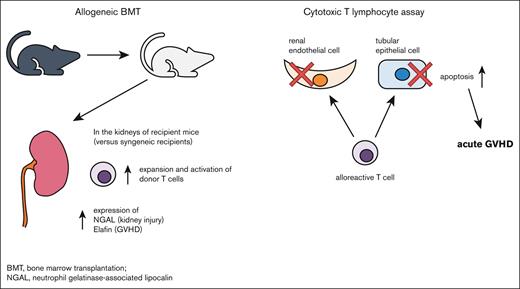
Key Points
Donor-derived T cells are increased and activated in the kidney after allo-HCT.
Allogeneic immune responses induced by donor T cells contribute to renal endothelial and tubular epithelial cell damage after allo-HCT.
Abstract
Acute kidney injury (AKI) is a frequent complication of allogeneic hematopoietic cell transplantation (allo-HCT). There are many causes of AKI after allo-HCT, but it is unknown whether renal acute graft-versus-host disease (aGVHD) caused by direct allogeneic donor T-cell–mediated renal damage contributes. Here, we tested whether allogeneic donor T cells attack kidneys in murine models of aGVHD. To avoid confounding effects of nephrotoxic agents, we did not administer immunosuppressants for GVHD prophylaxis. We found that urinary N-acetyl-β-D-glucosaminidase, a marker of tubular injury, was elevated in allogeneic recipients on day 14 after allogeneic bone marrow transplantation. Donor major histocompatibility complex–positive cells were present and CD3+ T cells were increased in the glomerulus, peritubular capillaries, interstitium, and perivascular areas in the kidneys of allo-HCT recipient mice. These T cells included both CD4+ and CD8+ cells with elevated activation markers, increased exhaustion markers, and greater secretion of proinflammatory cytokines and cytotoxic proteins. Consistent with allo-T-cell–mediated renal damage, expression of neutrophil gelatinase-binding lipocalin, a marker of AKI, and elafin, a marker of aGVHD, were increased in renal tissue of allogeneic recipients. Because apoptosis of target cells is observed on histopathology of aGVHD target tissues, we confirmed that alloreactive T cells increased apoptosis of renal endothelial and tubular epithelial cells in cytotoxic T-lymphocyte assays. These data suggest that immune responses induced by donor T cells contribute to renal endothelial and tubular epithelial cell injury in allo-HCT recipients and that aGVHD may contribute to AKI after allo-HCT.
Introduction
Acute graft-versus-host disease (aGVHD) is a life-threatening complication of allogeneic hematopoietic cell transplantation (allo-HCT). The skin, liver, and gastrointestinal tract are the organs most often targeted and damaged by aGVHD.1 Although chronic GVHD can cause nephrotic syndrome and glomerulonephritis,2,3 the kidney is not generally recognized as a target of aGVHD.4
Acute kidney injury (AKI) is a frequent complication of allo-HCT occurring in >50% of patients.5 The differential diagnosis for AKI after allo-HCT is broad, ranging from drug-induced nephropathy to thrombotic microangiopathy (TMA).6,7 Clinical studies have found an association between aGVHD and the development of AKI8; however, it is unknown whether aGVHD directly causes AKI.
aGVHD is initiated by the activation of antigen-presenting cells (APCs) after conditioning-related tissue damage. Donor-derived T cells are then activated and help coordinate target organ injury.9 Strategies to prevent GVHD typically use immunosuppressive agents such as calcineurin inhibitors (CNIs), mycophenolate mofetil, and posttransplant cyclophosphamide.1 Many of these immunosuppressive agents are also nephrotoxic,10 which complicates efforts to determine whether aGVHD directly targets the kidneys. Determining the etiology of AKI after allo-HCT is further complicated by the frequent inability to obtain diagnostic kidney biopsies because of the increased risk of bleeding after HCT.10 Despite these challenges, several studies suggest that the kidneys may be targeted by aGVHD. For example, urine markers of AKI were increased, expression of inflammatory cytokines in the kidneys were increased, and cellular infiltration of renal tubules and interstitium were observed in allo-HCT recipient mice.11 In a rat model of bone marrow transplant (BMT), donor-derived leukocytes infiltrated the kidneys of recipient animals12; however, the classification and function of these cells were not determined. These prior studies indicate that alloimmune responses may contribute to kidney damage, but definitive evidence and mechanisms of direct alloimmune-mediated kidney damage have not been established.13
In this report, we tested whether donor allogeneic T cells cause kidney damage by using murine models of allo-HCT. We found that donor-derived T cells infiltrate the kidney of allo-HCT recipients and directly attack endothelial and epithelial cells, resulting in renal aGVHD. Thus, renal aGVHD may contribute to AKI after allo-HCT.
Methods
Mice
BALB/c (H-2d) and C57BL/6 (B6, H-2b) mice were purchased from CLEA Japan, Inc (Tokyo, Japan). B6D2F1 (H-2b/d) mice were purchased from Japan SLC, Inc (Hamamatsu, Japan). C3H.sw mice were bred in our facility. Age-matched female mice (10-14 weeks old) were used for all experiments. Animals were cared for according to the animal welfare regulations of Yamagata University, and the study protocol was approved by the Animal Subjects Committee of Yamagata University. The investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
BMT
BMTs were performed as previously described.14-16 Briefly, splenic T cells from donor animals were isolated by magnetic cell sorting using CD90.2 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). We used well-established HCT models. In a major histocompatibility complex (MHC)-mismatched model, BALB/c animals were used as recipients and received 7 or 4 Gy total body irradiation (TBI; TITAN-225S, Shimadzu Industrial Systems, Kyoto, Japan) on day −1 and were injected IV with 0.5 × 106 CD90.2+ T cells together with 5 × 106 bone marrow (BM) cells from either syngeneic or allogeneic C57BL/6 (B6) donors on day 0. In a parent-to-F1 model, B6D2F1 animals received 11 Gy TBI on day −1 and were injected with 3.5 × 106 CD90.2+ splenic T cells together with 5 × 106 BM cells from either syngeneic or allogeneic B6 donors on day 0. In an MHC-matched multiple minor antigen–mismatched model, C3H.sw animals received 10.5 Gy TBI on day −1 and were injected with 1 × 106 CD90.2+ splenic T cells together with 5 × 106 BM cells from either syngeneic or allogeneic B6 donors on day 0. The mice were randomly assigned to syngeneic or allogeneic transplant groups in each experiment. The investigators were not blinded to allocation during the experiments or outcome assessment.
