Key Points
Patients with lymphoma have increased fibrin formation and impaired fibrinolysis compared with healthy controls.
Antineoplastic treatment reduces clot propagation and clot strength measured by rotational thromboelastometry in patients with lymphoma.
Abstract
Thrombosis and bleeding are significant contributors to morbidity and mortality in patients with hematological cancer, and the impact of altered fibrinolysis on bleeding and thrombosis risk is poorly understood. In this prospective cohort study, we investigated the dynamics of fibrinolysis in patients with hematological cancer. Fibrinolysis was investigated before treatment and 3 months after treatment initiation. A dynamic clot formation and lysis assay was performed beyond the measurement of plasminogen activator inhibitor 1, tissue- and urokinase-type plasminogen activators (tPA and uPA), plasmin-antiplasmin complexes (PAP), α-2-antiplasmin activity, and plasminogen activity. Clot initiation, clot propagation, and clot strength were assessed using rotational thromboelastometry. A total of 79 patients were enrolled. Patients with lymphoma displayed impaired fibrinolysis with prolonged 50% clot lysis time compared with healthy controls (P = .048). They also displayed decreased clot strength at follow-up compared with at diagnosis (P = .001). A patient with amyloid light-chain amyloidosis having overt bleeding at diagnosis displayed hyperfibrinolysis, indicated by a reduced 50% clot lysis time, α-2-antiplasmin activity, and plasminogen activity, and elevated tPA and uPA. A patient with acute promyelocytic leukemia also displayed marked hyperfibrinolysis with very high PAP, indicating extreme plasmin generation, and clot formation was not measurable, probably because of the extremely fast fibrinolysis. Fibrinolysis returned to normal after treatment in both patients. In conclusion, patients with lymphoma showed signs of impaired fibrinolysis and increased clot strength, whereas hyperfibrinolysis was seen in patients with acute promyelocytic leukemia and light-chain amyloidosis. Thus, investigating fibrinolysis in patients with hematological cancer could have diagnostic value.
Introduction
Thrombosis and bleeding cause increased morbidity and mortality in patients with hematological cancer.1,2 Some risk factors of thrombosis and bleeding are well established, but the underlying mechanisms are generally poorly understood in these patients.1
Hematological cancers are a diverse group of cancers in which hematopoietic or lymphatic tissues undergo malignant transformation.3 They range from very indolent lymphomas and myeloproliferative neoplasms (MPNs) with expected survival measured in years, if not decades, to very aggressive leukemias and lymphomas in which patients succumb to the disease in a matter of months.4 The different diseases are classified based on immunohistochemical staining, flow cytometry, and cytogenetic and molecular genetic methods, with new subgroups emerging constantly as research advances and our understanding of disease mechanisms deepens. These classifications allow for more precise diagnosis and treatment selection, ultimately improving patient outcomes.3,5-8
As treatment for hematological cancer continues to improve and patient survival rates increase, there is a growing need to address the risks of cancer-associated thrombosis and bleeding. Large epidemiological studies from Denmark and Canada have found an increased risk of both arterial and venous thromboembolic events (ATE/VTE) and bleeding events leading to hospital admission in patients with hematological malignancies.2,9 In a study by Adelborg et al with patients with hematological cancer, the 10-year risk of ATE or VTE was 11.5%, and the 10-year risk of bleeding leading to hospital admission was 8.5%.2 Risk of thrombosis exceeded the risk of bleeding in all patients except those diagnosed with acute myeloid leukemia (AML), acute lymphoblastic leukemia, or myelodysplastic syndrome (MDS). Alam et al reported that 17.6% of patients with hematological cancer were diagnosed with VTE, and those diagnosed with VTE had a significantly lower median survival.9
Several risk factors and mechanisms can lead to bleeding and thrombosis in patients with hematological cancer.1 Evidence suggests that patients with cancer display a hypercoagulable state, with risk factors including thrombocytosis and leukocytosis and type of cancer.10,11 Other mechanisms such as neutrophil extracellular traps, procoagulant tumor proteins, chemotherapy, and changes in coagulation factor and plasminogen activator inhibitor 1 (PAI-1) have been associated with increased risk of thrombosis.12-16 In a study by Lisman et al investigating the association between clot lysis time (measured by a dynamic clot formation and lysis assay) and deep-vein thrombosis (DVT), the authors found that patients with clot lysis times above the 90th percentile for healthy controls had an odds ratio of 2 for DVT.17 Bleeding, however, is largely attributed to thrombocytopenia in patients with hematological cancer, with the exception of acute promyelocytic leukemia (APL) in which disseminated intravascular coagulation and hyperfibrinolysis plays a major role.18-20
The role of altered fibrinolysis in thrombosis and bleeding in hematological cancer is sparsely investigated, except for APL. In APL increased expression of annexin II on the surface of malignant promyelocytes catalyzes cleavage of plasminogen to plasmin, which leads to a hyperfibrinolytic state.20-22 In a recent systematic review regarding fibrinolysis in non-APL hematological cancer, we found that most studies were very small and only analyzed a subset of the proteins and enzymes involved in the fibrinolytic system.23 Only 3 studies used dynamic clot formation and lysis assays, and none of the studies associated the observed changes to fibrinolysis with thrombosis and bleeding.24-26 Rotational thromboelastometry (ROTEM) provides a dynamic investigation of clot formation, clot propagation, and clot strength. ROTEM has been used to evaluate bleeding risk in patients with APL and was found to predict bleeding risk and risk of early hemorrhagic death.27 Otherwise, to our knowledge, ROTEM has not been applied to investigate coagulation in hematological malignancies.
