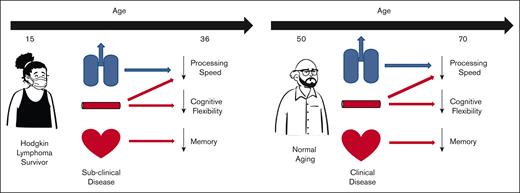
Key Points
Compared with matched community controls, long-term survivors of HL treated with chest radiation have worse neurocognitive outcomes.
Moderately decreased cardiac, pulmonary, and vascular concurrent function is associated with worse neurocognitive outcomes.
Abstract
Thoracic radiation is associated with significant cardiopulmonary morbidities in survivors of long-term Hodgkin lymphoma and may affect neurocognitive outcomes. Survivors (N = 204; 52.5% female; mean [standard deviation] age, 36.6 [8.01] years) treated with thoracic radiation and age-, sex-, and race/ethnicity-matched community controls (N = 205; 51.7% female; age, 36.7 [9.17] years) completed standardized neurocognitive testing, echocardiography, pulmonary function tests, and vascular studies during the same visit. Treatments were abstracted from medical records. Cardiac (ie, left ventricular ejection fraction [LVEF], global longitudinal strain [GLS]), vascular (ie, large and small artery elasticity [SAE]), pulmonary (ie, diffusing capacity of the lungs for carbon monoxide [DLCO] and forced expiratory volume [FEV1]), and chronic health conditions were evaluated for associations with age-adjusted neurocognitive performance using multivariable linear regression. Compared with controls, survivors had lower performance (P < 0.05) in visuomotor (0.11 vs 0.41), visual processing speed (0.25 vs 0.64), short-term recall (−0.24 vs 0.12), and flexibility (−0.04 vs 0.28). Survivors had lower pulmonary (FEV1, DLCOcorr), cardiac (LVEF, GLS), and vascular function (SAE) than controls (all P < 0.001). FEV1 was associated with visuomotor (P = .008) and visual processing speed (P = .05), and flexibility (P = .05). GLS was associated with short-term recall (P = .03). SAE was associated with flexibility (P = .007). Neurocognitive outcomes were also associated with moderate-to-severe neurologic chronic conditions (P < .05). Findings suggest a link between subclinical cardiopulmonary and vascular findings, neurologic morbidity, and neurocognitive impairments. Prevention of health morbidity may benefit neurocognitive outcomes.
Introduction
Hodgkin lymphoma (HL) is a cancer of the immune system that occurs primarily in adolescents and young adults, affecting ∼8500 people in the United States each year.1 Thoracic and neck radiation, anthracyclines, and bleomycin are mainstays of therapy and have been associated with chronic cardiac and pulmonary late effects.2,3 The Childhood Cancer Survivor Study found HL survivors treated with thoracic radiation had a 15-fold elevated risk for congestive heart failure, an 11-fold greater risk for heart valve abnormalities, and a 12-fold higher risk for myocardial infarction than their siblings.4,5 HL survivors exposed to pulmonary toxic therapies, such as bleomycin and/or thoracic radiation, are significantly more likely to have poorer lung function and lower carbon monoxide clearance 5 years after completion of therapy.6
We have previously demonstrated that treatment-related cardiac and pulmonary morbidities are associated with neurocognitive impairments7,8 as well as multifocal leukoencephalopathy and hemosiderin deposits, both indices of cerebrovascular pathology.9 However, no study to date has examined whether subclinical measures of cardiac and pulmonary function are associated with neurocognitive impairments in survivors of HL treated with thoracic radiation. For adult patients without cancer, studies have indicated that diminished gas exchange in the lungs increases cerebrovascular pressure, which can lead to damage in small cerebral arteries.10,11 Hypoxia has been shown to induce chronic low-grade systemic inflammation, which can also contribute to endothelial dysfunction and reduced small artery elasticity (SAE)12,13 These mechanisms can increase the risk of neurocognitive problems.14,15
The aim of this study was to perform the largest objective assessment of cardiopulmonary function, vascular health, chronic health conditions, and neurocognitive function in a well-defined cohort of adult survivors of childhood cancer treated with thoracic radiation to identify the predictors of neurocognitive impairment, including treatment exposures and health-related factors, in the survivors of HL. We explored clinically assessed markers of subclinical ventricular and endothelial dysfunction to detect previously unknown associations that could lead to better predictors of at-risk survivors and identify possible targets for early interventions.