Systemic analysis of GVHD and urinalysis
We monitored survival after HCT daily and assessed the degree of clinical GVHD weekly, as described previously.17 We performed weekly qualitative urinalysis with urine test strips that react to albumin (URIACE-KC, Terumo, Tokyo, Japan). Concentrations of N-acetyl-β-D-glucosaminidase (NAG) in the urine were measured 14 and 42 days after HCT by enzyme-linked immunosorbent assay using QuantiChrom β-N-acetylglucosaminidase Assay Kit (BioAssay Systems, Hayward, CA). Assays were performed according to the manufacturer’s protocol and read at 405 nm using a microplate reader (xMark; Bio-Rad Laboratories, Hercules, CA).
Histopathological analysis
Kidney tissue was fixed in 4% paraformaldehyde (FUJIFILM Wako Pure Chemical, Osaka, Japan) and embedded in paraffin. Tissues were sectioned (5 μm thickness) and periodic acid-Schiff (PAS)-stained for light microscopic examination. To detect donor-derived cells, immunofluorescence staining for H-2b was performed using a fluorescein isothiocyanate–conjugated mouse monoclonal anti-mouse MHC class I H-2Kb antibody (clone AF6-88.5, LS-C188568, LSBio, Seattle, WA) diluted 1:100 overnight at 4°C. Vector TrueVIEW Autofluorescence Quenching Kit (Vector Laboratories, Newark, CA) was used to diminish autofluorescence. Staining with 4′,6-diamidino-2-phenylindole (Bio-Rad Laboratories) was also performed on each section. To detect T cells and elafin-positive cells, paraffin-embedded kidney specimens were stained using an avidin-biotin-peroxidase complex technique. Primary anti-CD3 rabbit polyclonal antibody (17617-1-AP, proteintech, Rosemont, IL; 1:300),and anti-elafin rabbit polyclonal antibody (ab272906, Abcam, Cambridge, United Kingdom, 1:500) were incubated with specimens, overnight at 4°C. Secondary horseradish peroxidase–conjugated anti-rabbit immunoglobulin G (W4018, PROMEGA, Madison, WI) was diluted 1:200 and incubated for 1 hour at room temperature. Bound antibody was detected with 3,3'Diaminobenzidine (Takara Bio, Kusatsu, Japan). Slides were then counterstained with hematoxylin, dehydrated, and covered. Sections were observed using a microscope (OLYMPUS, Tokyo, Japan). For each sample, CD3+ cells in the glomerular, interstitial, and periarterial regions were counted from 10 randomly selected 200× magnification fields.
Cell isolation
Kidneys were minced and dissociated with collagenase D (Roche, Basel, Switzerland) and DNase I (Roche) at 37°C for 50 minutes to obtain single-cell suspensions. Cells were then strained through a 100-μm cell filter followed by incubation with red blood cell lysis buffer (BioLegend, San Diego, CA) at room temperature for 3 minutes.
Flow cytometry
Flow cytometry was performed as previously described.16,18,19 Briefly, to analyze donor T-cell expansion and activation status, on days 14 and 42 after HCT, kidney cells from animals that received transplantation were resuspended in 2% fetal bovine serum (Sigma-Aldrich) in phosphate-buffered saline and stained with conjugated monoclonal antibodies (mAbs). Zombie Aqua dye (BioLegend) was used to selectively stain dead cells according to the manufacturer’s protocol before incubation with primary antibodies. The following antibodies were used: fluorescein isothiocyanate–conjugated mAbs against mouse H-2Kd (clone SF1-1.1, 116606; 1:200) and H-2Kb (clone AF-6-88.5, 116506; 1:200); phycoerythrin (PE)-conjugated mAbs against mouse CD69 (clone H1.2F3, 104508; 1:200), programmed cell death protein 1 (PD-1) (clone 29F.1A12, 135206; 1:200), and CD62L (clone MEL-14, 104408; 1:200); APC-conjugated mAbs against mouse CD25 (clone PC61, 102012; 1:200), CD366 (Tim-3, clone RMT3-23, 119706; 1:200), and CD44 (clone IM7, 103012; 1:200); APC/cyanine7-conjugated mAb against mouse CD3 (clone GK1.5, 100428; 1:200); and pacific blue–conjugated mAbs against mouse CD4 (clone 1A8, 127614; 1:200) and CD8a (clone 53-6.7, 100725; 1:200) (BioLegend). After staining, the cells were washed twice and fixed with BD fluorescence-activated cell sorting lysing solution (Becton, Dickinson and Company, Franklin Lakes, NJ). For intracellular cytokine staining, cells were permeabilized after fixation and stained with PE-conjugated mAbs against mouse interferon gamma (IFN-γ; clone XMG1.2, 505808; 1:200) and granzyme B (clone QA16A02, 372208; 1:200) as well as APC-conjugated mAbs against mouse tumor necrosis factor α (TNF-α; clone MP6-XT22, 506308; 1:200) and perforin (clone S16009B, 154404; 1:200) (BioLegend). To detect regulatory T cells, cells were stained with PE-conjugated mAbs against mouse FOXP3 (clone MF-14, 126404; 1:200; BioLegend) after permeabilization with Perm/Wash Buffer (Becton, Dickinson and Company). Cells were run on a FACSMelody cell sorter (Becton, Dickinson and Company) and analyzed using FlowJo version 10.8.1.
Western blot analysis
Kidneys were lysed in radio-immunoprecipitation assay buffer, and total proteins were extracted as previously reported.20 Equal amounts of protein extracts were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 20 mM tris-HCl, pH 7.4, containing 150 mM NaCl, 0.1% Tween (tris-buffered saline with Tween 20), and 5% milk or 5% bovine serum albumin. The membranes were probed overnight at 4°C with the following primary antibodies: neutrophil gelatinase-associated lipocalin (NGAL; clone H-7, sc-515876, Santa Cruz, Dallas, TX; 1:500), elafin (ab272906, Abcam; 1:1000), and β-tubulin (no. 2146, Cell Signaling Technology, Danvers, MA; 1:3000). After incubation with horseradish peroxidase, conjugated secondary antibodies diluted in tris-buffered saline with Tween 20 containing 5% milk or 5% bovine serum albumin, immunoreactive bands were detected using Clarity Max Western ECL Substrate (Bio-Rad Laboratories). The bands were recorded using the Light Capture II system using CS Analyzer version 3.0 software (ATTO, Tokyo, Japan). Protein expression levels were normalized to β-tubulin.
Cell culture
BALB/c mouse primary kidney endothelial cells (BALB-5014; Cell Biologics, Chicago, IL) were cultured in complete mouse endothelial cell medium (M1168; Cell Biologics). BALB/c mouse primary proximal tubular epithelial cells (BALB-5015; Cell Biologics) were cultured in complete epithelial cell medium (M6621; Cell Biologics).