Increased knowledge about changes in the fibrinolytic activity and clot strength in patients with hematological cancer could identify patients who would benefit from antifibrinolytic therapy to decrease the bleeding risk; or, conversely, identify patients in whom inhibited fibrinolysis increases the risk of ATE and VTE and, thus, who could benefit from thromboprophylaxis.
In this prospective cohort study of patients diagnosed with various hematological malignancies we investigated whether patients displayed altered fibrinolysis and/or clot strength before chemotherapeutic treatment and 3 months after treatment start.
Research design and methods
Study population
Patients diagnosed at the Department of Hematology at Aarhus University Hospital, Aarhus, Denmark, between December 2021 and January 2023 were included. Inclusion criteria were (1) diagnosed with hematological cancer (ICD DD45x, DD46x, DD47x, DC8xx, or DC9xx); (2) had not received chemotherapy treatment yet, with the exception that prednisolone that was accepted for patients with lymphoma and myeloma, and hydroxyurea that was accepted for patient with acute leukemia, and 1 dose of all-trans retinoic acid was accepted for patients with APL; (3) planned chemotherapy treatment; (4) aged >18 years; and (5) ability to give informed consent. Exclusion criterion was treatment with antifibrinolytics.
Study design
We conducted a prospective cohort study in which patients were included at diagnosis, before treatment start, and followed-up for 3 months. Blood samples were drawn at inclusion and at the end of follow-up. Participants who opted out of, or were medically advised against, the planned chemotherapy treatment were still included in the study; their contribution was limited to the diagnostic sample. This was also the case for patients with MPNs who did not require physical consultations but rather were followed-up with telephone consultations.
Data collection
At inclusion, we recorded demographic data, history of bleeding and thrombosis, use of platelet inhibitors, use of anticoagulants, and type of hematological cancer. At follow-up, we recorded whether patients had experienced clinically relevant bleeding (World Health Organization [WHO] grade 2 or worse, as described by Slichter et al28,29), were diagnosed with ATE or VTE, use of platelet inhibitors and/or anticoagulants, current chemotherapy treatment, and remission status, if relevant.
Blood sampling and analysis
Blood was drawn from an antecubital vein through a 21-gauge butterfly needle using minimum stasis. Platelet-poor plasma (PPP) was prepared by centrifuging blood anticoagulated with 3.2% sodium citrate for 25 minutes at 3000g within 30 minutes of blood sampling. PPP was frozen at −80°C for later batch analyses.
Clot formation and lysis
Clot formation and lysis assay was performed as described by Larsen et al.30 In short, samples of PPP, produced as described earlier, were thawed and centrifuged for 3 minutes at 15 000g. Reagents used were as follows: tissue-type plasminogen activator (tPA), 116 μg/L (Calbiochem, San Diego, CA); CaCl2, 26.7 nM; recombinant tissue factor, diluted 1:5000 (Siemens Healthcare GmbH, Erlangen, Germany); and phospholipid solution, 4 μM (Rossix, Mölndal, Sweden). Samples were analyzed in duplicate, and an average of the 2 was reported. Results were reported as clot formation reflected by peak absorbance, lysis of the clot reflected by 50% clot lysis time (time in seconds from peak absorbance to 50% clot lysis), and a combination of clot formation and lysis reflected by area under the curve (AUC).
Data from 120 healthy controls, previously used for establishing the reference range for the assay, were used for comparison. Healthy controls were age and sex-matched at a ratio of 1:1, thus including 79 healthy controls in the analysis. Blood samples from healthy controls were prepared similarly to patient samples.31
Fibrinolysis enzymes and proteins
PAI-1, urokinase plasminogen activator (uPA) and tPA, and plasmin-antiplasmin (PAP) complexes were quantified by enzyme-linked immunosorbent assay (ELISA) using the following kits: TECHNOZYM PAI-1 Antigen ELISA Kit, TECHNOZYM tPA EDTA ELISA Kit, TECHNOZYM uPA ELISA Kit, and TECHNOZYM PAP Complex ELISA Kit (Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Austria). Plates were read using a Victor Reader X4 (Perkin Elmer, Waltham, MA). Plasminogen and α2-antiplasmin activity were analyzed on a CS5100 (Siemens Healthcare GmbH) using Berichrom Plasminogen Assay and Berichrom α2-Antiplasmin Assay (Siemens Healthcare GmbH). If results were outside the standard curve, samples were reanalyzed with appropriate dilutions. Samples were analyzed in duplicate, and if the difference between duplicates were >20%, samples we reanalyzed. Results were reported as the average between duplicates.
ROTEM
Dynamic whole blood coagulation was performed using ROTEM delta 4000 machines (Werfen, Barcelona, Spain). Analysis was performed on whole blood, anticoagulated with 3.2% sodium citrate. Analysis was performed between 30 to 60 minutes after blood sampling, using the standard reagents INTEM, EXTEM, and FIBTEM, as described by the manufacturer. For the EXTEM and INTEM assays, clot initiation was reflected by clotting time; clot propagation was reflected by clot formation time (CFT), maximum velocity (MaxVel), and time to MaxVel (tMaxVel). Clot strength was reflected in the INTEM, EXTEM, and FIBTEM assays by maximum clot firmness (MCF).