Methods
Participants
Survivors were recruited from the St. Jude Lifetime Cohort study, a large retrospectively identified cohort with longitudinal follow-up of patients with childhood cancer treated at St. Jude Children’s Hospital from 1962 to 2012.16 Survivors of HL treated with thoracic radiation therapy, <25 years of age at diagnosis and currently ≥18 years of age, and ≥5 years after diagnosis were eligible for this study. Community controls without a history of cancer were recruited and frequency-matched to survivors based on age, sex, and race/ethnicity. Survivors were excluded if they had a history of cranial or total body radiation therapy and intrathecal or high-dose IV antimetabolite therapy. Controls and survivors with a history of traumatic brain injury, genetic disorders associated with neurocognitive impairment, or congenital heart disease or who were pregnant at the time of the study were excluded. All participants provided informed consent, and the study was reviewed and approved by the institutional review board.
Treatment and chronic health data
Detailed diagnostic and treatment data were abstracted from the medical records. Cumulative radiation dosimetry to the chest and neck were modeled using treatment records and reconstruction radiation fields on age-scaled phantoms.17 Health behaviors (eg, tobacco use) and physiological events (eg, stroke and hypertension) were assessed by medical records, questionnaires, and comprehensive medical exams. Events were categorized using a modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (1 = mild, 2 = moderate, 3 = severe/disabling, and 4 = life-threatening).18 The highest-grade chronic conditions in survivors and controls at or before neurocognitive testing were used in analyses.
Cardiac, pulmonary, and vascular assessment
All participants underwent 3D echocardiography with Doppler and M-mode (VIVID 7 GE Medical Systems, Milwaukee, WI). Full systolic and diastolic assessments were performed, and measures of left ventricle function (global longitudinal strain [GLS], ejection fraction [LVEF]) were obtained. Complete pulmonary function testing, including lung volume measurements (total lung capacity), carbon monoxide diffusion capacity (DLCO), forced expiratory volume in 1-second (FEV1), and forced vital capacity, was performed for all participants. Abnormal values were defined based on the modified CTCAE criteria for obstructive lung problems (eg, grade 2 [moderate] = FEV1 69%-50% predicted), restrictive lung problems (eg, grade 2 [moderate] = Total Lung Capacity (TLC) 69%-60% predicted), and reduced diffusion capacity (eg, grade 2 [moderate] = DLCO < 60%-40% predicted). All outcomes were compared with predicted values based on age, race, sex, and height. Pulse wave velocity (m/s) and central systolic and diastolic blood pressure (mm Hg) were measured by the SphygmoCor CPV Clinical System (AtCor Medical, Naperville, IL). Large and SAE (mL/mm Hg × 100) were measured by the HDI/PulseWave CR-2000 Cardiovascular Profiling System (Hypertension Diagnostics, Minneapolis, MN).
Neurocognitive assessment
Participants completed a comprehensive standardized neurocognitive evaluation during the same week as cardiac, pulmonary, and vascular assessments. Testing included measures of intelligence (Wechsler Abbreviated Scale of Intelligence19), sustained attention (Conners Continuous Performance Test 220), memory (California Verbal Learning Test21), processing speed (Coding/Digit Symbol and Grooved Peg Board22,23), and executive function (Trail Making Test,24 Verbal Fluency Test,25 and Digit Span23). Raw scores were converted into age-adjusted z scores based on population normative data. Global neurocognitive impairment was defined as a z score ≤−1.28 on at least 3 different tests.
Statistical analysis
Descriptive variables were provided and compared between groups using χ2 for categorical variables and t test for continuous variables. Multivariable linear regression models, adjusted for sex, were used to compare neurocognitive outcomes between survivors and controls. False discovery rate–adjusted P values21 were used to account for multiple comparisons. A similar analysis was undertaken to compare vascular, cardiac, and pulmonary function between survivors and controls, with models adjusted for age and sex. Multivariable linear regression models were used to assess the effect of radiation dose to chest/neck (maximum dose of 24-30 Gy and >30 Gy compared with <24 Gy) on neurocognitive z scores, adjusting for age at diagnosis, time since diagnosis, sex, and chemotherapy exposures within 5 years of primary cancer diagnosis.
Chronic health conditions, categorized as mild (CTCAE grades none and 1) and moderate to life-threatening (CTCAE grades 2-4), were compared between survivors and controls using logistic regression, adjusted for sex. The prevalence of the most common neurologic conditions was compared between survivors and controls using the χ2 test. Means and standard deviations of age-adjusted neurocognitive z scores and chronic health condition outcomes for survivors and controls were reported. Linear regression, adjusted for sex, compared age-adjusted neurocognitive z scores of survivors and controls.
We used Elastic Net variable selection to identify the most important variables among sex, age at examination, and cardiac, pulmonary, and vascular function studies associated with neurocognitive outcomes.26 Elastic Net variable selection is resilient to extreme correlations among predictors and avoids the multiple comparison problem by choosing the most economical model, controlling for age at the time of neurocognitive testing and sex. The penalized approaches tend to shrink the estimates toward 0; therefore, the results of the linear regression model that included the variables selected by Elastic Net were reported.27 This approach was performed for each neurocognitive outcome.