In vitro allogeneic cytotoxic T-cell assay
Effector splenic T cells from naïve B6 and BALB/c animals were activated by coculture with irradiated (20 Gy) red blood cell–lysed splenocytes from BALB/c mice at an effector-to-target ratio of 5:2 for 6 days. Target cells consisted of 4 × 104 BALB/c-derived renal endothelial cells or proximal tubular epithelial cells that were cultured overnight on collagen-coated 24-well plates (AGC Techno Glass, Yoshida, Japan). After aspirating the medium, 2 × 106 effector cells suspended in RPMI supplemented with 10% fetal bovine serum were added to each well and incubated for 4 hours. After removing the floating cells and medium, the wells were washed with phosphate-buffered saline, followed by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL) using the CF 594 TUNEL Assay Apoptosis Detection Kit (Biotium, Fremont, CA). Staining with 4′,6-diamidino-2-phenylindole (Bio-Rad Laboratories) and wheat germ agglutinin 488 (Thermo Fisher Scientific, Waltham, MA) was also performed on each well. Cells were observed using a microscope (BZ-X700, KEYENCE, Osaka, Japan).
Statistical analysis
Bars and error bars represent the mean and standard error of the mean, respectively. We used unpaired t tests for statistical comparisons, except for survival analyses. P values < .05 were considered significant. Statistical significance of survival was determined using the log-rank test. All statistical analyses were performed using EZR version 1.5321 and GraphPad Prism 9. All sample sizes are detailed in each figure legend.
Results
Renal injury marker levels are elevated in allo-HCT mice
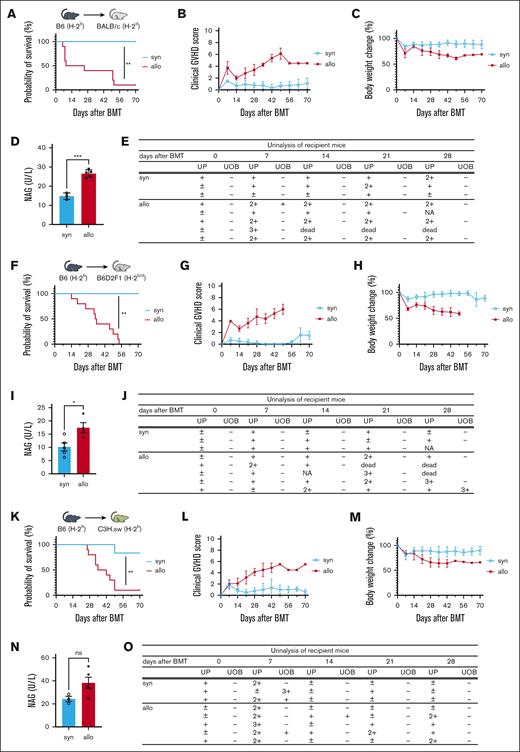
To test whether allogeneic immune responses cause renal injury, we used murine models of HCT. To avoid confounding nephrotoxic effects, GVHD prophylaxis was not used. As expected, survival was lower, clinical signs of aGVHD were more severe, and weight loss was more pronounced in BALB/c allogeneic recipients of MHC-mismatched C57BL/6 cells relative to that in syngeneic recipients (Figure 1A-C). Levels of rinary protein and NAG, a marker of tubular damage, which were measured 14 days after HCT, were significantly elevated in allo-HCT recipients compared with those in syngeneic mice (Figure 1D-E). To rule out strain-dependent effects, we used a parent-to-F1 (B6 into B6D2F1) model and observed similar results (Figure 1F-J). In addition, we observed a similar but nonsignificant increase in urine NAG in an MHC-matched multiple minor antigen–mismatched B6 into C3H.sw model (Figures 1K-O). These data collectively showed that urine markers of renal injury are increased in allo-HCT recipient mice, suggesting that alloimmune responses may directly cause renal damage.
Renal injury markers are elevated in allo-HCT mice. (A-E) BALB/c mice received 7 Gy of TBI on day −1, and received transplantation with 0.5 × 106 CD90.2+ splenic T cells together with 5 × 106 BM cells from either syngeneic (syn) BALB/c or allogeneic (allo) MHC-mismatched B6 donors. (A-C) n = 6 to 10 mice per group, pooled from 2 experiments. (D-E) n = 3 to 5 mice per group; representative data from 3 experiments are shown. (F-J) B6D2F1 mice received 11 Gy of TBI on day −1 and received transplantation with 3.5 × 106 CD90.2+ splenic T cells together with 5 × 106 BM cells from either syngeneic B6D2F1 or allogeneic parent-to-F1 B6 donors. (F-H) n = 6 to 10 mice per group, pooled from 2 experiments. (I) n = 4 to 5 mice per group, (J) n = 3 to 5 mice per group; representative data from 2 experiments are shown. (K-O) C3H.sw animals received 10.5 Gy of TBI on day −1 and received transplantation with 1 × 106 CD90.2+ splenic T cells together with 5 × 106 TCD-BM cells from either syn C3H.sw or MHC-matched multiple minor antigen–mismatched B6 donors. (K-M) n = 6 to 10 mice per group, pooled from 2 experiments. (N-O) n = 3 to 5 mice per group; representative data from 2 experiments are shown. Survival (A,F,K), clinical GVHD score (B,G,L), body weight change (C,H,M), and urinalysis (E,J,O) are shown. Urinary NAG levels of recipient mice on day 14 after BMT was significantly higher in the allogeneic group in the MHC-mismatched (D) and parent-to-F1 (I) models than in other models. (N) In the MHC-matched multiple minor antigen–mismatched model, urinary NAG levels tended to be higher in the allo group than in the syn group. Results summarize at least 2 independent experiments. Data represent the mean ± standard error of the mean (SEM). Student t test was used for statistical analysis; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. UOB, urinary occult blood; UP, urinary protein.