Other laboratory analyses
Standard blood analyses were performed at the Department of Clinical Biochemistry, Aarhus University Hospital, Denmark. Plasma creatinine and plasma C-reactive protein were measured using an Atellica CH (Siemens Healthcare GmbH). Total leukocyte count, hemoglobin, and platelet count were measured using an XN9000 (Sysmex, Kobe, Japan). Activated partial thromboplastin time (aPTT), D-dimer, fibrinogen, and international normalized ratio (INR) were measured using a CS5100i (Sysmex).
Data analysis
The primary outcome of the study was the difference between 50% clot lysis time at diagnosis and follow-up. Because no studies have reported data on clot lysis in patients with hematological cancer, we did not have the data to perform a proper sample size calculation, which was therefore not done. Continuous variables are presented as mean and 95% confidence interval if normally distributed, median and 95% confidence interval if normally distributed after log transformation, and median and interquartile range for nonnormally distributed data. Comparison between diagnosis and follow-up samples was done using paired tests (t test or Wilcoxon signed rank test). Linear regression was used to evaluate the association between protein and enzyme concentration or activity and clot formation and lysis parameters. Analysis of variance was used to evaluate whether the different cancer diagnoses influenced the clot formation and lysis parameters. If the analysis of variance F-test showed a significant difference, a pairwise t test with Bonferroni correction was used to evaluate whether the effect was significant for each diagnosis. R Studio version 2023.06.1 Build 524, with R version 4.3.0 was used for statistical analysis.
Ethical statement
The current study was conducted after approval from the regional ethics committee of the Central Region of Jutland (case number: 1-10-72-40-21). All patients gave written informed consent before participating in the study. The study was performed in accordance with the Declaration of Helsinki.
Results
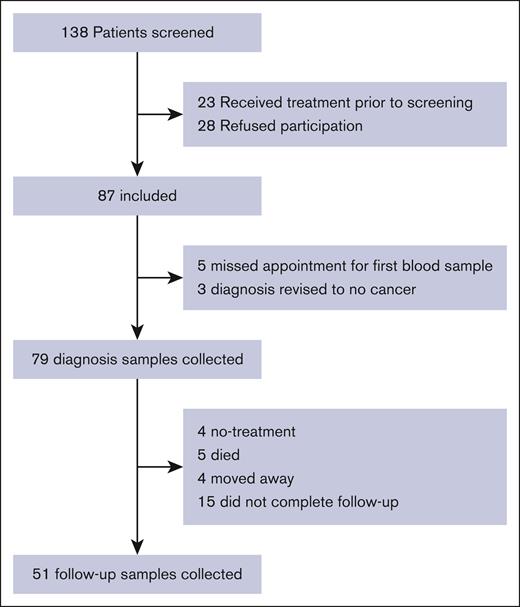
We identified 138 patients with newly diagnosed hematological cancer. Of these, 26 patients did not fulfill inclusion criteria, and 33 were excluded for other reasons, mainly unwillingness to participate in the study (Figure 1), leaving 87 patients to be included in the study. Of these, 5 were excluded because of missing the appointment for first blood sample, and 3 were excluded because their diagnosis was revised to no hematological cancer. This leaves 79 patients included in the study. As seen in Table 1, 39 (49%) patients were diagnosed with lymphoma, 19 (24%) had MPN, and 15 (19%) had multiple myeloma (MM) or amyloid light-chain (AL) amyloidosis. Six patients had AML/advanced MDS, with 1 patient having APL.
Flow diagram detailing inclusion and follow-up inpatients with hematological cancer.
Flow diagram detailing inclusion and follow-up inpatients with hematological cancer.
Demographic and baseline data of 79 patients with hematological cancer
| . | N = 79 . |
|---|---|
| Age, y, mean (95% CI) | 67 (63-71) |
| Male, n (%) | 30 (38) |
| BMI, kg/m2, mean (95% CI) | 27 (25-28) |
| Smoking status, n (%) | |
| Active smoker, | 4 (5) |
| Previous smoker | 28 (35) |
| Never smoked | 47 (59) |
| Platelet inhibitor and anticoagulation, n (%) | |
| Acetylsalicylic acid | 13 (16) |
| Clopidogrel | 4 (5) |
| Rivaroxaban | 4 (5) |
| Bleeding and thrombosis within previous 3 mo, n (%) | |
| Arterial thrombosis | 2 (3) |
| Venous thrombosis | 1 (1) |
| Bleeding WHO grade ≥2 | 2 (3) |
| Diagnosis, n (%) | |
| Lymphoma | 39 (49) |
| Myeloproliferative syndromes | 19 (24) |
| MM/amyloidosis | 15 (19) |
| AML/MDS | 6 (8) |
| ALL | 0 (0) |
| . | N = 79 . |
|---|---|
| Age, y, mean (95% CI) | 67 (63-71) |
| Male, n (%) | 30 (38) |
| BMI, kg/m2, mean (95% CI) | 27 (25-28) |
| Smoking status, n (%) | |
| Active smoker, | 4 (5) |
| Previous smoker | 28 (35) |
| Never smoked | 47 (59) |
| Platelet inhibitor and anticoagulation, n (%) | |
| Acetylsalicylic acid | 13 (16) |
| Clopidogrel | 4 (5) |
| Rivaroxaban | 4 (5) |
| Bleeding and thrombosis within previous 3 mo, n (%) | |
| Arterial thrombosis | 2 (3) |
| Venous thrombosis | 1 (1) |
| Bleeding WHO grade ≥2 | 2 (3) |
| Diagnosis, n (%) | |
| Lymphoma | 39 (49) |
| Myeloproliferative syndromes | 19 (24) |
| MM/amyloidosis | 15 (19) |
| AML/MDS | 6 (8) |
| ALL | 0 (0) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMI, body mass index; CI, confidence interval; MDS, myelodysplastic syndrome; MM, multiple myeloma.