Results
Of the 218 survivors of HL and 208 community controls confirmed to be eligible for this study, 204 survivors (93.6%) and 205 controls (98.6%) participated (Figure 1). No significant differences in sex, race, smoking status, body mass index, or age at assessment were found between survivors and controls (Table 1). This historic cohort was treated on protocols representative of their era (supplemental Table 1). Most (93%) of survivors received frontline-only therapy; 7% relapsed and received additional salvage therapy. The median time since diagnosis was 20.6 years (10.7-45.6), and the median age at diagnosis was 14.7 years (3.0-22.6) of age. The median maximum chest radiation dose was 26.0 (15.0-45.0) Gy.
Characteristics of survivors of HL and community controls
| . | Survivors N = 204 . | Controls N = 205 . | P value . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Sex | |||
| Male | 97 (47.5) | 99 (48.3) | .88 |
| Female | 107 (52.5) | 106 (51.7) | |
| Race | |||
| White | 172 (84.3) | 176 (85.9) | .66 |
| Other | 32 (15.7) | 29 (14.1) | |
| Smoking status | |||
| Current | 39 (19.1) | 29 (14.3) | .37 |
| Former | 35 (17.2) | 41 (20.2) | |
| Never | 130 (63.7) | 133 (65.5) | |
| Body mass index | |||
| Underweight | 7 (3.4) | 3 (1.5) | .23 |
| Normal weight | 51 (25.0) | 62 (30.2) | |
| Overweight | 73 (35.8) | 60 (29.3) | |
| Obese | 73 (35.8) | 80 (39.0) | |
| Age at assessment (y) | Mean (SD) | ||
| 36.6 (8.01) | 36.7 (9.17) | .84 | |
| Median (min-max) | |||
| Age at diagnosis (y) | 14.7 (3.0-22.6) | ||
| Time since diagnosis (y) | 20.6 (10.7-45.6) | ||
| Anthracyclines (n = 156) mg/m2 | 157.2 (91.0-400.0)∗ | ||
| IV methotrexate (n = 85) mg/m2 | 123.2 (20.3-167.7) | ||
| Bleomycin (n = 58) mg/m2 | 35.6 (5.0-95.0) | ||
| Corticosteroids (n = 140) mg/m2 | 2240 (250-4480) | ||
| Vincristine (n = 182) mg/m2 | 45.9 (9.2-151.2) | ||
| Cyclophosphamides (n = 113) mg/m2 | 3 655 (1332-22 889)† | ||
| Chest radiation (n = 204) Gy | 26.0 (15.0-45.0) | ||
| Neck radiation (n = 204) Gy | 26.0 (15.0-51.0) | ||
| N (%) | |||
| Relapsed | 14 (6.9) | ||
| Diagnosed with second malignancy | 51 (25.0) |
| . | Survivors N = 204 . | Controls N = 205 . | P value . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Sex | |||
| Male | 97 (47.5) | 99 (48.3) | .88 |
| Female | 107 (52.5) | 106 (51.7) | |
| Race | |||
| White | 172 (84.3) | 176 (85.9) | .66 |
| Other | 32 (15.7) | 29 (14.1) | |
| Smoking status | |||
| Current | 39 (19.1) | 29 (14.3) | .37 |
| Former | 35 (17.2) | 41 (20.2) | |
| Never | 130 (63.7) | 133 (65.5) | |
| Body mass index | |||
| Underweight | 7 (3.4) | 3 (1.5) | .23 |
| Normal weight | 51 (25.0) | 62 (30.2) | |
| Overweight | 73 (35.8) | 60 (29.3) | |
| Obese | 73 (35.8) | 80 (39.0) | |
| Age at assessment (y) | Mean (SD) | ||
| 36.6 (8.01) | 36.7 (9.17) | .84 | |
| Median (min-max) | |||
| Age at diagnosis (y) | 14.7 (3.0-22.6) | ||
| Time since diagnosis (y) | 20.6 (10.7-45.6) | ||
| Anthracyclines (n = 156) mg/m2 | 157.2 (91.0-400.0)∗ | ||
| IV methotrexate (n = 85) mg/m2 | 123.2 (20.3-167.7) | ||
| Bleomycin (n = 58) mg/m2 | 35.6 (5.0-95.0) | ||
| Corticosteroids (n = 140) mg/m2 | 2240 (250-4480) | ||
| Vincristine (n = 182) mg/m2 | 45.9 (9.2-151.2) | ||
| Cyclophosphamides (n = 113) mg/m2 | 3 655 (1332-22 889)† | ||
| Chest radiation (n = 204) Gy | 26.0 (15.0-45.0) | ||
| Neck radiation (n = 204) Gy | 26.0 (15.0-51.0) | ||
| N (%) | |||
| Relapsed | 14 (6.9) | ||
| Diagnosed with second malignancy | 51 (25.0) |
Max, maximum; min, minimum; SD, standard deviation.