Renal injury markers are elevated in allo-HCT mice. (A-E) BALB/c mice received 7 Gy of TBI on day −1, and received transplantation with 0.5 × 106 CD90.2+ splenic T cells together with 5 × 106 BM cells from either syngeneic (syn) BALB/c or allogeneic (allo) MHC-mismatched B6 donors. (A-C) n = 6 to 10 mice per group, pooled from 2 experiments. (D-E) n = 3 to 5 mice per group; representative data from 3 experiments are shown. (F-J) B6D2F1 mice received 11 Gy of TBI on day −1 and received transplantation with 3.5 × 106 CD90.2+ splenic T cells together with 5 × 106 BM cells from either syngeneic B6D2F1 or allogeneic parent-to-F1 B6 donors. (F-H) n = 6 to 10 mice per group, pooled from 2 experiments. (I) n = 4 to 5 mice per group, (J) n = 3 to 5 mice per group; representative data from 2 experiments are shown. (K-O) C3H.sw animals received 10.5 Gy of TBI on day −1 and received transplantation with 1 × 106 CD90.2+ splenic T cells together with 5 × 106 TCD-BM cells from either syn C3H.sw or MHC-matched multiple minor antigen–mismatched B6 donors. (K-M) n = 6 to 10 mice per group, pooled from 2 experiments. (N-O) n = 3 to 5 mice per group; representative data from 2 experiments are shown. Survival (A,F,K), clinical GVHD score (B,G,L), body weight change (C,H,M), and urinalysis (E,J,O) are shown. Urinary NAG levels of recipient mice on day 14 after BMT was significantly higher in the allogeneic group in the MHC-mismatched (D) and parent-to-F1 (I) models than in other models. (N) In the MHC-matched multiple minor antigen–mismatched model, urinary NAG levels tended to be higher in the allo group than in the syn group. Results summarize at least 2 independent experiments. Data represent the mean ± standard error of the mean (SEM). Student t test was used for statistical analysis; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. UOB, urinary occult blood; UP, urinary protein.
Donor T cells infiltrate the kidneys of allogeneic recipients and are associated with histopathological signs of kidney damage.
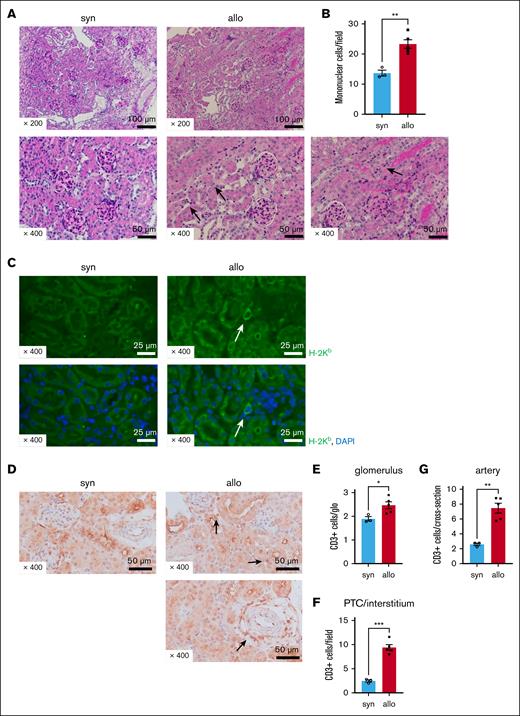
Next, we determined whether the increased urine markers of renal damage corresponded with renal histopathological changes. Renal tissue was harvested 14 days after allogeneic (C57BL/6 into BALB/c) or syngeneic HCT. We observed increased peritubular capillaritis (PTCitis) and tubulitis accompanied by mononuclear cell infiltration on PAS staining in the kidneys of allogeneic mice compared with in those of syngeneic mice (Figure 2A-B). The glomeruli appeared unaffected in both groups upon PAS staining, and no evidence of TMA was found (Figure 2A). We used immunostaining to characterize the inflammatory cellular infiltrate. H-2Kb–positive (donor-type) cells were found in the interstitium of allogeneic mice (Figure 2C), and the number of CD3+ T cells was significantly increased in the glomeruli, peritubular capillaries/interstitium, or periarterial areas of allo-HCT recipients compared with that of syngeneic recipients (Figures 2D-G). Thus, the kidneys of allo-HCT recipients had greater inflammation on histopathology than kidneys of syngeneic recipients and were infiltrated with greater amounts of donor and CD3+ cells.
Renal damage and donor T-cell infiltration are increased in allo-HCT recipients. BALB/c (H-2d) animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 (H-2b) donors. The kidneys were collected from recipient mice on day 14 after HCT. (A) Representative images of PAS-stained kidneys after HCT are shown. Compared with the syn group, there was increased mononuclear cell infiltration in the peritubular capillaries (PTCs; see arrows in center bottom row image) and tubules (see arrows in right bottom row image) of allogeneic recipients. (B) Quantification of infiltrating mononuclear cells (MNCs) in kidneys is shown. The number of infiltrating MNCs per 200× original magnification field is significantly higher in allo mice than in syn mice. (C) Representative H-2Kb (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue) immunofluorescence images are shown. H-2Kb–positive donor-derived cells were found in the kidneys of allogeneic recipients. (D) Representative images of immunohistological staining for CD3 are shown. Allogeneic recipients demonstrated increased CD3+ T-cell infiltration in the peritubular, perivascular, and glomerular areas relative to syngeneic recipients. (E-G) Quantification of T cells infiltrating the kidneys is shown. The absolute number of CD3+ T cells infiltrating the glomeruli (E), interstitium (F), and perivascular area (G) was significantly higher in allogeneic mice than in syngeneic mice; n = 3 to 5 mice per group. Data shown represent 2 experiments. Magnifications are shown in the figures. The bars represent the mean ± SEM. Student t test was used for statistical analysis; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for panels B,E-G.
Renal damage and donor T-cell infiltration are increased in allo-HCT recipients. BALB/c (H-2d) animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 (H-2b) donors. The kidneys were collected from recipient mice on day 14 after HCT. (A) Representative images of PAS-stained kidneys after HCT are shown. Compared with the syn group, there was increased mononuclear cell infiltration in the peritubular capillaries (PTCs; see arrows in center bottom row image) and tubules (see arrows in right bottom row image) of allogeneic recipients. (B) Quantification of infiltrating mononuclear cells (MNCs) in kidneys is shown. The number of infiltrating MNCs per 200× original magnification field is significantly higher in allo mice than in syn mice. (C) Representative H-2Kb (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue) immunofluorescence images are shown. H-2Kb–positive donor-derived cells were found in the kidneys of allogeneic recipients. (D) Representative images of immunohistological staining for CD3 are shown. Allogeneic recipients demonstrated increased CD3+ T-cell infiltration in the peritubular, perivascular, and glomerular areas relative to syngeneic recipients. (E-G) Quantification of T cells infiltrating the kidneys is shown. The absolute number of CD3+ T cells infiltrating the glomeruli (E), interstitium (F), and perivascular area (G) was significantly higher in allogeneic mice than in syngeneic mice; n = 3 to 5 mice per group. Data shown represent 2 experiments. Magnifications are shown in the figures. The bars represent the mean ± SEM. Student t test was used for statistical analysis; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 for panels B,E-G.