Fibrinolysis
The different clot formation and lysis parameters reflect different stages of fibrin formation and lysis. Peak absorbance is mainly influenced by fibrin formation, but hyperfibrinolysis will also lead to lower peak absorbance. AUC is a compound measure of both fibrin formation and breakdown, and 50% clot lysis time mainly reflects fibrinolysis but is also correlated to peak absorbance and cannot be seen as an isolated measure of fibrinolysis.
Comparison with healthy controls
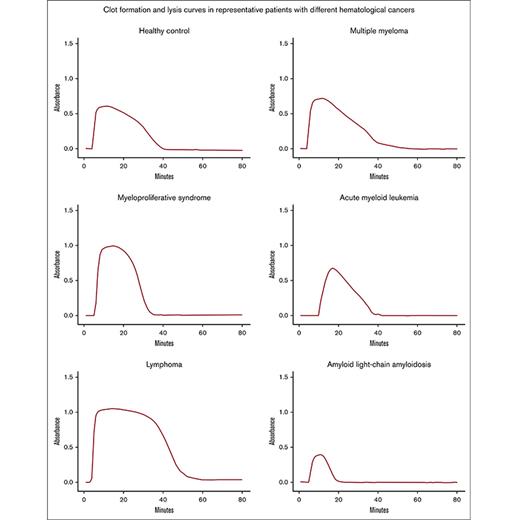
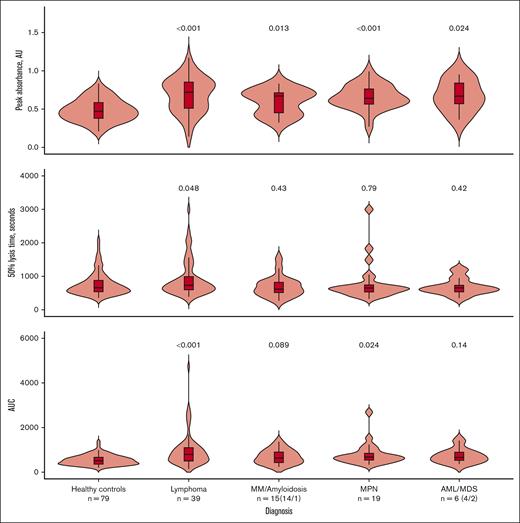
We compared the clot formation and lysis parameters between age- and sex-matched healthy controls and the patients with different hematological cancer diagnoses (Figure 2). All patients displayed signs of increased fibrin formation, reflected by increased peak absorbance. Patients with lymphoma were the only group displaying definite signs of impaired fibrinolysis, reflected by prolonged 50% clot lysis time. The lymphoma and MPN groups also had a higher AUC, which, in the MPN group likely reflects the increased fibrin formation, and, in the lymphoma group, a combination of prolonged clot lysis and increased fibrin formation. Representative curves for controls and each patient group are available in supplemental Figure 1.
Comparison of clot formation and lysis parameters between healthy controls and the different hematological cancer diagnoses. Comparisons were made using pairwise t tests with Bonferroni correction for multiple comparisons; P values shown are the adjusted P values. Comparisons are between healthy controls and each diagnosis. The median is shown by the horizontal line in the box; the box is the 25th and 75th percentiles, and the whiskers are 1.5 × the interquartile range (IQR). Points above or below 1.5 × IQR are displayed individually. AUC, area under the curve.
Comparison of clot formation and lysis parameters between healthy controls and the different hematological cancer diagnoses. Comparisons were made using pairwise t tests with Bonferroni correction for multiple comparisons; P values shown are the adjusted P values. Comparisons are between healthy controls and each diagnosis. The median is shown by the horizontal line in the box; the box is the 25th and 75th percentiles, and the whiskers are 1.5 × the interquartile range (IQR). Points above or below 1.5 × IQR are displayed individually. AUC, area under the curve.
Fibrinolysis at diagnosis and after 3 months
As seen in Table 2, the means or medians of all fibrinolysis markers were within the reference range, both at diagnosis and at follow-up. However, there was a significant increase in 50% clot lysis time and α2-AP activity and a decrease in PAP complexes from diagnosis to follow-up. Because PAP complexes are formed by α2-AP binding plasmin, lower PAP complexes despite increased α2-AP activity means that less plasmin is available for binding with α2-AP. Tables detailing changes in blood tests and fibrinolysis markers on the different diagnosis subgroups are available in supplemental Material.
