Key Points
Subtle enhanced VWF clearance is present in 20% of patients with low VWF and is associated with an attenuated bleeding phenotype.
In patients with low VWF, there is poor correlation between desmopressin fall-off rates and steady-state VWFpp/VWF:Ag ratios.
Abstract
Increased von Willebrand factor (VWF) clearance plays a key role in the pathogenesis of type 1 and type 2 von Willebrand disease (VWD). However, the pathological mechanisms involved in patients with mild to moderate reductions in plasma VWF:Ag (range, 30-50 IU/dL; low VWF) remain poorly understood. In this study, we investigated the hypothesis that enhanced VWF clearance may contribute to the pathobiology of low VWF. Patients with low VWF were recruited to the LoVIC study after ethics approval and receipt of informed consent. Desmopressin was administered IV in 75 patients, and blood samples were drawn at baseline and at the 1-hour and 4-hour time points. As defined by recent ASH/ISTH/NHF/WFH guidelines, 20% of our low-VWF cohort demonstrated significantly enhanced VWF clearance. Importantly, from a clinical perspective, this enhanced VWF clearance was seen after desmopressin infusion, but did not affect the steady-state VWF propeptide (VWFpp)-to-VWF antigen (VWF:Ag) ratio (VWFpp/VWF:Ag) in most cases. The discrepancy between the VWFpp/VWF:Ag ratio and desmopressin fall-off rates in patients with mild quantitative VWD may have reflected alteration in VWFpp clearance kinetics. Finally, bleeding scores were significantly lower in patients with low VWF with enhanced VWF clearance, compared with those in whom reduced VWF biosynthesis represented the principle pathogenic mechanism. This trial was registered at http://www.clinicaltrials.gov as #NCT03167320.
Introduction
A role for enhanced von Willebrand factor (VWF) clearance in the pathogenesis of VWD was first highlighted by postdesmopressin fall-off studies in patients with Vicenza VWD (p.R1205H).1 Subsequent studies showed that the steady-state VWFpp/VWF:Ag could also be used to identify patients with VWD with increased clearance.2,3 Using these methodologies, the US Zimmerman Program,4 the Willebrand in The Netherlands (WiN) study,5 and the MCMDM-1VWD European6 study observed pathological enhanced VWF clearance in >40% of patients with type 1 VWD (plasma VWF:Ag levels <30 IU/dL). These findings led to the recommendation that affected patients be subclassified as type 1C (1-clearance).2,7 More recently, enhanced VWF clearance has also been reported in patients with type 2 and type 3 VWD.5
The pathological mechanisms involved in patients with mild to moderate reductions in plasma VWF:Ag levels (range, 30-50 IU/dL; low VWF) remain poorly understood.8,9 Previous studies demonstrated that VWF gene pathogenic sequence variants are less common in patients with VWF plasma levels in the range of 30 to 50 IU/dL, when compared with those with levels <30 IU/dL.10,11 Moreover, linkage studies have shown that the condition is often not linked to the VWF locus on chromosome 12.12,13 Given that low VWF constitutes the commonest subtype of VWD,7,8 management of these patients continues to pose significant challenges. In this study, we investigated the hypothesis that increased VWF clearance may contribute to the pathobiology of low VWF. Subtle enhanced clearance was present in ∼20% of patients with low VWF. Importantly however, although this enhanced clearance was observed after desmopressin infusion, there was no increase in the steady-state VWFpp/VWF:Ag ratio in most patients. Finally, our data further suggest that the bleeding phenotype is attenuated in patients with low VWF with enhanced VWF clearance compared with those in whom reduced VWF biosynthesis represents the principle pathogenic mechanism.