Kidney-infiltrating donor T cells are activated and produce inflammatory cytokines
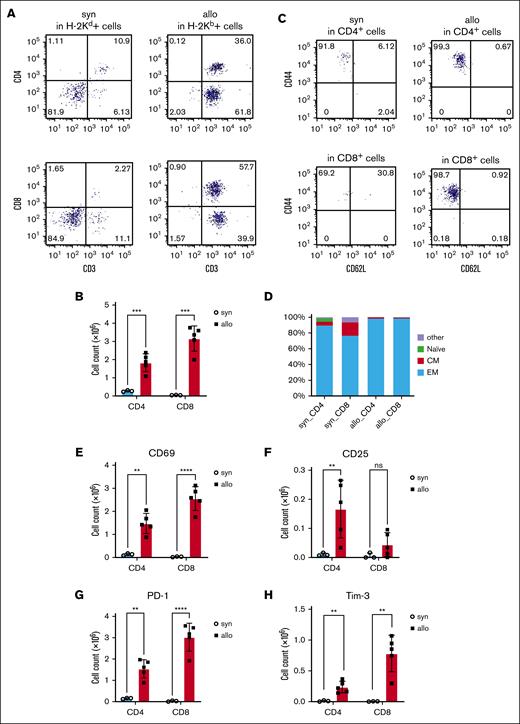
To determine whether the donor T cells infiltrating the kidneys of allo-HCT recipients expressed markers consistent with activation and inflammation, we performed flow cytometry on day 14 after HCT. We analyzed H-2Kd+ cells isolated from kidneys from syngeneic recipients and H-2Kb+ cells from kidneys of allogeneic recipients. The amount of kidney-infiltrating CD4+ and CD8+ T cells was increased in allogeneic mice (Figure 3A-B). CD4+ and CD8+ antigen–experienced effector memory T cells (CD44+CD62L−) were increased in kidneys of allo-HCT recipients. Consistent with this, activation markers (CD69 and CD25) and exhaustion markers (PD-1 and Tim-3) were also increased in T cells isolated from the kidneys of allo-HCT recipients (Figure 3C-H; supplemental Figure 1A-D). However, CD4+CD25+Foxp3+ regulatory T cells were not increased in the kidneys, even in the allogeneic recipients (supplemental Figure 2).
Activation and exhaustion markers were increased on kidney-infiltrating donor T cells from allogeneic recipients. BALB/c (H-2d) animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 (H-2b) donors. On day 14 after HCT, T cells infiltrating the kidneys (H-2Kd+ cells in syn recipients or H-2Kb+ cells in allo recipients) were analyzed by flow cytometry. (A) Representative dot plots of donor T-cell (CD3+) expansion in the kidneys are shown. (B) Absolute numbers of total CD3+CD4+ and CD3+CD8+ cells among gated live donor cells infiltrating the kidneys are shown. (C-D) Subsets of donor-derived CD4+ T cells (top) and CD8+ T cells (bottom) in the kidney of the allo-HCT recipients are shown. Representative dot plots (C) and percentages of each subset (D) are shown. (E-H) T-cell activation (CD69 and CD25) and exhaustion (PD-1 and Tim-3) markers were analyzed in CD3+CD4+ and CD3+CD8+ populations. CD69 (E), CD25 (F), PD-1 (G), and Tim-3 (H) were higher in the allo group; n = 3 to 5 mice per group. Data representing 3 experiments are shown. The bars depict the mean ± SEM. Student t test was used for statistical analysis for panels B,E-H. Naïve, naïve T cell (CD44−CD62L+); CM, central memory T cell (CD44+CD62L+); EM, effector memory T cell (CD44+CD62L−).
Activation and exhaustion markers were increased on kidney-infiltrating donor T cells from allogeneic recipients. BALB/c (H-2d) animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 (H-2b) donors. On day 14 after HCT, T cells infiltrating the kidneys (H-2Kd+ cells in syn recipients or H-2Kb+ cells in allo recipients) were analyzed by flow cytometry. (A) Representative dot plots of donor T-cell (CD3+) expansion in the kidneys are shown. (B) Absolute numbers of total CD3+CD4+ and CD3+CD8+ cells among gated live donor cells infiltrating the kidneys are shown. (C-D) Subsets of donor-derived CD4+ T cells (top) and CD8+ T cells (bottom) in the kidney of the allo-HCT recipients are shown. Representative dot plots (C) and percentages of each subset (D) are shown. (E-H) T-cell activation (CD69 and CD25) and exhaustion (PD-1 and Tim-3) markers were analyzed in CD3+CD4+ and CD3+CD8+ populations. CD69 (E), CD25 (F), PD-1 (G), and Tim-3 (H) were higher in the allo group; n = 3 to 5 mice per group. Data representing 3 experiments are shown. The bars depict the mean ± SEM. Student t test was used for statistical analysis for panels B,E-H. Naïve, naïve T cell (CD44−CD62L+); CM, central memory T cell (CD44+CD62L+); EM, effector memory T cell (CD44+CD62L−).
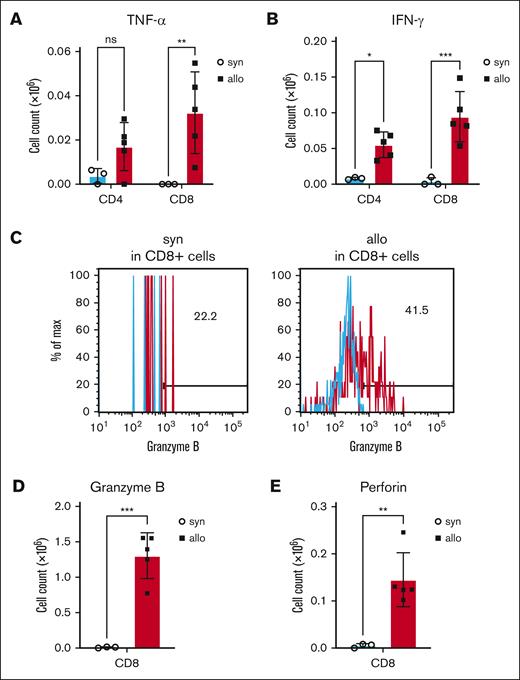
Production of the proinflammatory cytokines, TNF-α and IFN-γ, were also increased in T cells isolated from kidneys of allo-HCT recipients (Figure 4A-B; supplemental Figure 1E-F). Furthermore, CD8+ T cells from the kidneys of allo-HCT recipients secreted more cytotoxic markers, including granzyme B and perforin (Figure 4C-E; supplemental Figure 1G-H). Collectively, these data demonstrated that allogeneic donor T cells in the kidney were activated and expressed cytotoxic proteins and inflammatory cytokines that might have induced tissue damage.