Blood test results and fibrinolysis markers at diagnosis and follow-up for 79 patients with hematological cancer
| . | At diagnosis . | At follow-up . | P value . | Reference range . |
|---|---|---|---|---|
| Standard blood tests | ||||
| B–hemoglobin, mmol/L, mean (95% CI) | 7.9 (7.6-8.3) | 7.4 (6.9-7.8) | .27 | 7.3-10.5 |
| B–platelet count, 109/L, mean (95% CI) | 318 (275-361) | 231 (195-268) | .06 | 145-400 |
| B–leucocyte count, 109/L, median (95% CI) | 8.9 (7.4-10.6) | 4.7 (3.9-5.7) | .003 | 3.5-10 |
| P–creatinine, μmol/L, median (95% CI) | 71 (67-75) | 75 (69-82) | .52 | 45-105 |
| P–C-reactive protein, mg/L, median (IQR) | 4 (8.1) | 4 (2.5) | .09 | <8 |
| Coagulation tests | ||||
| P–INR, median (IQR) | 1 (0.1) | 1 (0.1) | .27 | <1.2 |
| P–aPTT, s, median (IQR) | 25 (5.8) | 25 (4.5) | .94 | 20-29 |
| P–D-dimer, mg/L, median (IQR) | 0.58 (0.9) | 0.44 (0.6) | .02 | <0.5 |
| P–fibrinogen, μmol/L, mean (95% CI) | 10.5 (9.8-11.3) | 10.66 (9.79-11.53) | .63 | 5.5-12.0 |
| Clot lysis assay | ||||
| Peak absorbance, AU, mean (95% CI) | 0.66 (0.61-0.71) | 0.68 (0.62-0.74) | .40 | 0.18-0.74 |
| 50% clot lysis time, s, median (95% CI) | 698 (620-786) | 817 (716-933) | .03 | 309-1565 |
| AUC, median (95% CI) | 691 (599-797) | 788 (670-927) | .007 | 219-1051 |
| Fibrinolysis enzymes and proteins | ||||
| P–α2-AP activity, % mean (95% CI) | 115 (111-120) | 125 (115-134) | .02 | 75-135 |
| P–PAI-1, ng/mL, median (95% CI) | 7.8 (6-10.1) | 9.3 (6.9-12.4) | .20 | 7.0-43.0 |
| P–PAP, ng/mL, median (95% CI) | 355 (296-426) | 235 (190-290) | .003 | 0-514 |
| P–PLG activity, % mean (95% CI) | 109 (104-113) | 112 (104-120) | .69 | 70-130 |
| P–tPA, ng/mL, median (95% CI) | 2.7 (2.5-2.9) | 3 (2.6-3.6) | .13 | 2.0-8.0 |
| P–uPA, ng/mL, median (95% CI) | 0.7 (0.6-0.7) | 0.7 (0.6-0.7) | .29 | 1.2-2.4 |
| . | At diagnosis . | At follow-up . | P value . | Reference range . |
|---|---|---|---|---|
| Standard blood tests | ||||
| B–hemoglobin, mmol/L, mean (95% CI) | 7.9 (7.6-8.3) | 7.4 (6.9-7.8) | .27 | 7.3-10.5 |
| B–platelet count, 109/L, mean (95% CI) | 318 (275-361) | 231 (195-268) | .06 | 145-400 |
| B–leucocyte count, 109/L, median (95% CI) | 8.9 (7.4-10.6) | 4.7 (3.9-5.7) | .003 | 3.5-10 |
| P–creatinine, μmol/L, median (95% CI) | 71 (67-75) | 75 (69-82) | .52 | 45-105 |
| P–C-reactive protein, mg/L, median (IQR) | 4 (8.1) | 4 (2.5) | .09 | <8 |
| Coagulation tests | ||||
| P–INR, median (IQR) | 1 (0.1) | 1 (0.1) | .27 | <1.2 |
| P–aPTT, s, median (IQR) | 25 (5.8) | 25 (4.5) | .94 | 20-29 |
| P–D-dimer, mg/L, median (IQR) | 0.58 (0.9) | 0.44 (0.6) | .02 | <0.5 |
| P–fibrinogen, μmol/L, mean (95% CI) | 10.5 (9.8-11.3) | 10.66 (9.79-11.53) | .63 | 5.5-12.0 |
| Clot lysis assay | ||||
| Peak absorbance, AU, mean (95% CI) | 0.66 (0.61-0.71) | 0.68 (0.62-0.74) | .40 | 0.18-0.74 |
| 50% clot lysis time, s, median (95% CI) | 698 (620-786) | 817 (716-933) | .03 | 309-1565 |
| AUC, median (95% CI) | 691 (599-797) | 788 (670-927) | .007 | 219-1051 |
| Fibrinolysis enzymes and proteins | ||||
| P–α2-AP activity, % mean (95% CI) | 115 (111-120) | 125 (115-134) | .02 | 75-135 |
| P–PAI-1, ng/mL, median (95% CI) | 7.8 (6-10.1) | 9.3 (6.9-12.4) | .20 | 7.0-43.0 |
| P–PAP, ng/mL, median (95% CI) | 355 (296-426) | 235 (190-290) | .003 | 0-514 |
| P–PLG activity, % mean (95% CI) | 109 (104-113) | 112 (104-120) | .69 | 70-130 |
| P–tPA, ng/mL, median (95% CI) | 2.7 (2.5-2.9) | 3 (2.6-3.6) | .13 | 2.0-8.0 |
| P–uPA, ng/mL, median (95% CI) | 0.7 (0.6-0.7) | 0.7 (0.6-0.7) | .29 | 1.2-2.4 |
Reference ranges for hemoglobin and platelet counts for men and women have been combined.
AUC, area under the curve; B, blood; CI, confidence interval; IQR, interquartile range; P, plasma; PLG, plasminogen.