Methods
Patients with low VWF levels were enrolled in the LoVIC study, as previously described.11,14 All patients had lowest plasma VWF levels (VWF:Ag, VWF:RCo, or VWF:CB) of 30 to 50 IU/dL, measured on 2 separate occasions at least 3 months apart. The LoVIC study was approved by the St James’ Hospital Research Ethics Committee, and written informed consent was provided by all participants. Bleeding phenotype was assessed with a physician-administered questionnaire of bleeding symptoms occurring before diagnosis.11 All hemostasis laboratory testing was performed in a single National Haemostatic Reference Laboratory. Plasma VWFpp levels were determined by enzyme-linked immunosorbent assay using the monoclonal antibodies CLB-Pro 35 and CLB-Pro 14.3-HRP (Sanquin, The Netherlands) on all patient samples at recruitment to the study, as previously described.11 Desmopressin (DDAVP) trials were performed in 75 patients with low VWF.15 In brief, DDAVP (0.3 μg/kg; maximum dose capped at 27 μg/kg) was administered IV, and blood samples were drawn for VWF:Ag, VWF:RCo, and FVIII:C at baseline (T0), 1 hour (T+1), and 4 hours (T+4). In a subset of 24 patients, additional samples were drawn at 24 hours (T+24). In those patients, elimination half-life (t1/2el) was estimated using the equation C(t) = C0e(-kt), where C(t) is plasma VWF:Ag level as a function over time, C0 is the plasma VWF:Ag level at baseline, k is the elimination phase first order rate constant and t is time, as previously described.16 Statistical analyses were performed with the Mann-Whitney U test and Pearson’s or Spearman’s rank correlation in Prism 8.4.3 (GraphPad Software, San Diego, CA), with P < .05 considered to indicate statistical significance. The association between VWF levels and bleeding score was assessed with linear regression analysis; outcomes are presented as the β coefficient and 95% confidence interval (CI).
Results and discussion
Subtle, enhanced VWF clearance plays an important role in the pathogenesis of low VWF
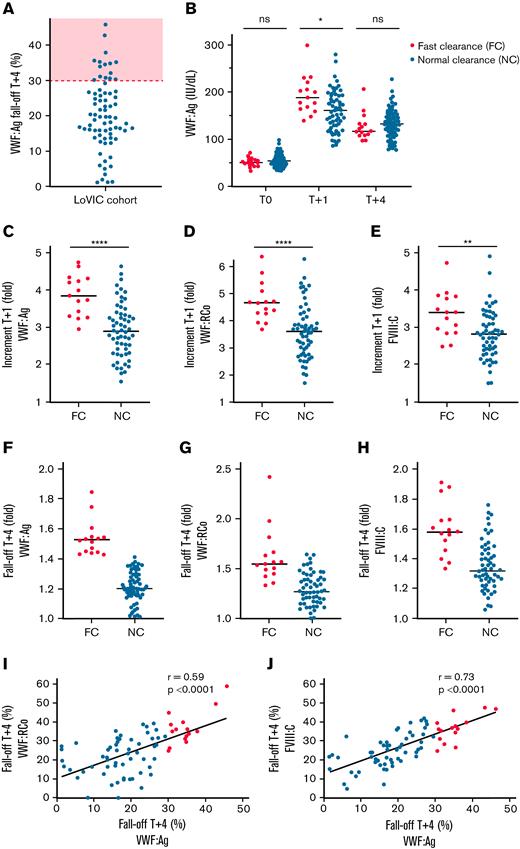
In keeping with ASH/ISTH/NHF/WFH guideline recommendations,7 enhanced VWF clearance was defined as plasma VWF:Ag fall-off >30% between peak (T+1 hours) and T+4 hours after desmopressin infusion. Of our low-VWF cohort, 20% (15 of 75) demonstrated enhanced clearance based on these criteria (Figure 1A). We further examined the subgroup of patients with low VWF with enhanced clearance (low VWFc) compared with patients with low VWF with normal clearance. Steady-state plasma VWF:Ag levels were similar between the 2 subgroups (Figure 1B; supplemental Figure 1A-B). Importantly however, the increase in plasma VWF:Ag levels at 1 hour after DDAVP was significantly higher in low VWFc compared with patients with low VWF with normal clearance (median, 3.84 vs 2.89-fold; P < .0001; Figure 1C). Similarly, DDAVP-induced increases in plasma VWF:RCo and FVIII:C levels at T+1 hour were both also significantly increased in the low-VWFc subgroup (Figure 1D-E). In contrast, patients with low VWFc demonstrated reduced VWF half-lives, such that the fall-off in VWF:Ag, VWF:RCo, and FVIII:C were all enhanced between 1 and 4 hours (Figure 1F-H). Postdesmopressin fall-offs in plasma VWF:RCo and FVIII:C correlated significantly with that in VWF:Ag (Figure 1I-J). Furthermore, peak VWF:Ag response at 1 hour correlated strongly with subsequent 4-hour fall-off rates (r = 0.70; P < .0001; supplemental Figure 1C). Together, these data clearly demonstrate that subtle enhanced VWF clearance plays an important pathogenic role in a significant subgroup of patients with low VWF.