Production of cytotoxic proteins and cytokines was increased in kidney-infiltrating donor T cells of allo-HCT recipients. BALB/c (H-2d) animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 (H-2b) donors. On day 14 after HCT, kidneys were harvested, and disrupted, and incubated for 5 hours with phorbol 12-myristate 13-acetate, ionomycin, brefeldin A, and monensin, followed by intracellular staining. (A-B) Donor TNF-α– (A) and IFN-γ–producing (B) T cells in the kidney at day 14 after allo-HCT are shown. (C-E) Donor granzyme B– (C-D) and perforin–producing (E) CD8+ T cells in the kidney on day 14 after allo-HCT are shown. Representative flow cytometry images of granzyme B (C) are shown. Blue histograms indicate isotype control. Production of granzyme B (D) and perforin (E) by renal-infiltrating cytotoxic T cells was significantly increased in allogeneic recipients; n = 3 to 5 mice per group. Data are representative of 3 experiments. The bars show the mean ± SEM. Student t test was used for statistical analysis; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Production of cytotoxic proteins and cytokines was increased in kidney-infiltrating donor T cells of allo-HCT recipients. BALB/c (H-2d) animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 (H-2b) donors. On day 14 after HCT, kidneys were harvested, and disrupted, and incubated for 5 hours with phorbol 12-myristate 13-acetate, ionomycin, brefeldin A, and monensin, followed by intracellular staining. (A-B) Donor TNF-α– (A) and IFN-γ–producing (B) T cells in the kidney at day 14 after allo-HCT are shown. (C-E) Donor granzyme B– (C-D) and perforin–producing (E) CD8+ T cells in the kidney on day 14 after allo-HCT are shown. Representative flow cytometry images of granzyme B (C) are shown. Blue histograms indicate isotype control. Production of granzyme B (D) and perforin (E) by renal-infiltrating cytotoxic T cells was significantly increased in allogeneic recipients; n = 3 to 5 mice per group. Data are representative of 3 experiments. The bars show the mean ± SEM. Student t test was used for statistical analysis; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Tissue injury markers were increased in the kidneys of allogeneic recipients
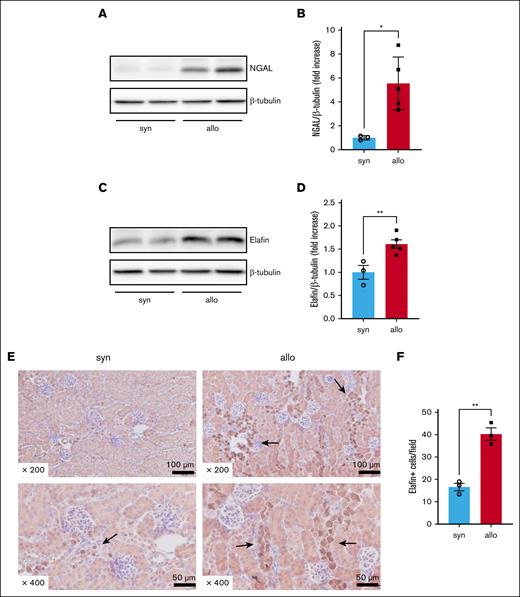
To determine whether alloimmune responses could produce kidney damage, we measured the expression of protein markers of kidney damage and alloimmune-mediated injury in the kidneys of recipient mice on day 14 after HCT. NGAL, an indicator of AKI, was significantly higher 14 days after HCT in kidney lysates of allogeneic compared with that in kidney lysates of syngeneic recipients (Figure 5A-B). Similarly, elafin, a tissue marker of acute GVHD,22 was higher in the allogeneic group (Figure 5C-D). Immunohistochemical staining to evaluate the localization of elafin revealed that elafin-positive cells were predominantly present in the distal tubular epithelium of allogeneic recipients (Figure 5E-F). These data suggested that the kidneys in allogeneic animals were damaged by alloimmune responses.
Alloimmune-mediated renal tissue damage was increased in allo recipients. BALB/c animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 donors. Kidneys were harvested on day 14 after HCT to investigate the expression of NGAL and elafin in whole-kidney lysates, and immunohistochemically stained for elafin. (A) Representative western blot image of NGAL is shown. (B) Quantification of NGAL protein level is shown. (C) Representative western blot image of elafin is shown. (D) Quantification of elafin protein level is shown. (E-F) Representative images (E) and quantification (F) of immunohistochemical staining for elafin are shown. Arrows highlight elafin-positive cells. Elafin staining was positive mainly in distal tubular epithelial cells. Magnifications are shown in the figures in panel E. The number of elafin-positive cells per 200× original magnification field was higher in the kidneys of allo mice than in those of syn mice in panel F. n = 3 to 5 mice per group. The data are representative of 2 experiments. The bars show the mean ± SEM. Student t test was used for statistical analysis for panels B,D,F.
Alloimmune-mediated renal tissue damage was increased in allo recipients. BALB/c animals were lethally irradiated and received transplantation with CD90.2+ splenic T cells together with BM cells from either syn BALB/c or allo MHC-mismatched B6 donors. Kidneys were harvested on day 14 after HCT to investigate the expression of NGAL and elafin in whole-kidney lysates, and immunohistochemically stained for elafin. (A) Representative western blot image of NGAL is shown. (B) Quantification of NGAL protein level is shown. (C) Representative western blot image of elafin is shown. (D) Quantification of elafin protein level is shown. (E-F) Representative images (E) and quantification (F) of immunohistochemical staining for elafin are shown. Arrows highlight elafin-positive cells. Elafin staining was positive mainly in distal tubular epithelial cells. Magnifications are shown in the figures in panel E. The number of elafin-positive cells per 200× original magnification field was higher in the kidneys of allo mice than in those of syn mice in panel F. n = 3 to 5 mice per group. The data are representative of 2 experiments. The bars show the mean ± SEM. Student t test was used for statistical analysis for panels B,D,F.
Renal damage caused by alloreactive T cells was present in the late phase of allo-HCT and persisted independent of the radiation dose used for conditioning
To determine whether the renal damage observed in recipient kidneys in the early phase of HCT (on day 14 after HCT) remained present at later time points, we also examined the kidneys on day 42 after HCT. In this later phase of the disease, the elevation of urinary NAG, mononuclear cell infiltration in histopathological samples, and increased NGAL expression were observed in allogeneic recipients (supplemental Figure 3). These data suggested that renal damage in the early phase continued in the late phase. Analysis of donor-derived T cells by flow cytometry showed that CD4+ T-cell infiltration was reduced to the same extent as in syngeneic recipients, but CD8+ T-cell infiltration and expression of activation (CD69) and exhaustion markers (PD-1 and Tim-3) were increased in allogeneic recipients similar to that observed in the acute phase (supplemental Figure 4). These data suggested that donor-derived allogeneic T cells remained activated and likely mediated kidney damage late in the disease course.