Anthracycline equivalent dose.
Cyclophosphamide equivalent dose.
Treatment-associated neurocognitive outcomes
Compared with controls, survivors had significantly lower scores on sustained attention, visuomotor processing speed, visual processing speed, motor processing speed, learning, short-term memory, long-term memory, and cognitive flexibility (Table 2). Survivors who received ≥30 Gy radiation therapy (RT) at the chest/neck performed significantly worse on cognitive flexibility (β [standard error], −0.74 [0.31]; P = .019), adjusting for sex, age at diagnosis, time since diagnosis, bleomycin, cyclophosphamide, IV methotrexate, prednisone, anthracycline dose, and vinblastine/vincristine, compared with survivors of HL who received <30 Gy RT to the chest/neck (supplemental Table 2).
Mean age-adjusted neurocognitive z scores of survivors and controls
| Domain . | Survivors . | Controls . | FDR P . |
|---|---|---|---|
| Mean (SD) . | Mean (SD) . | ||
| Attention | |||
| Focused | 0.34 (0.95) | 0.55 (0.84) | .061 |
| Span | 0.03 (1.02) | 0.20 (0.91) | .085 |
| Sustained | −0.13 (1.37) | 0.23 (0.85) | .011 |
| Variability | −0.24 (1.11) | −0.03 (1.06) | .089 |
| Processing speed | |||
| Reaction time | −0.17 (1.18) | −0.06 (1.00) | .29 |
| Visuomotor | 0.11 (0.97) | 0.41 (0.94) | .011 |
| Visual | 0.25 (0.98) | 0.64 (0.97) | .002 |
| Motor | −0.38 (1.05) | 0.00 (0.91) | .001 |
| Memory | |||
| Verbal learning | −0.08 (1.05) | 0.28 (1.02) | .001 |
| Short-term recall | −0.24 (1.01) | 0.12 (1.04) | .002 |
| Long-term recall | −0.32 (1.14) | 0.04 (1.10) | .002 |
| Selective reminding | −0.20 (1.11) | −0.02 (0.97) | .110 |
| Executive function | |||
| Working memory | −0.07 (0.94) | 0.05 (0.88) | .16 |
| Self-monitoring | −0.03 (1.15) | 0.18 (0.96) | .085 |
| Cognitive flexibility | −0.04 (1.25) | 0.28 (1.08) | .033 |
| Fluency | −0.07 (1.21) | 0.02 (1.05) | .47 |
| Domain . | Survivors . | Controls . | FDR P . |
|---|---|---|---|
| Mean (SD) . | Mean (SD) . | ||
| Attention | |||
| Focused | 0.34 (0.95) | 0.55 (0.84) | .061 |
| Span | 0.03 (1.02) | 0.20 (0.91) | .085 |
| Sustained | −0.13 (1.37) | 0.23 (0.85) | .011 |
| Variability | −0.24 (1.11) | −0.03 (1.06) | .089 |
| Processing speed | |||
| Reaction time | −0.17 (1.18) | −0.06 (1.00) | .29 |
| Visuomotor | 0.11 (0.97) | 0.41 (0.94) | .011 |
| Visual | 0.25 (0.98) | 0.64 (0.97) | .002 |
| Motor | −0.38 (1.05) | 0.00 (0.91) | .001 |
| Memory | |||
| Verbal learning | −0.08 (1.05) | 0.28 (1.02) | .001 |
| Short-term recall | −0.24 (1.01) | 0.12 (1.04) | .002 |
| Long-term recall | −0.32 (1.14) | 0.04 (1.10) | .002 |
| Selective reminding | −0.20 (1.11) | −0.02 (0.97) | .110 |
| Executive function | |||
| Working memory | −0.07 (0.94) | 0.05 (0.88) | .16 |
| Self-monitoring | −0.03 (1.15) | 0.18 (0.96) | .085 |
| Cognitive flexibility | −0.04 (1.25) | 0.28 (1.08) | .033 |
| Fluency | −0.07 (1.21) | 0.02 (1.05) | .47 |
Means and SDs presented as age-adjusted z scores, with population mean = 0 and SD = 1.0.
FDR P, false discovery rate–adjusted P values for multiple comparisons between survivors and controls; SD, standard deviation.
Elastic Net variable selection found that females were more likely to demonstrate problems in attention, processing speed, and executive function. Female survivors of HL demonstrated associations between prednisone and motor processing speed (−0.59[0.29]; P = .042). Although bleomycin was associated with visuomotor processing speed (−0.45[0.22]; P=.044) in female survivors of HL.