Enhanced clearance plays an important role in the pathogenesis of low VWF. (A) VWF:Ag fall-off (decrease in VWF:Ag from T+1 to T+4 hours after desmopressin infusion, as a percentage of T+1) is illustrated on the y-axis. The red dotted line and shaded area illustrate the ASH/ISTH/NHF/WFH guideline7 threshold of >30% for enhanced clearance. (B) Plasma VWF:Ag levels at 1 hour and 4 hours after desmopressin treatment in patients with low VWF with FC compared with patients with low VWF with NC rates. (C) Patients with low VWF with FC had a significantly greater increment in plasma VWF:Ag levels at 1 hour after desmopressin (median, 3.84-fold vs 2.89-fold; P < .0001). The increment is expressed as fold change (T+1/T0). Similarly, patients with low VWF with FC demonstrated significantly greater increments in plasma VWF:RCo (D) (median, 4.66-fold vs 3.60-fold; P < .0001) and plasma FVIII:C levels (E) (median, 3.40-fold vs 2.81-fold; P < .01) at 1 hour after desmopressin infusion. (F-H) Conversely, patients with low VWF with FC demonstrated greater fall-offs in plasma VWF:Ag, VWF:RCo, and FVIII:C levels between 1 hour and 4 hours after desmopressin. All fall-off values are expressed as fold change between the 1-hour and 4-hour time points. (I-J) In patients with low VWF, fall-off rates in plasma VWF:Ag levels after desmopressin treatment correlated significantly with fall-off rates in both VWF:RCo and FVIII:C (Pearson’s r), consistent with faster clearance of the VWF-FVIII complex from the circulation. Median values are illustrated by black lines. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Enhanced clearance plays an important role in the pathogenesis of low VWF. (A) VWF:Ag fall-off (decrease in VWF:Ag from T+1 to T+4 hours after desmopressin infusion, as a percentage of T+1) is illustrated on the y-axis. The red dotted line and shaded area illustrate the ASH/ISTH/NHF/WFH guideline7 threshold of >30% for enhanced clearance. (B) Plasma VWF:Ag levels at 1 hour and 4 hours after desmopressin treatment in patients with low VWF with FC compared with patients with low VWF with NC rates. (C) Patients with low VWF with FC had a significantly greater increment in plasma VWF:Ag levels at 1 hour after desmopressin (median, 3.84-fold vs 2.89-fold; P < .0001). The increment is expressed as fold change (T+1/T0). Similarly, patients with low VWF with FC demonstrated significantly greater increments in plasma VWF:RCo (D) (median, 4.66-fold vs 3.60-fold; P < .0001) and plasma FVIII:C levels (E) (median, 3.40-fold vs 2.81-fold; P < .01) at 1 hour after desmopressin infusion. (F-H) Conversely, patients with low VWF with FC demonstrated greater fall-offs in plasma VWF:Ag, VWF:RCo, and FVIII:C levels between 1 hour and 4 hours after desmopressin. All fall-off values are expressed as fold change between the 1-hour and 4-hour time points. (I-J) In patients with low VWF, fall-off rates in plasma VWF:Ag levels after desmopressin treatment correlated significantly with fall-off rates in both VWF:RCo and FVIII:C (Pearson’s r), consistent with faster clearance of the VWF-FVIII complex from the circulation. Median values are illustrated by black lines. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Limitations of VWFpp/VWF:Ag ratio in patients with low VWF
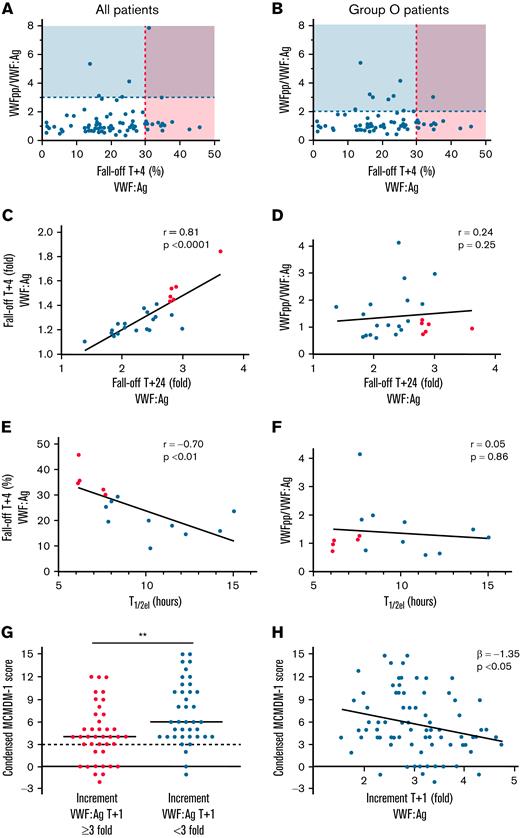