Regression coefficients for multiple linear regression of clot formation and lysis parameters, and proteins and enzymes involved with fibrinolysis in patients with hematological cancer and subgroup analysis of patients with lymphoma
| . | Peak absorbance . | 50% clot lysis time . | AUC . | |||
|---|---|---|---|---|---|---|
| All . | Lymphoma . | All . | Lymphoma . | All . | Lymphoma . | |
| Fibrinogen | 0.049∗∗∗ | 0.055∗∗∗ | 41.5∗∗ | 28.1 | 124.3∗∗∗ | 123.1∗∗∗ |
| PAI-1 | 0.001 | −0.002 | 14.2∗∗∗ | 38∗∗∗ | 13.9∗∗∗ | 36.7∗∗∗ |
| Plasminogen activity | 0 | 0 | −3.5 | −1.4 | −6.3∗ | −5 |
| t-PA | −0.004 | −0.005 | −22.3 | −161.6∗∗ | −10.6 | 83.5 |
| u-PA | −0.024 | 0.076 | 21 | −72.5 | 5.6 | −133.6∗ |
| α2-AP activity | −0.001 | 0.001 | 9.1∗∗∗ | 8.9∗ | 7.4∗∗ | 8.6 |
| . | Peak absorbance . | 50% clot lysis time . | AUC . | |||
|---|---|---|---|---|---|---|
| All . | Lymphoma . | All . | Lymphoma . | All . | Lymphoma . | |
| Fibrinogen | 0.049∗∗∗ | 0.055∗∗∗ | 41.5∗∗ | 28.1 | 124.3∗∗∗ | 123.1∗∗∗ |
| PAI-1 | 0.001 | −0.002 | 14.2∗∗∗ | 38∗∗∗ | 13.9∗∗∗ | 36.7∗∗∗ |
| Plasminogen activity | 0 | 0 | −3.5 | −1.4 | −6.3∗ | −5 |
| t-PA | −0.004 | −0.005 | −22.3 | −161.6∗∗ | −10.6 | 83.5 |
| u-PA | −0.024 | 0.076 | 21 | −72.5 | 5.6 | −133.6∗ |
| α2-AP activity | −0.001 | 0.001 | 9.1∗∗∗ | 8.9∗ | 7.4∗∗ | 8.6 |
∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Patients with clot formation and lysis parameters outside the reference range
Although the means and medians of the clot formation and lysis parameters were within the reference range for the patients as a whole, a subset of patients had parameters outside the reference range. Nine patients had 50% clot lysis time above the established reference range, indicating impaired fibrinolysis (range of 50% clot lysis time for the 9 patients: 1683-2343 s). All these patients had a combination of high-normal or elevated α2-AP activity and/or elevated PAI-1 concentration. These patients also showed signs of increased fibrin formation, with elevated peak absorbance and AUC. Of these 9 patients, 7 were diagnosed with lymphoma. All cases of increased 50% lysis time occurred at diagnosis, before antineoplastic treatment.
Overall, 16 patients presented 50% clot lysis time above the 90th percentile for healthy controls (1321 s). Patients with lymphoma were overrepresented in this group, with 31% (12 of 39) of patients with lymphoma having 50% clot lysis time above the 90th percentile of healthy controls.
One patient with AL amyloidosis displayed hyperfibrinolysis with a 50% clot lysis time (266 s) below the reference range (306-1565 s) at diagnosis. Along with fast 50% clot lysis time, the patient had low levels of α2-AP activity (46%), low plasminogen activity (50%), elevated tPA (9.2 ng/L), elevated uPA (2.8 ng/L), elevated D-dimer (9.2 mg/L), prolonged aPTT (35 s), elevated INR (2.3), and high levels of PAP complexes (925 ng/mL). This indicates hyperfibrinolysis with depletion of plasminogen and α2-AP. Prolonged aPTT and elevated INR indicated depleted coagulation factors. The patient had a history of increased bleeding tendency with WHO grade ≥2 bleeding episodes at diagnosis. At follow-up (after 3 months of treatment), the 50% clot lysis time and other fibrinolysis markers returned to normal, and the increased bleeding tendency had resolved.
The 1 patient with APL displayed extreme hyperfibrinolysis with severely elevated PAP complexes (12 885 ng/mL), suppressed α2-AP activity (46%), increased PAI-1 (47 ng/mL), low-normal plasminogen activity (74%), and low fibrinogen (3.7 μmol/L). The clot formation and lysis curve was completely flat at diagnosis, which can be explained by rampant hyperfibrinolysis and low fibrinogen levels. The clot formation and lysis assay returned to normal at follow-up, along with all the other fibrinolysis markers. The patient did not experience WHO grade ≥2 bleeding, possibly because of prompt recognition of the hyperfibrinolytic condition and, thus, early bleeding prophylaxis with platelet transfusion and fibrinogen concentrate.
ROTEM
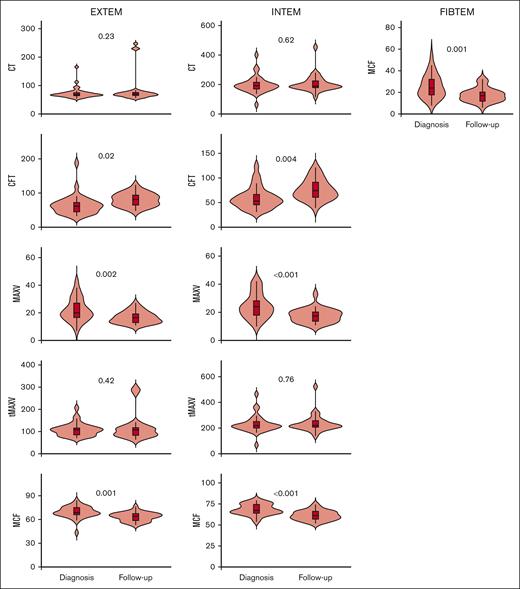
All patients displayed normal clot initiation and propagation measured by clotting time, CFT, MaxVel, and tMaxVel in the INTEM and EXTEM assays. Clot strength measured by MCF in the EXTEM, INTEM, and FIBTEM assays was also within reference range. We also investigated whether there was a change in clot initiation, propagation, and strength from diagnosis to follow-up. As seen in Figure 3, we found that clot formation and clot strength was reduced after treatment in patients with lymphoma. This is reflected by increased CFT and decreased MaxVel and MCF at follow-up vs at diagnosis. Platelet count and fibrinogen levels did not change between diagnosis and follow-up and, thus, does not explain this change in ROTEM parameters. No changes in clot strength or clot formation between diagnosis and follow-up was found in the other patient groups.