In addition, we performed BMT with a reduced radiation dose (4 Gy) to confirm that the events observed in the kidneys of HCT recipients were not due to radiation toxicity. Compared with myeloablative conditioning, recipients of reduced intensity conditioning had less severe GVHD and lower urinary protein and NAG. However, the trend toward more renal damage in allogeneic recipients than in syngeneic recipients persisted, and the number of T cells infiltrating the kidney remained significantly higher in renal glomeruli, interstitium, and perivascular regions of allogeneic recipients (supplemental Figure 5).
Allogeneic donor T cells directly attack renal cells
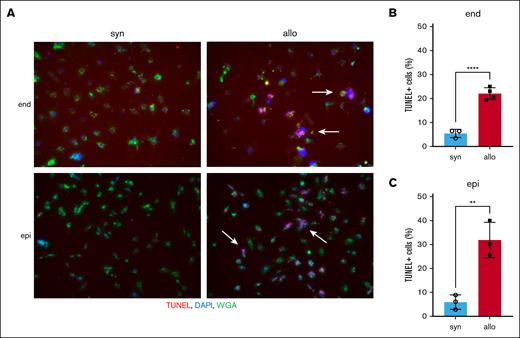
To confirm whether allogeneic donor T cells could directly attack renal cells, TUNEL staining was performed on histopathological specimens of the kidneys of recipient animals 14 days after HCT. In this in vivo experiment, few TUNEL-positive cells were present, even in the allogeneic group (supplemental Figure 6). Therefore, we performed an in vitro cytotoxic T-lymphocyte killing assay. We activated BALB/c (syngeneic) or B6 (allogeneic) effector T cells by culturing each with irradiated BALB/c splenocytes for 6 days. These effector T cells were then cocultured with target BALB/c-derived renal endothelial or tubular epithelial cells. Apoptotic target cells detected by TUNEL staining were significantly higher when cocultured with allogeneic vs syngeneic effector T cells. The increase in apoptosis after coculture with allogeneic T-cell effectors was similar between renal endothelial and epithelial target cells (Figure 6A-C). These data suggested that allogeneic donor T cells could directly attack renal cells and may mediate aGVHD in the kidney.
Allo donor T cells directly attack renal cells. Splenic effector T cells from either syn BALB/c or allo B6 mice were activated by coculturing with irradiated whole splenocytes from BALB/c mice for 6 days. Target BALB/c-derived renal endothelial cells (end) or proximal tubular epithelial cells (epi) were cocultured with these activated effector T cells for 4 hours. Target cell apoptosis was detected by TUNEL assay. (A) Representative TUNEL-stained images of renal endothelial cells (top) and proximal tubular epithelial cells (bottom) are shown. Arrows highlight TUNEL-positive cells. TUNEL, red; DAPI, blue; wheat germ agglutinin (WGA), green. (B-C) The proportion of TUNEL-positive cells among renal endothelial cells (B) and proximal tubular epithelial cells (C) is shown; n = 3 to 4 mice per group. The bars show the mean ± SEM. Student t test was used for statistical analysis in panels B-C.
Allo donor T cells directly attack renal cells. Splenic effector T cells from either syn BALB/c or allo B6 mice were activated by coculturing with irradiated whole splenocytes from BALB/c mice for 6 days. Target BALB/c-derived renal endothelial cells (end) or proximal tubular epithelial cells (epi) were cocultured with these activated effector T cells for 4 hours. Target cell apoptosis was detected by TUNEL assay. (A) Representative TUNEL-stained images of renal endothelial cells (top) and proximal tubular epithelial cells (bottom) are shown. Arrows highlight TUNEL-positive cells. TUNEL, red; DAPI, blue; wheat germ agglutinin (WGA), green. (B-C) The proportion of TUNEL-positive cells among renal endothelial cells (B) and proximal tubular epithelial cells (C) is shown; n = 3 to 4 mice per group. The bars show the mean ± SEM. Student t test was used for statistical analysis in panels B-C.
Discussion
AKI is a frequent complication of allo-HCT. The leading causes are thought to be nephrotoxic drugs or transplant complications.7 However, the etiology of renal injury is often not clear10 because renal biopsies early after allo-HCT are frequently contraindicated.23 One potentially underrecognized cause of AKI after allo-HCT is renal aGVHD. Previous studies reported an association between aGVHD and AKI,8,24-26 but it was not known whether this association was direct or indirect.
In this report, we tested whether alloimmune responses directly cause renal damage using murine models of HCT. To avoid the confounding effects of nephrotoxic drugs, we did not use GVHD prophylaxis. Compared with syngeneic recipients, allogeneic recipients developed increased renal glomerular and tubular injury–associated markers in the urine as well as greater donor T-cell infiltration of the renal glomeruli, tubulointerstitium, and perivasculature. These infiltrating donor T cells expressed higher levels of activation and exhaustion markers and secreted more inflammatory and cytotoxic cytokines than those from syngeneic controls. Furthermore, activated allogeneic T cells directly caused apoptosis of cocultured renal cells, suggesting that allogeneic donor T cells may directly mediate renal injury after allo-HCT.
Several prior studies have suggested that alloimmune responses cause renal damage after HCT. Similar to our data, these studies demonstrated kidney dysfunction indicated by increased urinary albumin11 and NAG11,12 in preclinical models of allo-HCT. These studies also showed renal infiltration of CD4+ T cells, CD8+ T cells, FoxP3+ regulatory T cells, and macrophages, primarily in the tubules and interstitium. However, these prior studies did not determine whether these cells were of donor or recipient origin. Here, we clearly showed that donor T cells infiltrated the kidneys of allo-HCT recipients. We further demonstrated that these donor T cells produced increased amounts of TNF-α and IFN-γ relative to T cells isolated from syngeneic recipients. This is consistent with the cytotoxic potential of these cytokines and prior reports showing their upregulation in the kidneys of animal models of allo-HCT.11,12 Moreover, we showed that these donor T cells expressed increased markers of activation and produced increased amounts of the cytotoxins granzyme B and perforin. These data collectively suggest that donor T cells may directly contribute to kidney dysfunction in allo-HCT recipients.
The histopathological findings we observed in the kidneys of allo-HCT recipients resembled those seen in the rejection of kidney allografts. For example, acute T-cell–mediated rejection often exhibits tubulitis, interstitial nephritis, intimal and transmural arteritis, and mild glomerulitis on histopathology, with findings of PTCitis in some cases.27 PTCitis, tubulitis, and mild glomerulitis were present in the kidneys of our aGVHD mouse model. Immunostaining suggested these infiltrating cells were most likely T cells. Thus, the pathology of T-cell–mediated allograft rejection and renal aGVHD are likely similar. However, we acknowledge that additional mechanisms of immunological attack, such as those associated with antibody-mediated rejection of kidney allograft,28 may also contribute to the renal damage we observed. These possibilities will need to be systematically examined in future studies.