Previous studies used a VWFpp/VWF:Ag ratio >3 to identify patients with type 1C VWD.2,3 However, biological factors influencing VWFpp half-life remain unknown, and it is not clear whether VWFpp biosynthesis and/or clearance may vary in some patients with VWD.17 Recent guidelines have highlighted the need for studies directly comparing the VWFpp/VWF:Ag ratio vs the desmopressin fall-off in VWD subgroups.7 In striking contrast to the desmopressin fall-off data demonstrating enhanced clearance in 20% of patients with low VWF, only 8% (6 of 75) had steady-state VWFpp/VWF:Ag ratios >3 (Figure 2A). Because the majority of our patients with low VWF were blood group O (64 of 75; 85.3%), we assessed the utility of a blood group O–specific VWFpp/VWF:Ag cutoff (ratio >2.15; 95% CI in blood group O normal subjects, 0.67-2.15).18 Even with this lower threshold, only 12% (9 of 75) of our low-VWF cohort had elevated VWFpp/VWF:Ag ratios (Figure 2B). Critically, we also found a clear discrepancy between patients with low VWF identified with enhanced clearance based on fast fall-off after desmopressin infusion, compared with those with abnormal steady-state VWFpp/VWF:Ag ratios (Figure 2A-B). Indeed, there was no correlation between VWFpp/VWF:Ag ratios and DDAVP 4-hour fall-offs (r = 0.21; P = .075; Figure 2A). Overall, our data demonstrate that the sensitivity of the VWFpp/VWF:Ag ratio for identifying enhanced clearance in the low-VWF cohort is lower than 15% when compared with the gold-standard assessment of desmopressin fall-off levels.
Limitations of VWFpp/VWF:Ag ratio in detection of fast VWF clearance in patients with low VWF and the impact of clearance on bleeding phenotype. (A) Only 8% (6 of 75) of patients with low VWF had VWFpp/VWF:Ag ratios >3 (above the dashed blue line). In contrast, 20% (15 of 75) of the patients with low VWF had enhanced clearance based on fall-off rates after desmopressin (to the right of the dashed red line: shaded area with >30% fall-off in VWF:Ag levels at 4 hours, per ASH/ISTH/NHF/WFH guidelines). The blue line illustrates VWFpp/VWF:Ag ratio >3. Patients with low VWF with suspected enhanced clearance, as defined by VWFpp/VWF:Ag >3 only are shown in the blue box. Patients with low VWF with confirmed enhanced clearance after desmopressin trial only are shown in the light red box. Finally, patients with low VWF with both positive VWFpp/VWF:Ag and enhanced clearance after desmopressin are in the purple box. (B) Because the majority of patients with low VWF were of blood group O (64 of 75; 85.3%), the VWFpp/VWF:Ag vs desmopressin fall-off comparison was repeated with a blood group O–specific VWFpp/VWF:Ag cutoff of >2.15 (corresponds to lower 97.5% CI for normal subjects in blood group O). Even with this lower cutoff, only 12% (9 of 75) of patients with low VWF had an elevated VWFpp/VWF:Ag ratio. (C) In patients with low VWF, 24 hour fall-off in plasma VWF:Ag levels (n = 24) strongly correlated with 4-hour fall-off of VWF:Ag levels (Spearman’s r). The 24-hour fall-off is expressed as fold change (T+24/T+1−1). (D) Conversely, there was no correlation between the 24-hour fall-off in VWF:Ag and steady-state plasma VWFpp/VWF:Ag ratios (Spearman’s r). (E-F) Elimination half-lives (t1/2el in hours) in patients with low VWF correlated inversely with the 4-hour fall-off plasma VWF:Ag levels after desmopressin (Pearson’s r) but did not correlate with VWFpp/VWF:Ag ratios (Spearman’s r). (G) Patients with low VWF with a threefold or higher increment in plasma VWF:Ag levels after desmopressin (illustrated as fold change, T+1/T+0) had significantly lower bleeding scores than patients with low VWF with attenuated desmopressin responses (median, 4 vs 6; P < .01). The upper limit of the normal condensed MCMDM-1 VWD score range is illustrated by the black dotted line (<3 for both males and females). (H) Increment in plasma VWF:Ag levels after desmopressin was significantly associated with the condensed MCMDM-1 VWD bleeding score. (C-H) Fast clearance, red dots; normal clearance, blue dots.