Chronic health condition–associated neurocognitive outcomes
Survivors demonstrated a greater prevalence of moderate to life-threatening (ie, CTCAE grade 2-4) cardiac (survivors, 31.9% vs controls, 10.7%; P < .001), vascular (survivors, 35.8% vs controls, 26.3%; P = .039), endocrine (survivors, 63.7% vs controls, 12.2%; P < .001), neurologic (survivors, 26.0% vs controls, 11.2%; P < .001), and pulmonary (survivors, 47.1% vs controls, 13.2%; P<.001) chronic conditions than controls (supplemental Table 3). Moderate-to-severe neurologic chronic conditions were associated with worse sustained attention (−0.75; P = .0008), visuomotor speed (−0.67; P < .001), visual processing speed (−0.35; P = .029), motor speed (−0.69; P < .0001), learning (−0.59; P = .0006), and cognitive flexibility (−0.67; P = .001; Table 3). Moderate-to-severe vascular chronic conditions were associated with poorer visuomotor speed (−0.29; P = .033) and motor speed (−0.34; P = .025). Moderate-to-severe respiratory chronic conditions were associated with worse sustained attention (−0.48; P = .012), and endocrine chronic conditions were associated with worse visual processing speed (−0.68; P = .012).
Linear regression model of associations between CTCAE grade 2 to 4 chronic conditions and neurocognitive outcomes of survivors, adjusted for sex
| Outcome∗ . | Cardiac . | Vascular . | Endocrine . | Neurologic . | Pulmonary . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Est . | P . | Est . | P . | Est . | P . | Est . | P . | Est . | P . | |
| Sustained attention | −0.126 | .55 | −0.333 | .095 | −0.061 | .77 | −0.750 | .0008 | −0.482 | .012 |
| Visuomotor speed | 0.005 | .97 | −0.294 | .033 | −0.149 | .30 | −0.676 | <.0001 | −0.191 | .15 |
| Visual speed | −0.052 | .72 | −0.195 | .17 | −0.368 | .012 | −0.351 | .029 | −0.244 | .074 |
| Motor speed | 0.200 | .20 | −0.340 | .025 | −0.142 | .37 | −0.697 | <.0001 | −0.102 | .49 |
| Learning | 0.162 | .31 | 0.057 | .71 | −0.219 | .16 | −0.591 | .0006 | −0.203 | .17 |
| Short-term recall | 0.259 | .096 | 0.027 | .86 | −0.123 | .43 | −0.199 | .25 | −0.056 | .70 |
| Long-term recall | 0.361 | .038 | −0.044 | .79 | −0.240 | .17 | −0.190 | .32 | −0.139 | .39 |
| Cognitive flexibility | 0.111 | .56 | −0.090 | .62 | −0.286 | .13 | −0.669 | .001 | −0.207 | .24 |
| Outcome∗ . | Cardiac . | Vascular . | Endocrine . | Neurologic . | Pulmonary . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Est . | P . | Est . | P . | Est . | P . | Est . | P . | Est . | P . | |
| Sustained attention | −0.126 | .55 | −0.333 | .095 | −0.061 | .77 | −0.750 | .0008 | −0.482 | .012 |
| Visuomotor speed | 0.005 | .97 | −0.294 | .033 | −0.149 | .30 | −0.676 | <.0001 | −0.191 | .15 |
| Visual speed | −0.052 | .72 | −0.195 | .17 | −0.368 | .012 | −0.351 | .029 | −0.244 | .074 |
| Motor speed | 0.200 | .20 | −0.340 | .025 | −0.142 | .37 | −0.697 | <.0001 | −0.102 | .49 |
| Learning | 0.162 | .31 | 0.057 | .71 | −0.219 | .16 | −0.591 | .0006 | −0.203 | .17 |
| Short-term recall | 0.259 | .096 | 0.027 | .86 | −0.123 | .43 | −0.199 | .25 | −0.056 | .70 |
| Long-term recall | 0.361 | .038 | −0.044 | .79 | −0.240 | .17 | −0.190 | .32 | −0.139 | .39 |
| Cognitive flexibility | 0.111 | .56 | −0.090 | .62 | −0.286 | .13 | −0.669 | .001 | −0.207 | .24 |
Each row denotes 1 independent model that includes all the chronic condition as explanatory variables.
Cardiac, pulmonary, and vascular function–associated neurocognitive outcomes
Survivors had significantly lower scores (P < .001) in pulmonary (FEV1, TLC, and DLCOcorr), cardiac (LVEF and GLS), and vascular function (SAE) (Table 4). Lower FEV1 (≤69% of the expected value) was associated with worse performance on visuomotor (0.11; P = .008) and visual processing speed (0.012; P = .05) as well as cognitive flexibility (0.013; P = .05; Table 5). Higher GLS was associated with short-term recall (0.056; P = .029). Poorer SAE was associated with cognitive flexibility (0.091; P = .007). Female survivors of HL with a higher FEV1/forced vital capacity (FVC) ratio had a lower risk for global neurocognitive impairment than those with a lower FEV1/FVC ratio (Relative Risk [RR], 0.08; 95% confidence interval, 0.01-0.95) after adjusting for other pulmonary, cardiac, and vascular factors.