Comparison of clot initiation, clot propagation, and clot strength analyzed by ROTEM at diagnosis and at 3 months of follow-up in 39 patients with lymphoma. The median is shown by the horizontal line in the box; the box is the 25th and 75th percentiles, and the whiskers are 1.5 × the interquartile range (IQR). Points above or below 1.5 × IQR are displayed individually. CT, clotting time (in s).
Comparison of clot initiation, clot propagation, and clot strength analyzed by ROTEM at diagnosis and at 3 months of follow-up in 39 patients with lymphoma. The median is shown by the horizontal line in the box; the box is the 25th and 75th percentiles, and the whiskers are 1.5 × the interquartile range (IQR). Points above or below 1.5 × IQR are displayed individually. CT, clotting time (in s).
Correlation between clot formation and ROTEM
There is a moderately strong, positive correlation between peak absorbance in the clot formation and lysis assay and MCF measured by ROTEM. However, after adjusting for fibrinogen using multiple regression analysis, the correlation was no longer significant.
Analyses were performed both including and excluding the patients treated with platelet inhibitors or anticoagulants, and the results did not differ significantly. Therefore, the results presented here include all patients regardless of platelet inhibitor or anticoagulant treatment.
Bleeding and thrombosis
At diagnosis
At diagnosis, 1 patient with lymphoma and 1 patient with AL amyloidosis had bleeding of WHO grade ≥2. The patient with lymphoma was diagnosed with Waldenström macroglobulinemia and had a high M component with immunoglobulin M of >45 g/L and increased plasma viscosity, which is known to be associated with increased risk of bleeding. The patient with AL amyloidosis displayed hyperfibrinolysis, as previously described.
Two patients with MPN had acute myocardial infarctions within the past 3 months, and 1 patient with MM was diagnosed with pulmonary embolism at the time of diagnosis. These patients did not display altered fibrinolysis or changes to clot formation or strength, but the patients with MPN had elevated platelet counts.
At follow-up
At follow-up, 2 patients had experienced clinically significant bleeding, 1 of whom being the patient with AL amyloidosis who was also bleeding at diagnosis and had overt hyperfibrinolysis. The other was a patient with lymphoma who did not experience bleeding at diagnosis but experienced WHO grade 2 bleeding during follow-up. However, in this case, the bleeding could be attributed to treatment with both platelet inhibitor (aspirin; because of ischemic heart disease) and low molecular weight heparin (because of atrial fibrillation).
No patients had experienced thromboembolic complications at follow-up.
Factors influencing clot formation and lysis
Using multiple linear regression analysis, we investigated which proteins and enzymes influenced the clot formation and lysis parameters. Peak absorbance was only affected by fibrinogen levels in this analysis. The 50% clot lysis time was affected by fibrinogen levels, PAI-1 concentration, and α2-AP activity, which could indicate that the limiting components in the fibrinolytic cascade are PAI-1 and α2-AP. AUC is a compound measure of both peak absorbance and 50% clot lysis time, which is illustrated by AUC being affected by both fibrinogen and PAI-1 concentrations and α2-AP activity (Table 3).
Discussion
The main finding of this study was that patients with lymphoma had impaired fibrinolysis compared with healthy controls; and, in patients with lymphoma, after 3 months of treatment, clot formation and strength was reduced compared with at the time of diagnosis. We also found hyperfibrinolysis in 2 patients with AL amyloidosis and APL. In patients with MM, MPN, non-APL AML, or MDS the evidence for changes in fibrinolysis was not as clear.
Because the major factors influencing clot formation and strength are fibrinogen and platelets, one might suspect that the change in clot formation and strength could be attributed to lower platelet counts due to chemotherapy treatment and lower fibrinogen levels due to decreased inflammation after treatment. However, we found no change in either platelet count or fibrinogen in the patients with lymphoma. Because samples were drawn just before patients received their next chemotherapy treatment, the direct effect of chemotherapy was minimized. Furthermore, the changes are evident in the INTEM, EXTEM, and FIBTEM assays, suggesting that neither platelets nor fibrinogen in isolation are responsible for the observed changes. We did not measure other coagulation factors, and changes in coagulation factor levels between diagnosis and follow-up might contribute to the observed changes in clot strength and formation; however, glucocorticoid treatment, which is a backbone in many lymphoma treatments, has been shown to increase coagulation factor levels in healthy persons.32
Lisman et al found that impaired fibrinolysis was associated with increased risk of DVT, but those patients were otherwise healthy and not diagnosed with cancer.17 Undas et al also found that increased clot lysis time was associated with pulmonary embolism, and patients with pulmonary embolism and high clot lysis time had an increased risk of death.33 ROTEM has not been investigated in patients with lymphoma alone but in mixed groups of patients with hematological cancer in a study investigating different doses of platelet transfusions.34 The authors found no difference in ROTEM parameters between low-dose and high-dose platelet transfusions.34 In patients who have undergone orthopedic and abdominal surgery, increased clot propagation and clot strength has been associated with increased VTE risk.35,36 According to Adelborg et al, patients with lymphoma have an increased risk of VTE,2 and Lisman et al and Undas et al found that impaired fibrinolysis based on increased lysis time was associated with increased VTE risk.17,33 Thus, our findings of impaired fibrinolysis and faster clot initiation and increased clot strength in patients with lymphoma could be contributing factors in the increased VTE risk.35-37 The number of VTE events in our study did not allow for correlative analysis between fibrinolysis or clot strength and VTE.