Limitations of VWFpp/VWF:Ag ratio in detection of fast VWF clearance in patients with low VWF and the impact of clearance on bleeding phenotype. (A) Only 8% (6 of 75) of patients with low VWF had VWFpp/VWF:Ag ratios >3 (above the dashed blue line). In contrast, 20% (15 of 75) of the patients with low VWF had enhanced clearance based on fall-off rates after desmopressin (to the right of the dashed red line: shaded area with >30% fall-off in VWF:Ag levels at 4 hours, per ASH/ISTH/NHF/WFH guidelines). The blue line illustrates VWFpp/VWF:Ag ratio >3. Patients with low VWF with suspected enhanced clearance, as defined by VWFpp/VWF:Ag >3 only are shown in the blue box. Patients with low VWF with confirmed enhanced clearance after desmopressin trial only are shown in the light red box. Finally, patients with low VWF with both positive VWFpp/VWF:Ag and enhanced clearance after desmopressin are in the purple box. (B) Because the majority of patients with low VWF were of blood group O (64 of 75; 85.3%), the VWFpp/VWF:Ag vs desmopressin fall-off comparison was repeated with a blood group O–specific VWFpp/VWF:Ag cutoff of >2.15 (corresponds to lower 97.5% CI for normal subjects in blood group O). Even with this lower cutoff, only 12% (9 of 75) of patients with low VWF had an elevated VWFpp/VWF:Ag ratio. (C) In patients with low VWF, 24 hour fall-off in plasma VWF:Ag levels (n = 24) strongly correlated with 4-hour fall-off of VWF:Ag levels (Spearman’s r). The 24-hour fall-off is expressed as fold change (T+24/T+1−1). (D) Conversely, there was no correlation between the 24-hour fall-off in VWF:Ag and steady-state plasma VWFpp/VWF:Ag ratios (Spearman’s r). (E-F) Elimination half-lives (t1/2el in hours) in patients with low VWF correlated inversely with the 4-hour fall-off plasma VWF:Ag levels after desmopressin (Pearson’s r) but did not correlate with VWFpp/VWF:Ag ratios (Spearman’s r). (G) Patients with low VWF with a threefold or higher increment in plasma VWF:Ag levels after desmopressin (illustrated as fold change, T+1/T+0) had significantly lower bleeding scores than patients with low VWF with attenuated desmopressin responses (median, 4 vs 6; P < .01). The upper limit of the normal condensed MCMDM-1 VWD score range is illustrated by the black dotted line (<3 for both males and females). (H) Increment in plasma VWF:Ag levels after desmopressin was significantly associated with the condensed MCMDM-1 VWD bleeding score. (C-H) Fast clearance, red dots; normal clearance, blue dots.
VWF testing was repeated at 24 hours after desmopressin infusion in 24 patients (24 of 75; 32%). The decrease in plasma VWF:Ag from peak to T+24 hours strongly correlated with T+4-hour fall-off data (Figure 2C) but not with steady-state VWFpp/VWF:Ag ratios (Figure 2D). Using these 24-hour data, VWF t1/2el was determined for 15 patients with low VWF. The t1/2el was significantly reduced in the low-VWFc subgroup compared with low the VWFn group (median 6.2 vs 10.2 hours; P = .0007). In addition, t1/2el inversely correlated with the 4-hour fall-off data (Figure 2E) but not with VWFpp/VWF:Ag ratios (Figure 2F). Collectively, these novel findings demonstrate that the VWFpp/VWF:Ag ratio and desmopressin fall-off rate correlate poorly in patients with low VWF. Thus, although the VWFpp/VWF:Ag ratio is useful in identifying patients with type 1C disease with markedly increased VWF clearance (t1/2 in the range of 1-4 hours), it is less sensitive in patients with more modest, reduced VWF half-lives (t1/2 ∼6 hours). We hypothesize that differences between VWFpp/VWF:Ag ratio and desmopressin fall-off data in some patients with low VWF may result from alterations in VWFpp clearance kinetics, either as a result of abnormal posttranslational modification within endothelial cells (ECs) before secretion, or because of alterations in specific clearance pathways.19 Consistent with this hypothesis, our preliminary results (n = 17 patients with low VWF) suggest that there is significant variability in VWFpp fall-off at 4 hours (data not shown).