Vascular, cardiac, and pulmonary function in survivors and controls at the time of neurocognitive testing
| Test . | Survivors . | Controls . | P value∗ . |
|---|---|---|---|
| Mean (SD) . | Mean (SD) . | ||
| Pulmonary function test | |||
| FEV1 (% predicted) | 82.69 (15.95) | 96.23 (13.31) | <.001 |
| FEV1/FVC (%) | 0.99 (0.09) | 1.00 (0.08) | .240 |
| TLC (% predicted) | 87.27 (15.33) | 98.97 (12.89) | <.001 |
| DLCOcor (% predicted) | 83.31 (16.70) | 98.03 (14.93) | <.001 |
| Cardiac function test | |||
| Ejection fraction (3D ECHO) (%) | 0.56 (0.06) | 0.60 (0.05) | <.001 |
| GLS (%) | −18.30 (2.88) | −20.37 (2.24) | <.001 |
| Vascular studies | |||
| Pulse wave velocity (m/s) | 6.89 (1.70) | 7.24 (4.04) | .300 |
| Large artery elasticity (mL/mm Hg) | 17.44 (6.03) | 18.23 (5.01) | .060 |
| SAE (mL/mm Hg) | 6.94 (3.39) | 8.25 (3.43) | <.001 |
| Systolic blood pressure (mm Hg) | 124.75 (15.94) | 126.02 (12.51) | .410 |
| Diastolic blood pressure (mm Hg) | 74.34 (10.55) | 76.23 (9.87) | .078 |
| Test . | Survivors . | Controls . | P value∗ . |
|---|---|---|---|
| Mean (SD) . | Mean (SD) . | ||
| Pulmonary function test | |||
| FEV1 (% predicted) | 82.69 (15.95) | 96.23 (13.31) | <.001 |
| FEV1/FVC (%) | 0.99 (0.09) | 1.00 (0.08) | .240 |
| TLC (% predicted) | 87.27 (15.33) | 98.97 (12.89) | <.001 |
| DLCOcor (% predicted) | 83.31 (16.70) | 98.03 (14.93) | <.001 |
| Cardiac function test | |||
| Ejection fraction (3D ECHO) (%) | 0.56 (0.06) | 0.60 (0.05) | <.001 |
| GLS (%) | −18.30 (2.88) | −20.37 (2.24) | <.001 |
| Vascular studies | |||
| Pulse wave velocity (m/s) | 6.89 (1.70) | 7.24 (4.04) | .300 |
| Large artery elasticity (mL/mm Hg) | 17.44 (6.03) | 18.23 (5.01) | .060 |
| SAE (mL/mm Hg) | 6.94 (3.39) | 8.25 (3.43) | <.001 |
| Systolic blood pressure (mm Hg) | 124.75 (15.94) | 126.02 (12.51) | .410 |
| Diastolic blood pressure (mm Hg) | 74.34 (10.55) | 76.23 (9.87) | .078 |
P value from a linear regression model adjusted for age and sex.
DLCOcor, diffusion capacity of the lungs for carbon monoxide corrected for hemoglobin; FVC, forced vital capacity; TLC, total lung capacity.
Mean difference in the neurocognitive z score associated with cardiac, pulmonary, and vascular function from multivariable models with Elastic Net variable selection among survivors of HL
| . | Attention . | Processing speed . | ||||||
|---|---|---|---|---|---|---|---|---|
| Sustained . | Visuomotor . | Motor . | Visual . | |||||
| β . | P . | β . | P . | β . | P . | β . | P . | |
| Female | — | 0.42 | .002 | 0.40 | .006 | 0.33 | .015 | |
| Age at examination | — | — | — | — | ||||
| FEV1 | — | 0.011 | .008 | — | 0.012 | .005 | ||
| TLC | — | — | — | — | ||||
| GLS | — | −0.031 | .23 | — | −0.05 | .052 | ||
| SAE | — | — | — | — | ||||
| Large artery elasticity | — | — | — | 0.0379 | .0011 | |||
| . | Attention . | Processing speed . | ||||||
|---|---|---|---|---|---|---|---|---|
| Sustained . | Visuomotor . | Motor . | Visual . | |||||
| β . | P . | β . | P . | β . | P . | β . | P . | |
| Female | — | 0.42 | .002 | 0.40 | .006 | 0.33 | .015 | |
| Age at examination | — | — | — | — | ||||
| FEV1 | — | 0.011 | .008 | — | 0.012 | .005 | ||
| TLC | — | — | — | — | ||||
| GLS | — | −0.031 | .23 | — | −0.05 | .052 | ||
| SAE | — | — | — | — | ||||
| Large artery elasticity | — | — | — | 0.0379 | .0011 | |||
| . | Memory . | Executive function . | ||||||
|---|---|---|---|---|---|---|---|---|
| Verbal learning . | Short-term recall . | Long-term recall . | Cognitive flexibility . | |||||
| β . | P . | β . | P . | β . | P . | β . | P . | |
| Female | — | −0.19 | 0.20 | — | 0.52 | 0.012 | ||
| Age at examination | — | — | 0.028 | 0.047 | ||||
| FEV1 | — | — | 0.013 | 0.05 | ||||
| TLC | — | −0.010 | 0.039 | −0.012 | 0.024 | — | ||
| GLS | — | 0.056 | 0.029 | — | — | |||
| SAE | — | — | 0.091 | 0.007 | ||||
| Large artery elasticity | — | — | — | |||||
| . | Memory . | Executive function . | ||||||
|---|---|---|---|---|---|---|---|---|
| Verbal learning . | Short-term recall . | Long-term recall . | Cognitive flexibility . | |||||
| β . | P . | β . | P . | β . | P . | β . | P . | |
| Female | — | −0.19 | 0.20 | — | 0.52 | 0.012 | ||
| Age at examination | — | — | 0.028 | 0.047 | ||||
| FEV1 | — | — | 0.013 | 0.05 | ||||
| TLC | — | −0.010 | 0.039 | −0.012 | 0.024 | — | ||
| GLS | — | 0.056 | 0.029 | — | — | |||
| SAE | — | — | 0.091 | 0.007 | ||||