Previous studies of fibrinolysis and fibrin clot properties in patients with MM have found evidence of hypofibrinolysis, reflected by increased clot lysis time, both at diagnosis25 and during induction chemotherapy.24,37 Undas et al37 found that in 48 patients with MM, of which 25% experienced a thromboembolic event, the clot permeability and maximum D-dimer release rate in an in-house assay predicted thromboembolic events. All patients received thalidomide-containing regimens and thromboprophylaxis with either acetylsalicylic acid (n = 42) or low molecular weight heparin (n = 6). In the study by van Marion et al,24 patients received either a thalidomide-containing regimen or a vincristine-containing regimen. However, thalidomide has in many institutions been superseded by the thalidomide derivative lenalidomide, which also carries an increased VTE risk, albeit lower than for thalidomide.38 All patients with MM included in this study received lenalidomide-containing regimens. Studies should confirm these important findings in patients treated with lenalidomide.
AL amyloidosis have previously been associated with hyperfibrinolysis and increased bleeding risk but only in a subset of patients.39-41 In accordance with previous studies and case reports,39-41 we found very low levels of α2-AP, and elevated tPA and uPA in a patient with AL amyloidosis and a history of bleeding. Uchiba et al reported that increased levels of uPA in bone marrow plasma cells were responsible for hyperfibrinolysis in 5 patients with AL amyloidosis.42 Treatment with nafamostat mesylate, a potential uPA inhibitor, reversed the hyperfibrinolysis in 1 patient. The authors also argued that uPA and plasmin are involved in amyloidogenisis.42 Thus, awareness of hyperfibrinolysis in patients with AL amyloidosis with bleeding tendency is warranted.
In this study, the most extreme case of hyperfibrinolysis was found in a patient with APL, with no fibrin formed on the clot formation and lysis assay despite adequate levels of fibrinogen. Hyperfibrinolysis in APL has been extensively studied, and the current evidence indicates that increased annexin II expression on APL cells causes increased plasmin formation and depletion of α2-AP, which is consistent with our findings.20 Other studies have reported increased tPA and uPA concentrations,43,44 however, we found these parameters to be within the reference range. Annexin II was also associated with hyperfibrinolytic coagulopathy and bleeding diathesis in a recent case report of a patient with acute myelomonocytic leukemia (AMML),45 and, moreover, Olwill et al found that annexin II was highly expressed in APL, AMML, and erythroid leukemia cell lines.45,46 Several case reports spanning multiple decades have also associated AMML with disseminated intravascular coagulation and hyperfibrinolysis47-49 Thus, annexin II–associated hyperfibrinolysis does not seem to be exclusive to APL.
In accordance with the literature, we found that the limiting factors in fibrinolysis in patients with hematological cancer is α2-AP activity and PAI-1.50 However, in amyloidosis and APL this may not be the case because of abnormally high concentrations of uPA and plasmin.
The major strength of this study is that it, to our knowledge, constitutes the most comprehensive examination of the fibrinolytic system in patients with hematological cancer to date, including investigation by a dynamic clot formation and lysis assay. This is also, to our knowledge, the first study demonstrating impaired fibrinolysis in patients with lymphoma. The subgroup analysis is impaired by small group size, with the largest subgroups being of patients with lymphoma, MM, amyloidosis, and MPN.
Conclusions
This study of fibrinolysis in patients with hematological cancer demonstrated that one-third of patients with lymphoma displayed impaired fibrinolysis, which previously has been associated with an increased VTE risk; however, larger studies including clinical outcomes are needed to confirm this. A case of hyperfibrinolysis in AL amyloidosis stresses the importance of examining the fibrinolysis in this patient group if they display bleeding tendency. Thus, investigating fibrinolysis in patients with hematological cancer could have diagnostic value, with potential to aide decisions on bleeding and thrombosis prophylaxis.
Acknowledgments
The authors thank laboratory technicians Vivi Bo Mogensen and Tine Kusk Jørgensen for their invaluable assistance in the laboratory.
Authorship
Contribution: S.T.B., A.-M.H., H.B.O., and C.F.-E. formulated the idea and developed the protocol for the study; S.T.B. collected data and blood samples, performed some of the laboratory analyses, and performed the statistical analysis; S.T.B. wrote the first draft of the manuscript; and A.-M.H., H.B.O., and C.F.-E. edited the manuscript.
Conflict-of-interest disclosure: S.T.B. received unrestricted research support from CSL Behring, outside the submitted work. A.-M.H. received unrestricted research support from CSL Behring, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Søren Thorgaard Bønløkke, Department of Clinical Biochemistry, Aarhus University Hospital, Palle Juul-Jensens Blvd 99, 8200 Aarhus N, Denmark; e-mail: sobo@clin.au.dk.
References
Author notes
Because of data protection laws, supporting data from this study are not available.
The full-text version of this article contains a data supplement.