Transplantation-associated TMA (TA-TMA) is a known cause of AKI after allo-HCT. TA-TMA is thought to be due to endothelial damage from TBI, chemotherapy, and CNIs.10 Recent studies indicate that aGVHD of the vascular endothelium also contributes to TA-TMA.29,30 Although we found no evidence of pathological TA-TMA in the kidneys of allo-HCT recipients, Ma et al observed findings suggestive of TA-TMA, including mesangiolysis, mesangial proliferation, mesangial edema, subendothelial thickening, fibrinoid necrosis of afferent arterioles, microthrombi, and complement deposition.31 Therefore, the histopathological changes of renal aGVHD may more closely resemble that of TA-TMA rather than the characteristic aGVHD histopathological findings of epithelial cell apoptosis and lymphocyte infiltration seen in the main aGVHD target organs (ie, the skin, liver, and gut).32 Consistent with this, we confirmed that donor-derived cytotoxic T cells directly attack and induce apoptosis in renal endothelial and tubular cells in vitro. We did not observe significant renal or endothelial cell apoptosis in vivo. Apoptosis of target tissue cells is not always observed on histopathologic analysis, particularly for liver aGVHD.33 However, the lack of apoptotic renal cells in vivo may also be because of the early examination time points. Future studies will need to explore the evolution of renal aGVHD histopathology over time. In addition, comparing the renal histopathological changes we observed with that observed in other potential nonclassical target organs of aGVHD (ie, the lungs, central nervous system, and BM34) may reveal new insights into the pathogenesis and spectrum of allogeneic immune–mediated tissue injury.
Elafin is a specific marker for cutaneous GVHD.22 It is a small protein abundantly expressed in epithelial tissues and inhibits neutrophil elastase and proteinase-3.35 Elafin increased in the urine of patients after HCT,36 suggesting that it is involved in the pathogenesis of kidney disease in HCT recipients. Interestingly, we found that elafin expression was elevated in the kidneys of allogeneic mice after HCT. These results suggest that alloimmune responses induce markers of alloimmune-mediated tissue injury in the kidneys of allo-HCT recipient mice. Whether urinary elafin would be useful for identifying renal aGVHD as a cause of AKI after allo-HCT will need further study in prospective trials.
There are several limitations of our studies. First, because we cannot specifically label donor-derived cells in syngeneic recipients in our BMT models, the origin of T cells in the kidneys of syngeneic mice cannot be strictly determined. Second, we did not use any GVHD prophylaxis, which differs from clinical practice. We omitted GVHD prophylaxis to avoid confounding nephrotoxic effects of these agents. By doing so, we might have unmasked renal aGVHD that is not clinically significant in the setting of GVHD prophylaxis. Although there are reports of inflammatory cell infiltration in the renal pathology of patients who receive allo-HCT who are on immunosuppressive drugs,6 the origin and type of cells have not been studied in detail. Indeed, the kidney is not typically recognized as a major aGVHD target organ despite being susceptible to other autoimmune-mediated diseases such as systemic lupus erythematosus and antineutrophil cytoplasmic antibody–associated vasculitis that can result in AKI.37 One hypothesis for why the intensity of alloimmune responses varies among organs is that organs differ in their capacity to withstand immune attack.38 Indeed, renal tissue repair after an AKI follows a robust pathway involving dedifferentiation, proliferation, and redifferentiation of surrounding epithelial cells.39 Differences in the microbiome among organs may also influence alloimmune responses and contribute to differences in alloimmune-mediated damage among organs.38 Notably, the kidneys are typically sterile, unlike the main aGVHD target organs. Hence, the kidneys may have a relatively high tolerance for alloimmune responses because of a sterile environment that impedes the establishment of a severe alloimmune reaction and the presence of robust intrinsic repair pathways. Future studies will need to confirm that renal aGVHD develops in the setting of aGVHD prophylaxis.
Additional limitations of our study include that TBI conditioning might have contributed to the renal injury we observed; however, we controlled for this by including a syngeneic group also conditioned with TBI. Prerenal AKI due to dehydration from expected greater diarrhea in the allo-HCT recipients also might have contributed to the renal damage we observed but would not account for the activated donor T-cell infiltration seen in allo-HCT recipient mice.
Our data indicate that the kidney is targeted by aGVHD and open several avenues of future study that may affect renal function outcomes after allo-HCT. One future avenue is the development of a noninvasive biomarker for renal aGVHD. Establishing such a biomarker would help clinicians tailor the treatment of AKI to the underlying etiology in each patient. For instance, the treatment of CNI nephropathy includes CNI withdrawal,40 which is the exact opposite treatment of aGVHD.41,42 In addition to identifying renal aGVHD biomarkers, further research is needed to elucidate the unique aspects of renal aGVHD pathogenesis that may lead to the development of specific therapies.
Collectively, our data demonstrated that donor T-cell–mediated immune responses (aGVHD) exacerbate renal endothelial and tubular epithelial injury in allo-HCT recipients. Uncovering kidney-specific mechanisms of aGVHD may lead to improved renal outcomes after allo-HCT.
Acknowledgments
The authors thank Emiko Nishidate, Hiroko Sasaki, and Tomomi Seino for their excellent technical assistance.
This work was supported by JSPS KAKENHI grants JP20K08704 (T. Toubai) and JP21K08410 (K. Ichikawa), a Japanese Society of Hematology Research grant (T. Toubai), Takeda Science Foundation Research grant (T. Toubai), the Amy Strelzer Manasevit Research Program, which is funded through the Be The Match Foundation (D.P.), and National Heart, Lung, and Blood Institute of the National Institutes of Health grant K08 HL157619 (D.P.).
Authorship
Contribution: M.M. designed and performed experiments, analyzed the data, and wrote the manuscript; E.M., T. Takehara, Y.H., and E.S. performed experiments; D.P. critically reviewed and edited the manuscript; P.R. provided advice on the experimental design and critically reviewed the manuscript; M.W. and K. Ishizawa provided advice on the experimental design; and T. Toubai designed and supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomomi Toubai, Division of Hematology and Cell Therapy, Department of Internal Medicine III, Yamagata University School of Medicine, 2-2-2, Iida-Nishi, Yamagata 990-9585, Japan; e-mail: toubai@med.id.yamagata-u.ac.jp; and Kazunobu Ichikawa, Department of Cardiology, Pulmonology, and Nephrology, Yamagata University School of Medicine, 2-2-2, Iida-Nishi, Yamagata 990-9585, Japan; e-mail: ichikawa-k@med.id.yamagata-u.ac.jp.
References
Author notes
Data are available on request from the corresponding author, Tomomi Toubai (toubai@med.id.yamagata-u.ac.jp).
The full-text version of this article contains a data supplement.