| Large artery elasticity | — | — | — | |||||
Note: empty cells represent variables not selected by the Elastic Net process; thus, the estimates could not be calculated.
Discussion
In this large, well-characterized cohort, we found that HL survivors, decades older since the time of diagnosis, had significantly worse neurocognitive performance, cardiopulmonary and vascular function, and chronic health conditions compared with similarly aged community controls. Risk factors for poorer neurocognitive performance included concurrent restrictive (TLC) and obstructive (FEV1) pulmonary disease, impaired cardiac function (GLS), and impaired small artery function. Our findings suggest that processing speed, executive function, and memory outcomes are associated with both lung and heart function and that measures such as GLS, a more sensitive marker of cardiomyopathy in cancer survivors than LVEF, may identify at-risk survivors.28 This is similar to findings in populations without cancer, which have demonstrated links between low FEV1 and declines in executive function, processing speed, and memory outcomes29,30 and GLS with worse memory and language performance.31 However, the severity of cardiac dysfunction in the noncancer population is generally higher or the impact occurs at an older age. Subclinical changes in cardiac, pulmonary, and/or vascular changes in survivors of HL may have a significant impact on neurocognitive declines occurring far earlier (35 years vs 53-79 years of age) than in populations without cancer. As such, promotion of cardiopulmonary health and management of cardiopulmonary function before the onset of clinical chronic conditions may be important for neurocognitive health.
GLS is a measure of left ventricular function that is sensitive to subclinical disease and is a recommended metric to predict the risk of chemotherapy-related left ventricular systolic dysfunction.32,33 The association between GLS and short-term memory outcomes implies that subclinical disease of the left ventricle may be affecting perfusion to the hippocampus. The hippocampus, critical for short-term memory, is supplied with blood vessels from the posterior (via the vertebral arteries) and anterior circulation (via the carotid artery)34 and is highly susceptible to global ischemia. Previous studies in humans and animals have demonstrated that subclinical reductions in cardiac output, like those measured by GLS, reduce blood flow through these vessels.35-38 Further, restricted flow may be induced by radiation.39 Reduced SAE was associated with impaired cognitive flexibility, further suggesting vasculopathy in small vascular beds may contribute to neurocognitive dysfunction in this population.
Moderate-to-severe chronic neurologic conditions were associated with impairments in a wide range of neurocognitive domains, including attention, processing speed, memory, and executive function. The most common neurologic condition in both male and female survivors was moderate-to-severe dizziness and vertigo (supplemental Table 4). Survivors also reported moderate-to-severe motor and sensory neuropathy. The prevalence of peripheral neuropathy, along with symptoms of serious dizziness and vertigo, raises the possibility that the link between neurologic conditions and neurocognitive outcomes may be associated with autonomic dysfunction, which places survivors at risk of poor cerebral blood flow regulation.40 Several important autonomic nervous system structures lie within the neck and chest that could be adversely affected by radiation.41 The carotid and vertebral arteries are at risk of radiation-induced stenosis.42 Moreover, the carotid bifurcation includes chemoreceptors that monitor blood pressure, pH, CO2, and O2 levels.43,44 Via sympathetic tone, these structures help regulate the body’s cardiovascular and respiratory function and contribute to the regulation of cerebral blood flow.45 A similar organ also exists at the aortic arch, which lies within the sternal and mantle fields.46 Dysregulation of cerebral perfusion due to radiation damage to these structures could lead to lightheadedness, syncope, and unexplained falls. In the context of radiation to the neck and chest, carotid and/or aortic arch injury and subsequent cerebral perfusion dysregulation are potential etiologies for chronic neurologic conditions and associated neurocognitive problems.