Clinical significance of enhanced clearance in patients with low VWF
Desmopressin response provides a measure of endogenous VWF and FVIII stores within ECs and has been proposed as a surrogate marker of capacity to respond to hemostatic challenge. Moreover, previous studies in carriers of hemophilia A and patients with type 1 VWD (levels <30 IU/dL) reported associations between desmopressin response and bleeding phenotype.20,21 Castaman et al previously defined a threefold or greater VWF level as a measure of desmopressin responsiveness.22 Interestingly, we observed that condensed MCMDM-1 (Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWF) bleeding scores were significantly reduced in patients with low VWF with a threefold or greater increase in VWF:Ag at T+1 hour post desmopressin, compared with those with attenuated desmopressin responses (median condensed MCMDM-1 score 4 vs 6; P = .002; Figure 2G). Consistent with previous studies,10,11 baseline VWF levels were not associated with bleeding score on linear regression analysis (β = 5.97; 95% CI −1.46 to 13.41). However, the VWF response to desmopressin was significantly associated with bleeding score (β = −1.35, 95% CI −2.58 to −0.12; Figure 2H). Moreover, this association strengthened after adjustment for potential confounders of age at diagnosis and sex (β = −1.39; 95% CI −2.54 to −0.23).
In summary, our findings demonstrate that enhanced VWF clearance plays an important role in the pathogenesis of low VWF. Importantly, from a clinical perspective, this enhanced VWF clearance is apparent after desmopressin infusion, but does not affect the VWFpp/VWF:Ag ratio in many cases. We hypothesize that the bleeding phenotype is less severe in patients with low VWF with enhanced clearance pathophysiology, because these patients have enhanced EC stores that can be used to respond to hemostatic challenge.
Acknowledgments
This work was performed within the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board (Grant Number 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division, Northern Ireland. The study was also supported by funds from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grant HL081588 for the Zimmerman Program and a Science Foundation Ireland Principal Frontiers for the Future (FFP) award (20/FFP-A/8952).
Authorship
Contribution: D.D., M.L., M.B., J.M.O., K.R., N.M.O., and J.S.O. performed experiments; and all authors designed the research, analyzed the data, and were involved in writing and reviewing the manuscript.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma and on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and has received research grants from Baxter, Bayer, Pfizer, Shire, Takeda, and Novo Nordisk. M.L has served as a consultant for Sobi and CSL Behring and has received indirect funding for development of educational content from Takeda. J.M.O. has received research grant funding from LEO Pharma and Grifols. P.J. has received research funding from CSL Behring, Takeda, and Bayer. The remaining authors declare no competing financial interests.
A complete list of the members of the Zimmerman Program Investigators appears in the “Appendix.”
Correspondence: James O’Donnell, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, Ardilaun House, 111 St Stephen’s Green, Dublin 2, Ireland; e-mail: jamesodonnell@rcsi.ie.
Appendix
The Zimmerman Program Investigators are as follows: Principal Investigators—R. Montgomery, V. Flood, S. Haberichter, T. Abshire, H. Weiler, Versiti Blood Research Institute, Milwaukee, WI; D. Lillicrap, P. James, Queen’s University, Kingston, ON, Canada; J. O’Donnell, Royal College of Surgeons in Ireland, Dublin, Ireland, C. Ng, University of Colorado, Denver, CO; J. Di Paola, B. Sadler, Washington University in St. Louis, St. Louis, MO. Directors of the primary centers—T. Abshire, C. Bennett, R. Sidonio, Emory University School of Medicine, Atlanta, GA; M. Manco-Johnson, J. Di Paola, C. Ng, Mountain States Regional Hemophilia and Thrombosis Center, Aurora, CO; J. Journeycake, A. Zia, UT Southwestern, Dallas, TX; J. Lusher, M. Rajpurkar, Wayne State University, Detroit, MI; A. Shapiro, Indiana Hemophilia & Thrombosis Center, Indianapolis, IN; S. Lentz, University of Iowa, Iowa City, IA; J. Gill, V. Flood, Comprehensive Center for Bleeding Disorders, Milwaukee, WI; C. Leissinger, Tulane University Health Sciences Center, New Orleans, LA; M. Ragni, University of Pittsburgh, Pittsburgh, PA; M. Tarantino, J. Roberts, Bleeding & Clotting Disorders Institute, Peoria, IL; P. James, Queen’s University, Kingston, ON, Canada.
References
Author notes
Original data are available by e-mail request to the corresponding author, James S. O'Donnell (jamesodonnell@rcsi.ie).
The full-text version of this article contains a data supplement.