger@uabmc.edu).

![Subsequent ACR among participant with first ACR >100 mg/g. The median of the first episode of ACR >100 mg/g was 175.8 (IQR, 135-242), while next ACR was 117.3 (IQR, 64-292). Among the participants 9 (22%) has an increase (median increase, 72% [IQR, 13%-157%] and 32 [78%]) had a decrease (median decrease, 45% [IQR, 13%-74%]) in their susequent ACR.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/22/10.1182_bloodadvances.2023010101/3/m_blooda_adv-2023-010101-gr3.jpeg?Expires=1771336227&Signature=qoNJG2nt2MmuJxDwMFrIoBT~g~Pr~QTuPOCKwcP1zzss-Jw1CzncIlH8lTkMsuH8K~DGm8d~5HUqfY3zPNDb5auzYfLvBlzey0cHE1Zr000Tby6ROr2BMCbCP9FmMs-Xxi~14~OQ50FkYlDeTcZH0BrDajWL7v8H3~sK~1dYvbizq1fQdYfZENa7ZbnWE29uAbmPQSnGs3EoT~Epjsj3JMLU8PhVdZ3kTLrUWHKhKfq1rc9yokZNmBE~iuseNEkPmxfAbaYHQ9zeyYS2WxiSuSqX0Ic2X5YkPLau1Vqmn-YXU5m9VktrP9KuQpC0IJyg0w~saxYLfy8QXidGFl5gyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)