Associations were found between prednisone dose, attention, and processing speed in female survivors. This is similar to correlations we found in other pediatric populations with cancer that link glucocorticoid exposure and structural and functional brain changes to processing speed and executive function impairments in female survivors of childhood cancer.47,48 It is suspected that female survivors may be more susceptible to the effects of glucocorticoid steroids, given evidence that glucocorticoid receptors exhibit sex differences in their temporal distribution.49 The detrimental effects of glucocorticoids on neurocognitive outcomes may be linked to inhibition of key proteins in the brain’s antioxidant pathway, exacerbation of excitotoxic injury, and myelin disruption.
Several shortcomings limit the interpretation of some of these findings.50 We were unable to investigate the link between radiation-induced damage to neurovascular bundles (ie, carotid sinus, etc.) in the neck and at the root of the great vessels and brain perfusion. Additionally, this cohort underwent historical therapeutic strategies used at a single institution and may not reflect current treatments used at other sites. However, the cardiopulmonary late effects in those treated with more modern therapies, including proton therapy, which can target significant portions of the bronchus, upper lung, accessory muscles, and diaphragm, and brentuximab, are currently unknown in adolescent and young adult survivors and merit investigations similar to those outlined in this study.51,52 Finally, this cohort was treated with pediatric classical HL consortium protocols that routinely included chest and neck radiation (supplemental Table 1). As such, only a few patients with HL (n = 33) received no radiation and survived to participate in this study. Power analysis revealed that this is an insufficient population (25% power) for comparison so were not included. However, the no-radiation survivors of HL received much higher concentrations of anthracyclines, which would lead to more severe cardiovascular risks and would not have diminished the link we found between neurocognitive outcomes and cardiac, pulmonary, and vascular function.
This study presents evidence that measures of subclinical left ventricular dysfunction are associated with poor memory in survivors of HL treated with chest and neck radiation. Additionally, our findings suggest that a complex link between abnormal systolic function before loss of ejection fraction, arterial stiffness, and lung function may contribute to the link between HL treatment and late onset neurocognitive impairments. These findings provide support for new guideline recommendations that endorse follow-up neurocognitive evaluations among survivors of HL survivors with subclinical cardiopulmonary or vascular morbidities late in survivorship. Given the risk for memory decline with normal aging, interventions targeted to improve brain perfusion in patients with HL with subclinical left ventricular dysfunction that may prevent accelerated memory loss in this at-risk population are a priority. Such a trial might evaluate the efficacy of vasopressors vs antihypertensive drugs to preserve or improve cerebral blood flow in survivors of HL. Finally, future research should focus on improving our understanding of the link between cardiopulmonary toxicity and brain function. This would include studies to determine whether altered cerebral perfusion is linked to progressive restricted lung disease, reduced cardiac output, restricted blood vessels, or impaired chemoreceptors damaged by neck radiation.
Acknowledgments
The authors thank the participants and their families without whom this study would not have been possible. The authors especially thank the senior biostatistics analyst Mingjuan Wang for her contributions to this manuscript.
This work was supported by the National Cancer Institute (CA174794 [K.R.K. and N.D.S., principal investigators] and CA157838 [M.M.H., principal investigator]). Support to St. Jude Children's Research Hospital also provided by the Cancer Center Support grant (CA21765) (C. Roberts, principal investigator) and the American Lebanese Syrian Associated Charities.
The funding organizations had no role in the design, collection, management, analysis, or interpretation of the data and preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: This study concept and design were developed by K.R.K. and N.S.P.; data preparation was conducted by W.L.; statistical analysis was conducted by W.L. and K.L.S.; interpretation of data was conducted by N.S.P., K.R.K., N.D.S., and A.M.W.; and all authors contributed to, drafted, or edited the manuscript for submission and are accountable for all aspects of this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kevin R. Krull, Department of Psychology and Biobehavioral Sciences, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS 740, Memphis, TN 38105-3678; e-mail: kevin.krull@stjude.org; and Noah D. Sabin, Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS 220, Memphis, TN 38105; e-mail: Noah.sabin@stjude.org.
References
Author notes
∗N.D.S. and K.R.K. contributed equally as senior authors.
All data collected for this study are available to researchers, to facilitate the development of treatments and cures for childhood cancer and its long-term effects, at https://sjlife.stjude.org/data-sharing.html or from St. Jude Hospital (sjlife@stjude.org).
The full-text version of this article contains a data supplement.